2022 Volume 256 Issue 4 Pages 291-301
2022 Volume 256 Issue 4 Pages 291-301
Vasohibin-1 (VASH1) is an angiogenesis inhibitor, while vasohibin-2 (VASH2) is a proangiogenic factor. The roles of VASH1 and VASH2 expression in gastroenterological cancers remain unclear. We searched for relevant literature, specifically studies on gastroenterological cancer, and evaluated the relationship between VASH expression and clinical outcomes. Nine studies on VASH1 involving 1,574 patients were included. VASH1 expression was associated with the TNM stage [OR (odds ratio) 2.05, 95% CI (confidence interval) 1.24-3.40], lymph node metastasis (OR 1.79, 95% CI 1.24-2.58), lymphatic invasion (OR 1.95, 95% CI 1.41-2.68), and venous invasion (OR 2.49, 95% CI 1.60-3.88); poor clinical outcomes were associated with high VASH1 expression. High VASH1 expression was associated with a significantly shorter overall survival (OS) [HR (hazard ratio) 1.69, 95% CI 1.25-2.29] and disease-free survival (DFS) (HR 2.01, 95% CI 1.28-3.15). Three studies on VASH2 involving 469 patients were analyzed. VASH2 expression was associated with the TNM stage (OR 4.21, 95% CI 1.89-9.51) and venous invasion (OR 2.10, 95% CI 1.15-3.84); poor clinical outcomes were associated with high VASH2 expression. High VASH2 expression was associated with a significantly lower OS (HR 1.61, 95% CI 1.09-2.37). In conclusion, high VASH1 and VASH2 expression levels were associated with poor clinical outcomes and prognosis in patients with gastroenterological cancers.
Vasohibins (VASH), which are novel angiogenesis regulators, consist of two subtypes; asohibin-1 (VASH1), and its homologue Vasohibin-2 (VASH2) (Watanabe et al. 2004; Shibuya et al. 2006). VASH1 is an endogenous angiogenesis inhibitor that was originally identified in a microarray analysis of genes upregulated by vascular endothelial growth factor (VEGF) in vascular endothelial cells (ECs) (Watanabe et al. 2004). VASH1 is mainly expressed in ECs in the termination zone to halt angiogenesis. Meanwhile, VASH2 is an endogenous proangiogenic factor, and the amino acid sequence of human VASH2 protein is 52.5% homologous to that of human VASH1 protein (Shibuya et al. 2006). VASH2 is mainly expressed on cancer cells and on CD11b-positive mononuclear cells mobilized from the bone marrow at the sprouting front to stimulate angiogenesis. Both VASH1 and VASH2 have been reported as prognostic factors for several types of cancer (Tamaki et al. 2009; Miyazaki et al. 2012; Takahashi et al. 2012; Kosaka et al. 2013; Zhang et al. 2014; Mikami et al. 2017; Torii et al. 2017). However, the role of VASH1 and VASH2 expression in gastroenterological cancers remains unclear. In this study, we reviewed and conducted a meta-analysis to clarify the relationship between vasohibin expression in gastroenterological tumors and clinicopathological factors.
This meta-analysis followed the Prisma guidelines (Moher et al. 2010).
Literature search strategyWe searched for relevant articles in Google Scholar and PubMed using the following keywords: “vasohibin,” “vasohibin-1,” “vasohibin-2,” “cancer,” “carcinoma,” and “expression.” Also, the reference lists of the included studies and related comments were manually filtered to search for new studies of possible relevance.
Inclusion and exclusion criteriaStudies were included if they (1) were studies on gastroenterological cancer, (2) evaluated the relationship between VASH expression and clinical outcomes, (3) reported the clinicopathological parameters of the subjects, and (4) were written in English. The exclusion criteria were as follows: (1) studies on cancers other than gastroenterological cancer, and (2) studies in which the clinical course or clinicopathological factors were not reported.
Data extractionThe following data were recorded from all eligible studies; (1) the first author’s name and year of publication, (2) the study’s nationality, (3) cancer types, (4) sample and pathology type, (5) the cut-off value and assay method, (6) follow-up period (in months), and (7) case numbers with high vasohibin expression and survival outcomes.
Statistical methodsThe free downloadable software EZR was used to calculate all the statistical analyses (Kanda 2013). All reported P values were two-sided, with P < 0.05 regarded as being statistically significant. The odds ratio (OR) or hazard ratio (HR) and the 95% confidence interval (CI) were measured using fixed-effects or random-effects models. Publication bias was assessed using funnel plots.
The flowchart in Fig. 1 shows the literature retrieval and selection process. In a search of Google Scholar and PubMed, we initially collected a total of 167 studies; however, 151 of them were excluded after screening the titles and abstracts. After a further review of the remaining studies, 5 studies were excluded because they involved animal research or were not relevant to the current analysis. Eventually, 10 studies (9 studies on VASH1, and 3 studies on VASH2 including 2 duplicates) were identified as meeting our inclusion criteria (Wang et al. 2012; Yan et al. 2014; Kitajima et al. 2014; Murakami et al. 2014; Liu et al. 2015; Kim et al. 2015; Shen et al. 2016; Ma et al. 2017; Ninomiya et al. 2018; Hara et al. 2020). Detailed information on these studies is shown in Tables 1 and 2.
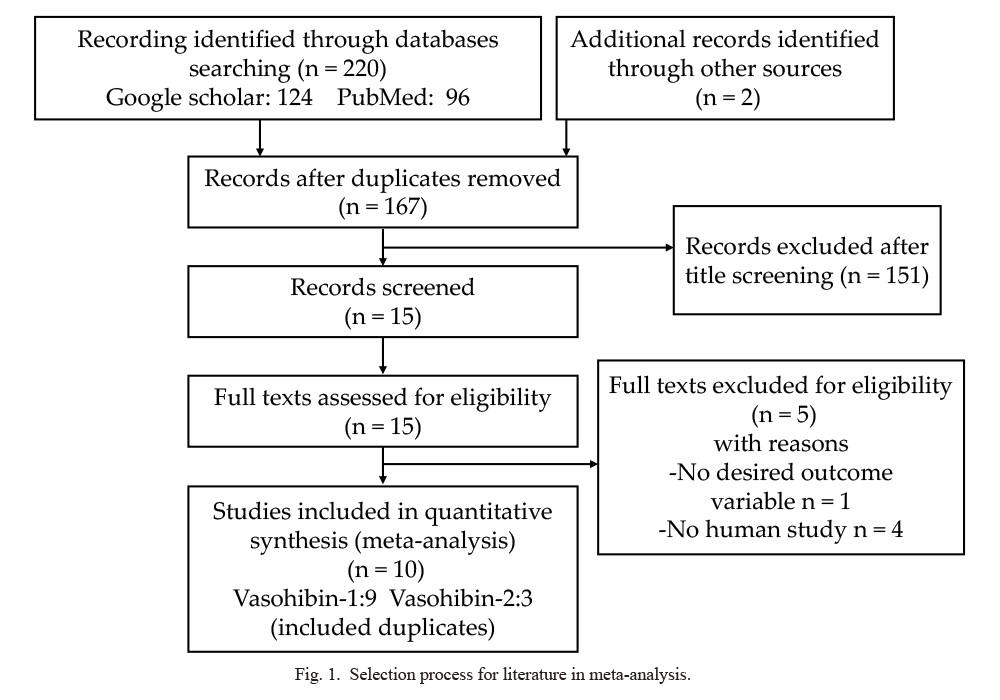
Selection process for literature in meta-analysis.
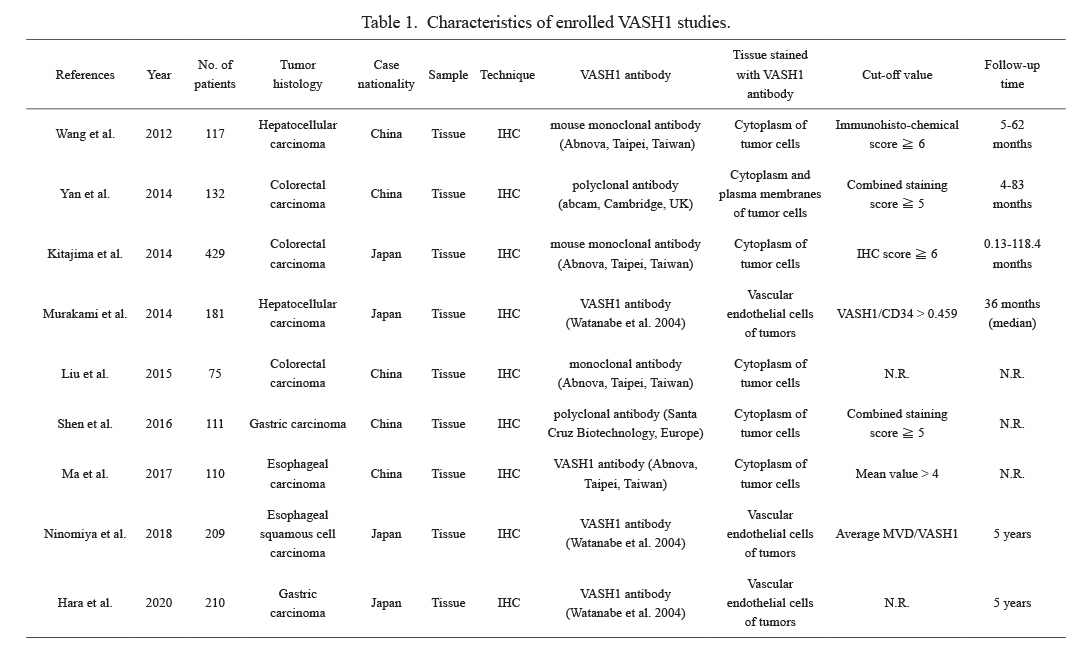
Characteristics of enrolled VASH1 studies.
VASH, vasohibin; IHC, immunohistochemistry; MVD, microvessels density; N.R., not reported.
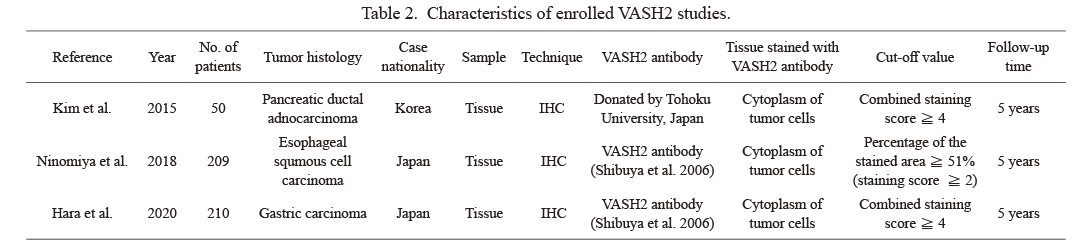
Characteristics of enrolled VASH2 studies.
VASH, vasohibin; IHC, immunohistochemistry.
A total of 9 studies on VASH1 involving 1,574 patients were analyzed; 2 on esophageal cancer, 2 on gastric cancer, 3 on colorectal cancer, and 2 on hepatocellular carcinoma. After referring to each of the studies, “VASH1-positive” was defined as VASH1-positive or high VASH1 expression in the text, and “VASH1 negative” was defined as VASH1-negative or low VASH1 expression in the text (Table 3). VASH1 expression was associated with the tumor-node-metastasis (TNM) stage (OR 2.05, 95% CI 1.24-3.40), lymph node metastasis (OR 1.79, 95% CI 1.24-2.58), lymphatic invasion (OR 1.95, 95% CI 1.41-2.68), and venous invasion (OR 2.49, 95% CI 1.60-3.88), and poor clinical outcomes were associated with high VASH1 expression (Fig. 2). VASH1 expression was associated with a significantly shorter overall survival (OS) (HR 1.69 95% CI 1.25-2.29) and disease-free survival (DFS) (HR 2.01, 95% CI 1.28-3.15).
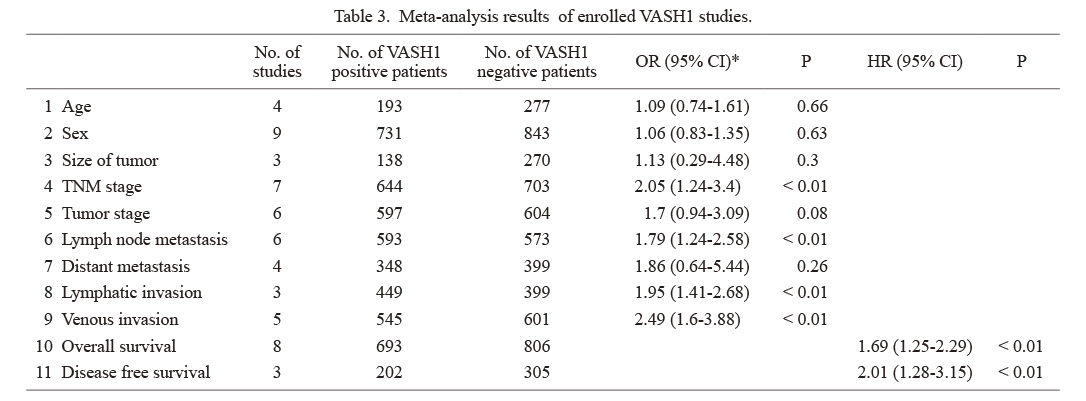
Meta-analysis results of enrolled VASH1 studies.
*Random effects models.
VASH, vasohibin; OR, odds ratio; CI, confidence interval; HR, hazard ratio; TNM stage, tumor-node-metastasis stage.
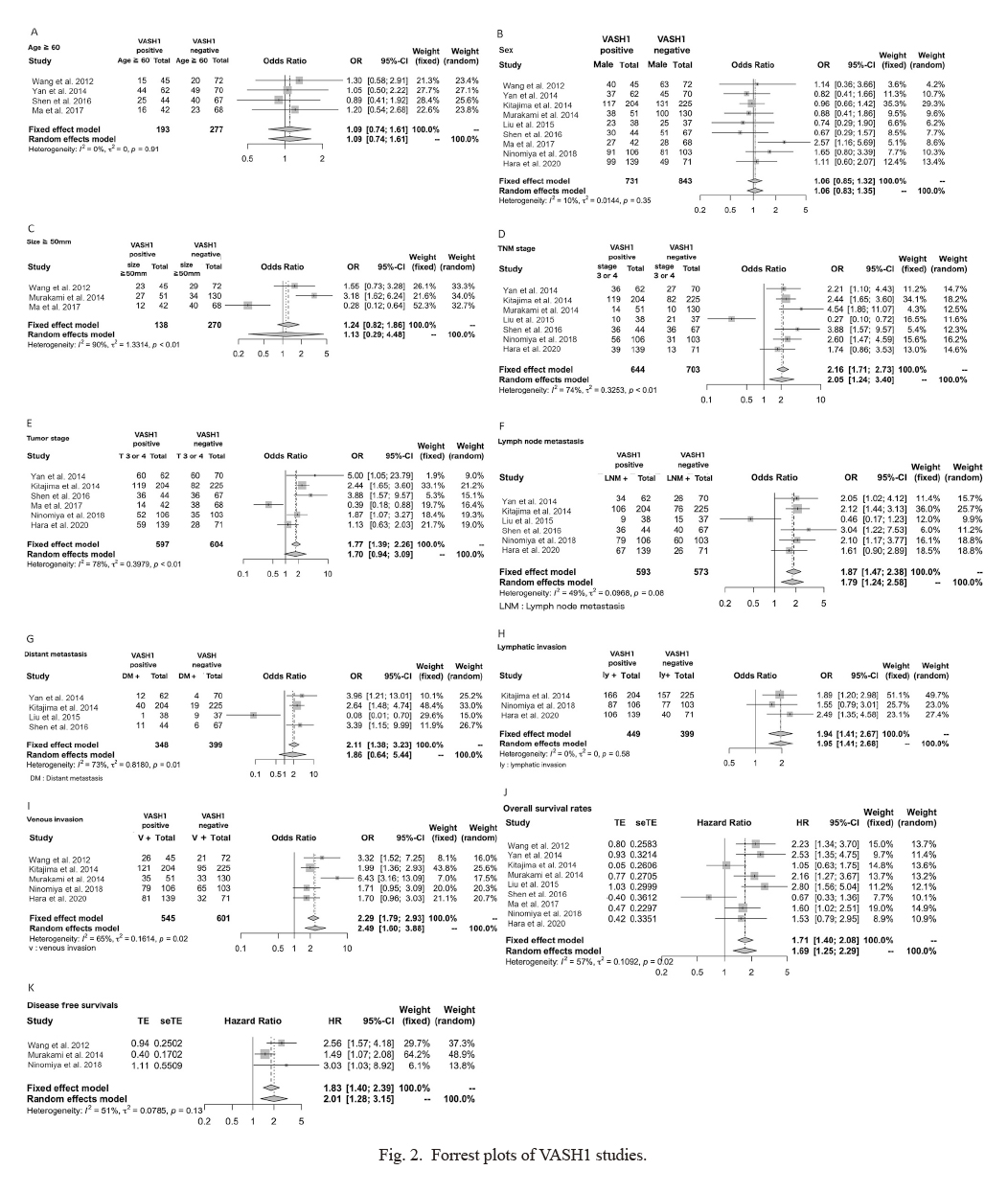
Forrest plots of VASH1 studies.
(A) Age, (B) sex, (C) size, (D) tumor-node-metastasis (TNM) stage, (E) tumor stage, (F) lymph node metastasis, (G) distant metastasis, (H) lymphatic invasion, (I) venous invasion, (J) overall survival rates, and (K) disease free survival rates.
VASH, vasohibin; OR, odds ratio; HR, hazard ratio; CI, confidence interval; LNM, lymph node metastasis; DM, distant metastasis; ly, lymphatic invasion; v, venous invasion.
A total of 3 studies on VASH2 involving 469 patients were analyzed: 1 on esophageal cancer, 1 on gastric cancer, and 1 on pancreatic carcinoma. After referring to each of the studies, “VASH2-positive” was defined as VASH2-positive or high VASH2 expression in the text, and “VASH2-negative” was defined as VASH2-negative or low VASH2 expression in the text (Table 4). VASH2 expression was associated with the TNM stage (OR 4.21, 95% CI 1.89-9.51) and venous invasion (OR 2.10, 95% CI 1.15-3.84), and poor clinical outcomes were associated with high VASH2 expression (Fig. 3). VASH2 expression was associated with a significantly shorter (HR 1.61, 95% CI 1.09-2.37).
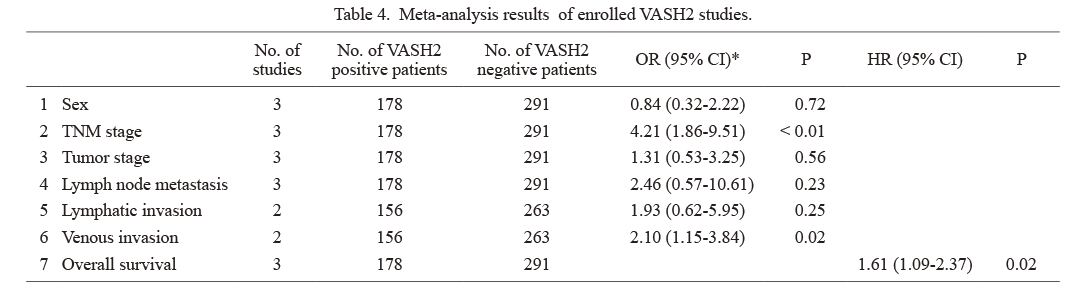
Meta-analysis results of enrolled VASH2 studies.
*Random effects models.
VASH, vasohibin; OR, odds ratio; CI, confidence interval; HR, hazard ratio; TNM stage, tumor-node-metastasis stage.
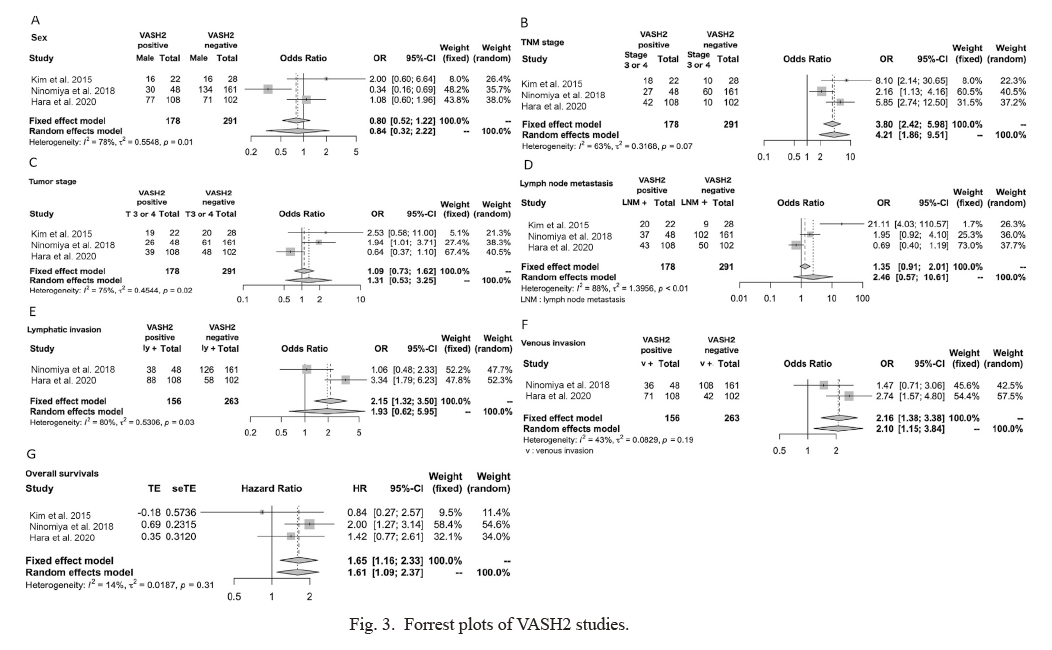
Forrest plots of VASH2 studies.
(A) Sex, (B) tumor-node-metastasis (TNM) stage, (C) tumor stage, (D) lymph node metastasis, (E) lymphatic invasion, (F) venous invasion, and (G) overall survival rates.
VASH, vasohibin; OR, odds ratio; HR, hazard ratio; CI, confidence interval; LNM, lymph node metastasis; ly, lymphatic invasion; v, venous invasion.
We analyzed publications on VASH1 according to cancer type (Table 5, Fig. 4). In hepatocellular carcinoma, VASH1 expression was associated with tumor size (OR 2.26, 95% CI 1.12-4.57), venous invasion (OR 4.72, 95% CI 2.47-9.0), OS (HR 2.20, 95% CI 1.52-3.17), and DFS (HR 1.89, 95% CI 1.12-3.21). In colorectal cancer, VASH1 expression was associated with tumor stage (OR 2.55, 95% CI 1.75-3.71). In gastric cancer, VASH1 expression was related to TNM stage (OR 2.47, 95% CI 1.13-5.37), lymph node metastasis (OR 2.0, 95% CI 1.11-3.62), and OS (HR 2.11, 95% CI 1.17-3.81). Among all the factors examined for each cancer type, high VASH1 levels were associated with poor clinical outcomes and prognosis. In esophageal cancer, no significant differences in clinicopathological factors were seen when patients were compared according to VASH1 expression. Because of the small number of publications on VASH2, an analysis according to cancer type was not possible.
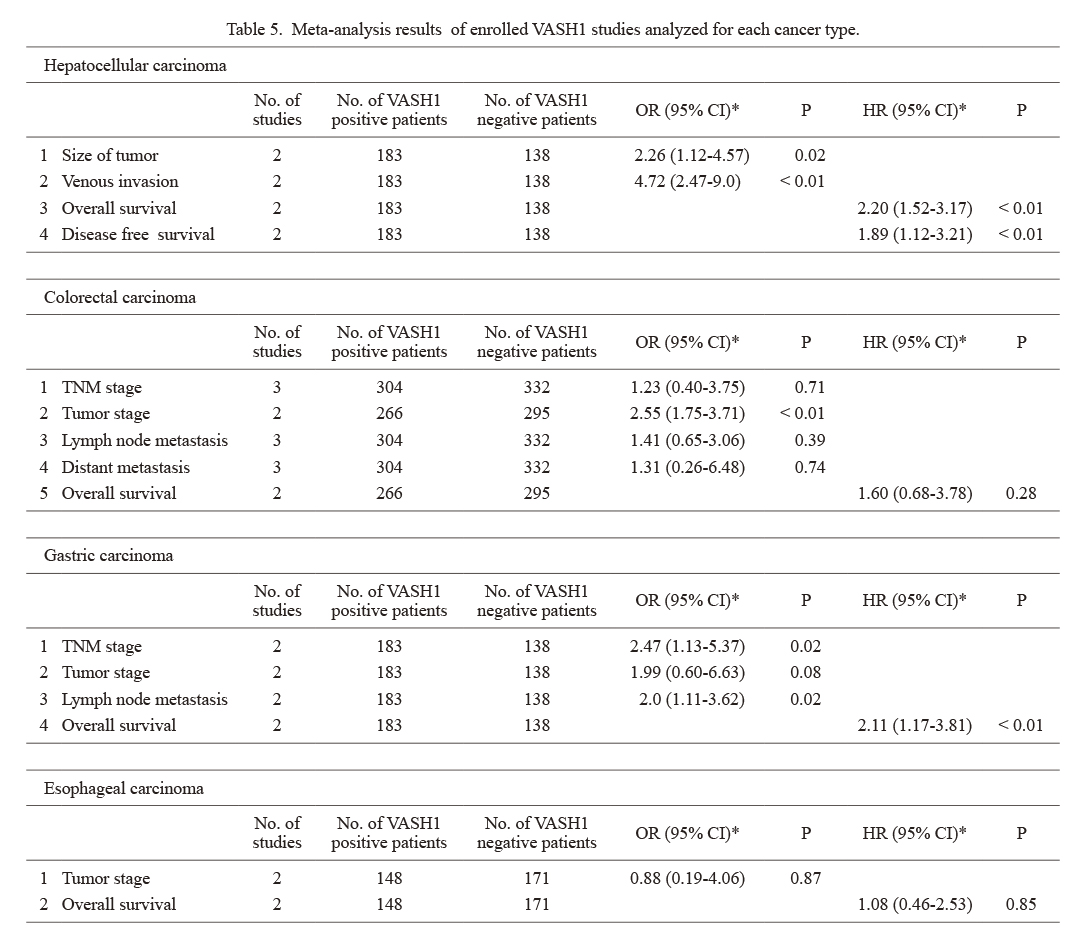
Meta-analysis results of enrolled VASH1 studies analyzed for each cancer type.
*Random effects models.
VASH, vasohibin; OR, oddds ratio; CI, confidence interval; HR, hazard ratio; TNM stage, tumor-node-metastasis stage.

Forrest plots of VASH1 studies analyzed for each cancer type.
(A) Hepatocellular carcinoma, tumor size, (B) hepatocellular carcinoma, venous invasion, (C) hepatocellular carcinoma, overall survival rates, (D) hepatocellular carcinoma, disease free survival rates, (E) colorectal cancer, tumor stage, (F) gastric cancer, TNM stage, (G) gastric cancer, lymph node metastasis, and (H) gastric cancer, overall survival rate.
VASH, vasohibin; OR, odds ratio; HR, hazard ratio; v, venous invasion; LNM, lymph node metastasis.
In the VASH1 meta-analysis, heterogeneity in the size, TNM stage, tumor stage, lymph node metastasis, distant metastasis, venous invasion and OS were observed among the studies (Fig. 2). However, no heterogeneity was observed for age, sex, lymphatic invasion and DFS.
In the VASH2 meta-analysis, sex, TNM stage, tumor stage, lymph node metastasis and lymphatic invasion were heterogeneous (Fig. 3). However, heterogeneity was not observed for venous invasion and OS.
Evaluation of publication biasFunnel plots to examine publication bias are shown in Figs. 5 and 6. Since the number of articles was less than 10 for both VASH1 and VASH2, the asymmetry investigation was not thought to provide valid data.
Funnel plots of VASH1.
(A) Age, (B) sex, (C) size, (D) tumor-node-metastasis (TNM) stage, (E) tumor stage, (F) lymph node metastasis, (G) distant metastasis, (H) lymphatic invasion, (I) venous invasion, (J) overall survival rates, and (K) disease free survival rates.

Funnel plots of VASH2.
(A) Sex, (B) tumor-node metastasis (TNM) stage, (C) tumor stage, (D) lymph node metastasis, (E) lymphatic invasion, (F) venous invasion, and (G) overall survival rates.
This study was the first meta-analysis of articles to examine the clinical significance of VASH expression in gastroenterological cancers. In this meta-analysis, we found that a VASH1-positive status (high VASH1 expression) and a VASH2-positive status (high VASH2 expression) in the tumor tissues of gastroenterological cancers were both associated with poor clinical outcomes.
VASH1 is mainly expressed in ECs in the termination zone to halt angiogenesis (Watanabe et al. 2004). Because VASH1 is an angiogenesis inhibitor, a high tumor expression level of VASH1 would be expected to be associated with a better prognosis. However, most studies on breast cancer (Tamaki et al. 2009), urothelial carcinoma (Miyazaki et al. 2012), prostate cancer (Kosaka et al. 2013), non-small cell lung cancer (Zhang et al. 2014), renal cell carcinoma (Mikami et al. 2017), and head and neck squamous cell carcinoma (Torii et al. 2017) have shown that an increased intensity of VASH1 immunostaining in the tumor vessels was associated with poor clinical outcomes. Similarly, in this meta-analysis of 1,574 gastroenterological cancers, a high VASH1 expression was associated with high malignancy and poor clinical outcomes. This implies that tumors with a higher angiogenic ability produce more VASH1. The expression of VASH1 seems to reflect the response of the ECs to angiogenic stimulation. In two articles that reported conflicting results, in which a high VASH1 expression was associated with a better prognosis, immunohistochemical staining showed that VASH1 was positive in the tumor cells, and not in ECs (Liu et al. 2015; Ma et al. 2017). On the other hand, four other papers reported that high VASH1 expression in tumor cells, as shown using immunohistochemical staining, was associated with poor clinical outcomes (Wang et al. 2012; Yan et al. 2014; Kitajima et al. 2014; Shen et al. 2016). The different antibodies used for immunohistochemical staining in each article may have affected the heterogeneity of the meta-analysis results. In the future, a larger number of cases will need to be evaluated using the same type of antibodies.
VASH2 has been found to be expressed in many tumors and is associated with angiogenesis and tumor growth (Kimura et al. 2009). VASH2 is a pro-angiogenic factor that is mainly expressed in cancer cells (Shibuya et al. 2006). In this study, we performed a meta-analysis of 469 cases of gastroenterological cancer and found that high VASH2 expression was associated with poor clinical outcomes and poor prognosis. In malignant tumors other than gastroenterological cancer, high tissue expression levels of VASH2 have been reported to be a poor prognostic factor in serous ovarian cancer (Takahashi et al. 2012). VASH2 has been reported to be involved in the epithelial-mesenchymal transition (EMT) by regulating TGF-β signaling (Norita et al. 2017). In addition, VASH2 has been suggested to play an important role in tumor progression via the stromal activation of cancer-associated fibroblasts (CAF) (Suzuki et al. 2017). Thus, VASH2 likely plays a variety of roles in the tumor microenvironment in addition to its pro-angiogenic activity.
In the analysis of VASH1 expression according to cancer types, high VASH1 levels were associated with poor clinical outcomes and a poor prognosis in patients with hepatocellular carcinoma, colorectal carcinoma, or gastric carcinoma. The present analysis suggested that high VASH1 levels may predict a poor prognosis in patients with each of the above-mentioned types of cancer. On the other hand, in esophageal cancer, no significant differences in clinicopathological factors were seen when patients were compared according to VASH1 expression. In two reports on esophageal cancer, Ma et al. (2017) used a VASH1 antibody that stained the cytoplasm, while Ninomiya et al. (2018) used a VASH1 antibody that stained ECs. This difference in antibodies may have led to conflicting results, and a direct comparison of the two studies might be difficult. An analysis of VASH2 expression according to cancer types could not be performed because of the small number of publications. Because of the relatively small number of cases and accompanying lack of statistical accuracy, the further accumulation of cases is needed.
In recent years, the diverse roles of vasohibin in the tumor microenvironment have attracted much attention (Norita et al.2017; Suzuki et al. 2017; Sato 2018). Although VASH1 was discovered as an angiogenesis inhibitor, it also reportedly promotes stress tolerance in vascular ECs and is involved in the maintenance of vascular homeostasis to prevent pathological angiogenesis (Sato 2018). In cancer tissues with immature tumor blood vessels, hypoxia is maintained because of circulatory failure (Pugh and Ratcliffe 2003). Hypoxia increases the invasive and metastatic potential of cancer, and immature tumor blood vessels provide a pathway for cancer cells to invade blood vessels. The maturation of tumor blood vessels is thought to generate hypoxia in cancer tissues, improve the tumor reachability of chemotherapeutic agents, increase the sensitivity to radiation therapy, and increase the reachability of immune cells (Kashiwagi et al. 2008; Hosaka et al. 2009). VASH1 is considered to promote the maturation of tumor blood vessels and to inhibit cancer growth and metastasis (Hosaka et al. 2009). On the other hand, VASH2 has also been reported to play an important role in tumor growth via stromal activation, such as EMT and CAF proliferation (Norita et al.2017; Suzuki et al. 2017). These findings indicate that VASH2 has not only a pro-angiogenic activity, but also plays diverse roles in the tumor microenvironment and may be a new molecular target for preventing tumor EMT and inhibiting CAF activation.
In the context of the diverse roles of vasohibin beyond angiogenesis, research is underway to apply vasohibin clinically and to develop molecularly targeted drugs. In VASH1 knockout mice, angiogenesis is not terminated, and the blood vessels remain in an immature state with poor EC coverage; in VASH2 knockout mice, on the other hand, angiogenesis is markedly attenuated, especially at the site of sprouting (Kimura et al. 2009). In addition, when cancer cells are transplanted into VASH1 knockout mice, tumor growth and metastasis are enhanced; when VASH1 is exogenously applied to such lesions, however, immature tumor blood vessels lacking ECs are converted into mature blood vessels coated with ECs, and tumor growth and metastasis are effectively suppressed. Reportedly, the knockout of VASH2 expression in cancer cells markedly suppresses tumor growth (Takahashi et al. 2012). Monoclonal antibodies against human VASH2 have been developed, and an anti-tumor activity comparable to that of bevacizumab, which inhibits VEGF and is widely used clinically, was confirmed in a xenograft mouse model (Koyanagi et al. 2017). VEGF is required for the development and maintenance of vascular endothelial cells, and VEGF knockout mice exhibit multiple organ failure due to vascular endothelial cell dysfunction (Ferrara et al. 1996; Carmeliet et al. 1996). This impairment of blood vessels is a side effect of VEGF inhibitors. On the other hand, VASH2 knockout mice are viable, suggesting that VASH2 antibody therapy may be safer than VEGF inhibitors with fewer adverse effects (Koyanagi et al. 2017). Curing gastroenterological cancer through non-surgical treatment remains difficult. The identification of therapeutic target molecules will be important for improving the prognosis of patients with gastroenterological cancer (Moehler et al. 2016; Jaiswal 2017). The findings of this meta-analysis, in which the expressions of VASH1 and VASH2 were shown to be prognostic factors, suggest the importance of the clinical application of VASH in gastroenterological cancer.
Reports which discussed the relationship between VASH1 and VASH2 have been limited. The VASH literature in the field of gastric cancer suggests a slight correlation between the expression levels of VASH1 and VASH2 in tumors (Hara et al. 2020). Further analysis of VASH1 and VASH2 expression levels in the same patients and an investigation of their relationships are needed.
This study has some limitations. First, the antibodies used for immunohistochemical staining differed among the studies that were surveyed, resulting in differences in tissue staining. In addition, the evaluation and the setting of cutoff values for immunohistochemical staining were inconsistent. These points may have contributed to the heterogeneity of the findings. In addition, the number of articles on VASH1 and VASH2 was 9 and 3, respectively, which are both relatively small and do not allow for sufficient evaluation of the publication bias using funnel plots. In the future, large-scale studies are required.
In recent years, the usefulness of liquid biopsies using bodily fluids, such as blood, for the development of new biomarkers has been attracting attention. However, very few reports have analyzed the blood concentrations of VASH. We previously reported that the plasma VASH1 and VASH2 concentrations in esophageal cancer patients are useful as biomarkers (Yamamoto et al. 2020). To the best of our knowledge, this was the first report to analyze plasma VASH2 levels. The further analysis of plasma VASH levels in many patients with different types of cancer is anticipated.
In conclusion, this study demonstrated that VASH1 and VASH2 expressions were relevant to more aggressive clinicopathological parameters and were associated with a poor OS in patients with gastroenterological cancers.
The authors declare no conflict of interest.