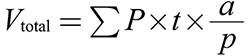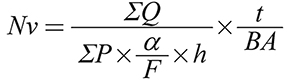Abstract
This research investigated the histopathological changes in the tissue of the lung, heart and liver, hepatocyte cell death, autophagy, and the apoptosis inductions in the postmortem cases. Since December 2019, coronavirus disease 2019 (COVID-19) has become a significant global health concern. In order to clarify the changes in tissues of the lung, heart and liver by COVID-19, samples were taken from five patients who died of COVID-19 and five control cases, and the pathological changes in the lung, liver, and heart tissue were studied by X-ray, computed tomography, histological studies, and stereological analysis. The formation of hyaline membranes, alveolar wall edema, and fibrin exudate was seen on histological analysis of the lungs in the COVID-19 group. Stereological analysis illustrated the number of hepatocytes, volume of the sinusoid, and volume of the liver have been decreased, however the pathological changes in the heart tissue were not observed. Serum levels of alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, and angiotensin-converting enzyme significantly increased. Real-time PCR results showed that the Bcl2, Caspase3, ATG5, and LC3 decreased while the Bax increased. COVID-19 causes fibrotic changes in the lung tissue and hepatocyte mortality in the liver tissue. Besides, it elevates the level of apoptosis and autophagy markers.
Introduction
Since the end of December 2019, the coronavirus disease 2019 (COVID-19) pandemic outbreak has been detected in Wuhan, China, as a result of severe acute respiratory Syndrome coronavirus 2 (SARS-CoV-2) infections, and led to a serious public health threat (Boroujeni et al. 2021). Officially, Iran confirmed the first case of COVID-19 on February 19, 2020. Hence, by June 12, 2020, more than 180,000 Iranian cases of COVID-19 were reported (Moghimi et al. 2021).
COVID-19 can result in substantial morbidity and mortality in infected individuals (van der Hoek et al. 2004). Based on the computed tomographic (CT) scans, the majority of patients who were suffering from dry cough, fever and dyspnea also had bilateral ground-glass opacity (GGO) (Xu et al. 2020; Zhou et al. 2020).
Based on clinical and laboratory studies, the COVID-19 falls into many clinical manifestations according to its spectrum, including the initial, acceleration, and recovery phases. Multiple target organs such as the heart, lungs, and liver were damaged during the acceleration phase, and a cytokine storm was occurred. (Cao et al. 2020; Tian et al. 2020).
However, the virus mainly infected the lungs, which may manifest as acute respiratory distress syndrome and mortality due to massive alveolar damage and progressive respiratory failure (Tomashefski 2000; Jain 2020). Besides, cardiovascular disease was almost common among the COVID-19 patients. Patients who were hospitalized with COVID-19 had been confirmed to have a myocardial injury with elevated troponin levels, which appears to be related to the outcome (Guo et al. 2020).
Ischemia caused by thrombotic coronary occlusion can cause myocardial damage. It must be noted that other factors such as heart failure, pulmonary embolism, and tachycardia can cause it too (Hartikainen et al. 2020). The COVID-19 patients with mild to serious illnesses are more likely to experience hepatic injuries (Zhang et al. 2020). According to increasing evidence, COVID-19 affect various phases of autophagy, and autophagy, in turn, may play a critical role in the viral lifecycle (Cottam et al. 2011, 2014; Gassen et al. 2019).
Autophagy is a complex process, and necessitates a large number of proteins with significant redundancy. Therefore, it explains why there are contradicting results when it comes to viral multiplication and autophagy inhibition (Darabi et al. 2017, 2018). However, the exact mechanisms underlying these interactions between the COVID-19 and autophagy are not entirely clear. Although several studies describe clinical features and characteristic radiographic findings, not enough data is provided about pathological changes and genes involved in autophagy and apoptosis in patients infected by novel coronavirus based on autopsy.
This study examined histopathologic changes in the liver, lungs, heart tissue, hepatocytes number, the liver enzyme levels and the expression of genes associated with autophagy and apoptosis of the COVID-19 patients who died. The findings could provide a better insight into the main pathological changes of the disease in the aforementioned organs and improve clinical strategies against the disease.
Methods
Patient selection and patient criteria
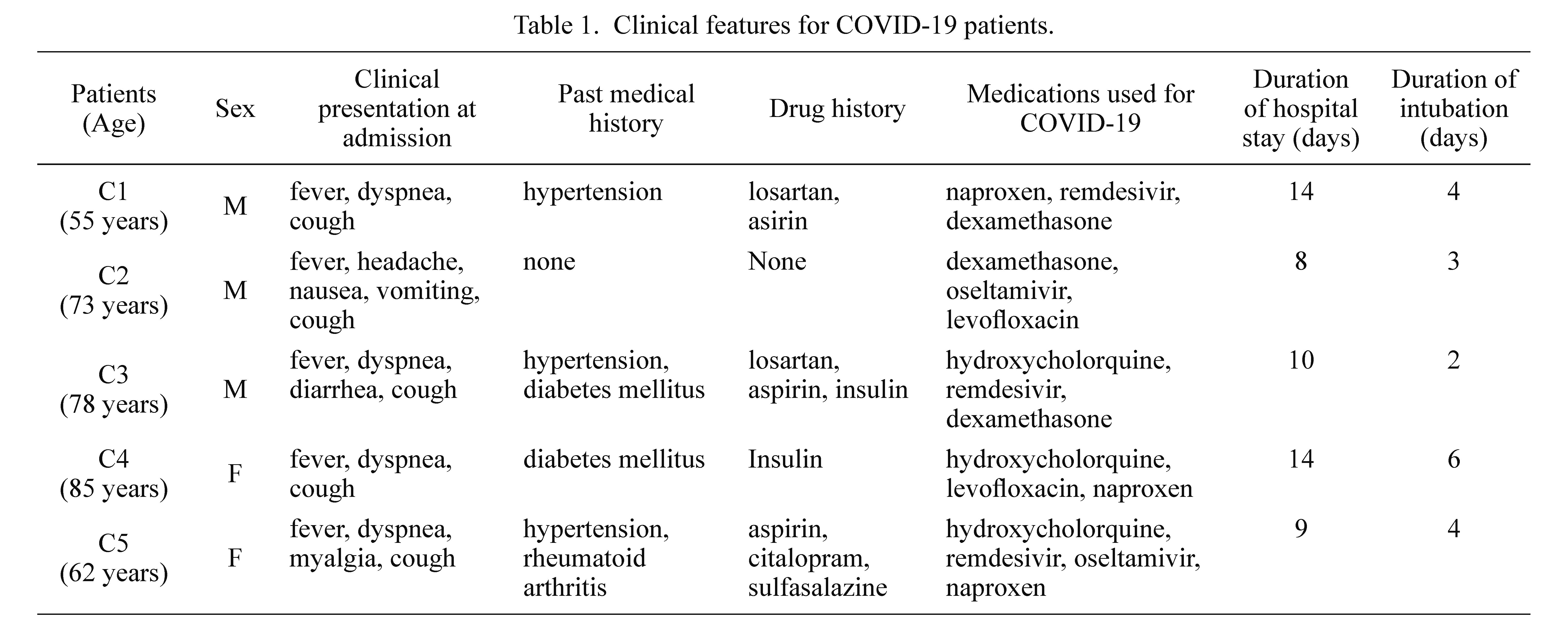
Five COVID-19 patients (3 males and 2 females) (Table 1), as well as five control patients (3 males and 2 females) participated in the present study. For the control group, we chose people whose age ranged from 55 to 85 years old who did not have any serious illness, and their death causes were accidents, carbon monoxide poisoning, and electric shock and so on. The causes of death were respiratory failure and septic shock due to COVID-19 in Case 1, 2, 4 and 5, and pneumonia, acute respiratory distress syndrome, and myocardial infarction due to COVID-19 in Case 3.
The informed consent was obtained from the patient family according to the Iranian Legal Medicine Organization, Tehran, Iran. The protocols of this study are confirmed by the Ethical Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1400.063).
Post-mortem analysis and lung, liver, and heart sampling
Lungs, liver, and heart tissue samples were collected from 5 COVID-19 patients, and 5 control subjects from the Iranian legal organization in Tehran, Iran, between June 2020 and March 2021. According to the World Health Organization’s provisional guidelines, the real-time PCR examination of nasopharyngeal swab samples were taken at the time of hospital admission confirmed the COVID-19 infection in all the 5 patients. Clinical characteristics including medication history, CT scans, laboratory reports, and illness duration were all illustrated in Table 1 and Fig. 1.
In addition, autopsies were carried out between 8 and 10 hours after the death in a room which was equipped with sufficient ventilation, and all the staffs were using personal protective equipments. A 3 × 3 cm2 tissue sample was collected by incisive autopsy for histological, molecular, and cellular investigations in 5 COVID-19 patients and 5 controls as well.
Histological evaluation
Tissue samples were fixed immediately in 4% paraformaldehyde for 72 h before going through routine tissue processing. Following that, histopathological evaluations were performed by sectioning and staining with hematoxylin and eosin (H&E) (Moghimi et al. 2021).
Stereological study
A microtome was utilized to perform serial slices which had 5 µm thickness to estimate the volume and 25 µm thickness to estimate the number by stereological procedures. Systematic Uniform Random Sampling (SURS) was used to select 10 portions of each sample by selecting a random number of 1 to 10.
Estimating the volume of the liver and sinusoid: To calculate the volume of the liver and sinusoid using the Cavalieri method as an estimator, the following formula was used (Gundersen et al. 1988a, b):
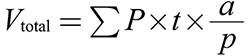
In this formulation, ΣP is the total number of points that hit the liver sections. The region associated with a point is a/p, and the distance between sample sections is t
Estimating the number of cardiomyocytes and hepatocytes: The total variety of hepatocytes and cardiomyocytes was examined by the optical disector method. The equation below was used to compute the numerical density (Nv) of various types of cells (Gundersen et al. 1988a):
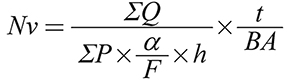
In the formula, ΣP is the total number of the microscopic fields, a/f is the surface per frame, h is the height of director, t in the numerator shows the actual thickness of the section, while BA in the denominator of the 2nd split is the advance of the microtome block. Then, the numerical value of the cell types was determined using the following equation: (N = Nv × V total)2.
Real-time PCR (qPCR)
On a Step One TM-Plus method, gene RT-qPCR was performed on samples PE, (Applied Biosystems, Waltham, MA, USA). For a whole volume of 20 μl, the sample reactions included SYBR green PCR master mixture (TaKaRa, Kusatsu, Japan), cDNA template, primer forward and reverse, and distilled water. For each primer, the PCR cycle conditions were: 30 denaturation cycles at 94°C for 30 seconds, annealing temperature for 30 seconds at 60, 62, 59, 65, 58 and 63°C for the Bax, Bcl2, Caspase 3, GAPDH, autophagy-related gene 5 (ATG5) and LC3, respectively, extension at 72°C, 30 seconds and the last extension stage at 72°C for 5 minutes. The level of relative gene expression was measured using the ΔΔCq method. GAPDH was used to normalize gene expression. Each sample was examined twice 20. The primers used are listed as follows in Table 2.
Enzyme-linked immunosorbent assay (ELISA)
The enzyme levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALKP), creatinine, and blood urea nitrogen (BUN) were calculated using the instructions which were provided by the kit company Biorbyt (Cat #: orb159085, Cambridgeshire, UK). In a brief, the required numeral of blank wells, samples, and standards were designated and arranged on the dish. 100 μL of samples and calibrators were added to the wells. After removing any unbound substances, the biotinylated antibody is added to the wells, which were stored for 60 minutes at 27℃. After washing,the wells were then filled with 100 μL of Streptavidin and incubated at normal room temperature for 45 minutes. After incubation for 30 minutes with TMB One-Step Substrate Component, 50 μL of Stop Solution was applied to each well and read at 450 nm immediately (Boroujeni et al. 2021).
Reactive oxygen species in liver tissue
Subsequently separating cells from liver tissue, they were treated with trypsin-EDTA, the resultant suspension was centrifuged in phosphate buffered saline (PBS) by 1,400 ×g for 5 minutes at 4°C. After the addition of 2, 7-dichlorofluorescein diacetate (DCFDA) to the sample at a concentration of 20 μM in a 100 μl aliquot, it was incubated for 45 minutes at 37°C. Lastly, the sample was studied by using a flowcytometer at 495 nm (Boroujeni et al. 2021).
Glutathione disulfide content assessments
Glutathione peroxidase (GPX) test kit (Zellbio GmbH, Lonsee, Germany) was used for detecting GPX in liver tissue samples. In this analysis, the GPX activity was measured based on the total of sample that catalyze the decomposition of 1 μmol of GSH in one minute. Aliquots of the liver cells suspension containing either O-Phthalaldehyde (OPA) and N-Ethylmaleimide (NEM) probes were harvested from the incubation media using centrifuging at 1,000 rpm for 1 min (Boroujeni et al. 2021).
TUNEL assay
After the fixation of liver tissues, the samples were embedded into paraffin, sectioned, and mounted on glass slides using gelatin. They were preserved by Xylene which dissolved and removed paraffin. Subsequently washing the slices with water, the Tunel staining was done. Image J was also used to measure Tunel-positive cells in normal and COVID groups (Boroujeni et al. 2021).
Statistical analysis
The mean and standard deviation (SD) were used to present data that were regularly distributed, and the data, which is not having distribution, was presented as medians. Counts and percentages were used to describe categorical variables. Statistical analysis was completed using Microsoft Excel.
Results
Characteristics of patients
In the present investigation, five deceased patients with verified COVID-19 diagnoses and five control cases were studied. The patients were 3 males and 2 females. The patients’ average age was 71.2 years (ranging from 58 to 85 years). The average stay in the hospital was 9.5 days (ranging from 4 to 15 days). All of the patients had been intubated for 4.5 days. The main symptoms of these patients were fever, dyspnea and cough, as shown in Table 1. The chest X-rays of COVID-19 positive patients demonstrated undiversified consolidation in peripheral distribution on each lung with obscuration of each costo-phrenic angle in the middle and lower zones (Fig. 1, upper panel). In all of the 5 patients, CT scan results revealed bilateral peripheral ground-glass opacities that were largely dispersed along basal segments. Other occurrences such as sleek interlobular septal thickening, fibrotic bands, and collapse consolidation in each lung were determined likewise (Fig. 1, lower panel).
Histopathological findings
The formation of hyaline membranes, alveolar wall edema, and fibrin exudate was seen on histological analysis of the lungs in the five COVID-19 patients. Interstitial fibroblastic proliferation and type II pneumocyte hyperplasia, as well as the development of bronchial respiratory epithelium desquamation and cytopathic syncytial cells were observed (Fig. 2). As seen in Fig. 3, the COVID-19 autopsy specimens had no clinical symptoms of heart diseases or signs of heart failure. Wholly, all five COVID-19 cases in this study had indicated congestion and sinusoidal dilation sinuses in liver slices, which were reached from mild to severe forms. Hepatocytes in all of the cases were mildly expanding. Bile plugs, both focal and dispersed, and hepatocellular regenerative changes were discovered (Fig. 4).
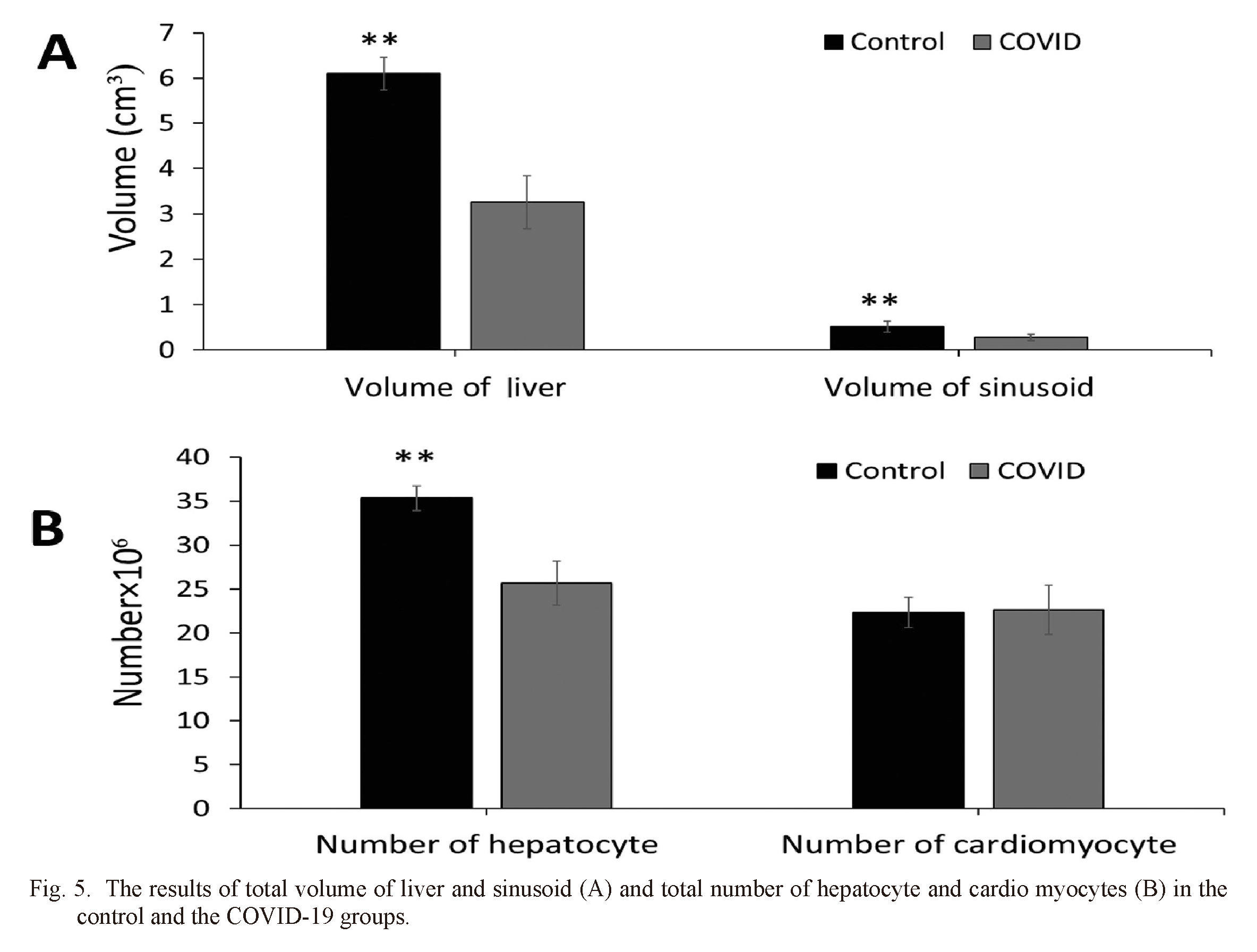
Stereological parameters analysis
Fig. 5A shows the results of comparing different groups. The volume of the liver and sinusoid was significantly different between COVID-19 and the control group. As shown in Fig. 5B, the control group revealed a significant difference in hepatocyte count compared to the COVID-19 group. In heart samples, no significant differences were found concerning the number of cardiomyocytes.
Real time PCR analysis
To evaluate mRNA expression of target genes at the molecular level, the number of transcriptions of two autophagy-related genes (ATG5 and LC3) and three genes which were involved in apoptosis, Bcl2, Bax, and Caspase 3, were examined in the liver samples. According to the statistical results, Bcl2 expression levels in the COVID-19 group were notably lower than in the control group (P < 0.0001). Finally, the Bax and Caspase 3 expression levels in COVID-19 group were significantly higher than the control group (P < 0.0001). When the COVID-19 group was compared to the control group, the expression levels of ATG5 and LC3 were significantly higher (P < 0.0001) (Fig. 6).
ELISA results
The results of liver markers (ALT, AST and ALKP) and kidney markers (BUN and creatinine) revealed that the COVID-19 group showed considerably higher levels of these markers than the control group (Table 3).
Reactive oxygen species (ROS) production
In order to clarify oxidative stress results in the COVID-19 samples, the effects of COVID-19 on the ROS formation were investigated by ROS assay. The ROS formation in the COVID-19 group displayed an increase in comparison with the control group (P < 0.5) (Fig. 7A).
GSH analysis
As Fig. 5B showed, the concentration of GSH in the COVID-19 group was significantly decreased in comparison to the control group (P < 0.05) (Fig. 7B).
Percentage of apoptotic cells
Tunel-positive cell results showed a considerable increase in the apoptotic cells in the COVID-19 group in comparison with control group (P < 0.001) (Fig. 7C).
Discussion
The results of the present study showed that COVID-19 can induce autophagy and apoptosis. Besides, it causes hepatocyte cell death, and liver tissue damage. During the COVID-19 pandemic, numerous investigations were conducted to better understand the disease progression and therapeutic approach (Machhi et al. 2020). The COVID-19 is most commonly associated with the respiratory and immunological systems, although it can also damage the cardiovascular and gastrointestinal systems, particularly in the elderly, and more frequently when comorbidities are present.
In this study, the majority of the pathologic results in autopsy lung, kidney and liver samples of cadaver SARS-CoV-2 diseased persons displayed the severe phase of diffuse alveolar damage. Hyaline membrane creation, alveolar wall edema, and fibrinoid exudate are all symptoms of diffuse alveolar damage in its acute phase (Kligerman et al. 2013). More advanced stages of diffuse alveolar damage, including proliferative and fibrotic phases, have been identified too. This result is constant by previous pathologic discoveries in patients through severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), where diffuse alveolar damage was the most protuberant pathological discovery (Franks et al. 2003; Ng et al. 2016; Beigmohammadi et al. 2021). In their investigation, they mentioned hyaline membrane formation, desquamated pneumocytes, the attendance of mononuclear leukocytes, and pulmonary edema (Beigmohammadi et al. 2021).
Liver dysfunction is widespread in COVID-19 patients, according to the laboratory test results of serum liver enzymes, BUN and creatinine levels, especially in severe cases. All the COVID-19 cases in the present research had elevated levels of liver markers. Although the specific mechanism of liver injury caused by the SARS-CoV-2 infection is unknown; medication poisonousness and a general inflammatory reaction have been proposed. All liver autopsy specimens from the dead COVID-19 patients had pathologic characteristics ranging from mild to severe in the current investigation. In a 2003 research of coronavirus-infected individuals, they found numerous mitoses and substantial hepatocytes proliferation (Chau et al. 2004); nevertheless, in this study a decrease in hepatocytes in liver tissue samples from SARS-CoV-2-infected individuals was found.
The ischemic injury was highly suggested in the liver, because only a low grade of inflammation was observed in portal tracts and lobules. The results of the present study are consistent with explanations by Beigmohammadi et al. (2021). Microvesicular steatosis, portal, lobular and sinusoidal inflammation, and multifocal necrosis were seen in liver biopsy samples of COVID-19 patients, which were understood as also SARS-CoV-2-induced injury (Beigmohammadi et al. 2021). According to molecular findings, the expression of genes involved in apoptosis and autophagy increased, which is in line with previous studies (Tan et al. 2007; Kudchodkar and Levine 2009).
Apoptosis induction is a hallmark of SARS-CoV-2 infection. Failure to activate apoptosis will not only prevent cell death and tissue damage, but will also slow SARS-CoV-2 clearance from infected cells (Ye et al. 2008). SARS-CoV-2 belongs to the beta coronaviruses (b-Cov) family, which has been found to cause ATG5-dependent increase of autophagosome production, then afterward impede their maturation (Cottam et al. 2014; Chen et al. 2014 ).
Upregulation of LC3 during b-Cov infection might suggest viral hijacking of an alternative pathway, endoplasmic reticulum-associated degradation (Knoops et al. 2008). Understanding how this and other intracellular pathways affect SARS-CoV-2 is crucial in dealing with coronavirus outbreaks.
In conclusion, COVID-19 promotes fibrosis alterations in lung tissue and hepatocyte mortality in liver tissue, as well as inducing the expression of genes related to autophagy, apoptosis, and increase of ROS.
Acknowledgments
We are thankful for the funding provided by laser applications in the Medical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran, and Iranian Legal Medicine Organization, Tehran, Iran.
Author Contributions
H.A. designed the present research and drafted the manuscript. A.S.H., G.R.M., B.A., and M.F. provided the clinical information and samples and were involved in the draft of the manuscript. M.A.A. and H.A.A. conducted statistical analyses and were involved in the draft of the manuscript. M.A.A., M.R.T. and S.H.A. accomplished the histological study and were involved in the draft of the manuscript. Each of the authors read and confirmed the resulting paper.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Beigmohammadi,
M.T.,
Jahanbin,
B.,
Safaei,
M.,
Amoozadeh,
L.,
Khoshavi,
M.,
Mehrtash,
V.,
Jafarzadeh,
B. &
Abdollahi,
A.
(2021) Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int. J. Surg. Pathol., 29, 135-145.
-
Boroujeni,
M.E.,
Simani,
L.,
Bluyssen,
H.A.R.,
Samadikhah,
H.R.,
Zamanlui Benisi,
S.,
Hassani,
S.,
Akbari Dilmaghani,
N.,
Fathi,
M.,
Vakili,
K.,
Mahmoudiasl,
G.R.,
Abbaszadeh,
H.A.,
Hassani Moghaddam,
M.,
Abdollahifar,
M.A. &
Aliaghaei,
A.
(2021) Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem. Neurosci., 12, 2143-2150.
-
Cao,
W.,
Liu,
X.,
Bai,
T.,
Fan,
H.,
Hong,
K.,
Song,
H.,
Han,
Y.,
Lin,
L.,
Ruan,
L. &
Li,
T.
(2020) High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect. Dis., 7, ofaa102.
-
Chau,
T.N.,
Lee,
K.C.,
Yao,
H.,
Tsang,
T.Y.,
Chow,
T.C.,
Yeung,
Y.C.,
Choi,
K.W.,
Tso,
Y.K.,
Lau,
T.,
Lai,
S.T. &
Lai,
C.L.
(2004) SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology, 39, 302-310.
-
Chen,
X.,
Wang,
K.,
Xing,
Y.,
Tu,
J.,
Yang,
X.,
Zhao,
Q.,
Li,
K. &
Chen,
Z.
(2014) Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell, 5, 912-927.
-
Cottam,
E.M.,
Maier,
H.J.,
Manifava,
M.,
Vaux,
L.C.,
Chandra-Schoenfelder,
P.,
Gerner,
W.,
Britton,
P.,
Ktistakis,
N.T. &
Wileman,
T.
(2011) Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy, 7, 1335-1347.
-
Cottam,
E.M.,
Whelband,
M.C. &
Wileman,
T.
(2014) Coronavirus NSP6 restricts autophagosome expansion. Autophagy, 10, 1426-1441.
-
Darabi,
S.,
Tiraihi,
T.,
Noori-Zadeh,
A. &
Abbazadeh,
H.A.
(2017) Creatine and retinoic acid effects on the induction of autophagy and differentiation of adipose tissue-derived stem cells into GABAergic-like neurons. J. Babol Univ. Med. Sci., 19, 7-15.
-
Darabi,
S.,
Noori-Zadeh,
A.,
Rajaei,
F.,
Abbaszadeh,
H.A.,
Bakhtiyari,
S. &
Roozbahany,
N.A.
(2018) SMER28 attenuates dopaminergic toxicity mediated by 6-hydroxydopamine in the rats via modulating oxidative burdens and autophagy-related parameters. Neurochem. Res., 43, 2313-2323.
-
Franks,
T.J.,
Chong,
P.Y.,
Chui,
P.,
Galvin,
J.R.,
Lourens,
R.M.,
Reid,
A.H.,
Selbs,
E.,
McEvoy,
C.P.,
Hayden,
C.D.,
Fukuoka,
J.,
Taubenberger,
J.K. &
Travis,
W.D.
(2003) Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol., 34, 743-748.
-
Gassen,
N.C.,
Niemeyer,
D.,
Muth,
D.,
Corman,
V.M.,
Martinelli,
S.,
Gassen,
A.,
Hafner,
K.,
Papies,
J.,
Mosbauer,
K.,
Zellner,
A.,
Zannas,
A.S.,
Herrmann,
A.,
Holsboer,
F.,
Brack-Werner,
R.,
Boshart,
M.,
et al. (2019) SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun., 10, 5770.
-
Gundersen,
H.J.,
Bagger,
P.,
Bendtsen,
T.F.,
Evans,
S.M.,
Korbo,
L.,
Marcussen,
N.,
Moller,
A.,
Nielsen,
K.,
Nyengaard,
J.R.,
Pakkenberg,
B.,
Sorensen,
F.B.,
Vesterby,
A. &
West,
M.J.
(1988a) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS, 96, 857-881.
-
Gundersen,
H.J.,
Bendtsen,
T.F.,
Korbo,
L.,
Marcussen,
N.,
Moller,
A.,
Nielsen,
K.,
Nyengaard,
J.R.,
Pakkenberg,
B.,
Sorensen,
F.B.,
Vesterby,
A. &
West,
M.J.
(1988b) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS, 96, 379-394.
-
Guo,
T.,
Fan,
Y.,
Chen,
M.,
Wu,
X.,
Zhang,
L.,
He,
T.,
Wang,
H.,
Wan,
J.,
Wang,
X. &
Lu,
Z.
(2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol., 5, 811-818.
-
Hartikainen,
T.S.,
Sorensen,
N.A.,
Haller,
P.M.,
Gossling,
A.,
Lehmacher,
J.,
Zeller,
T.,
Blankenberg,
S.,
Westermann,
D. &
Neumann,
J.T.
(2020) Clinical application of the 4th Universal Definition of Myocardial Infarction. Eur. Heart J., 41, 2209-2216.
-
Jain,
A.
(2020) COVID-19 and lung pathology. Indian J. Pathol. Microbiol., 63, 171-172.
-
Kligerman,
S.J.,
Franks,
T.J. &
Galvin,
J.R.
(2013) From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics, 33, 1951-1975.
-
Knoops,
K.,
Kikkert,
M.,
Worm,
S.H.,
Zevenhoven-Dobbe,
J.C.,
van der Meer,
Y.,
Koster,
A.J.,
Mommaas,
A.M. &
Snijder,
E.J.
(2008) SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol., 6, e226.
-
Kudchodkar,
S.B. &
Levine,
B.
(2009) Viruses and autophagy. Rev. Med. Virol., 19, 359-378.
-
Machhi,
J.,
Herskovitz,
J.,
Senan,
A.M.,
Dutta,
D.,
Nath,
B.,
Oleynikov,
M.D.,
Blomberg,
W.R.,
Meigs,
D.D.,
Hasan,
M.,
Patel,
M.,
Kline,
P.,
Chang,
R.C.,
Chang,
L.,
Gendelman,
H.E. &
Kevadiya,
B.D.
(2020) The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol., 15, 359-386.
-
Moghimi,
N.,
Eslami Farsani,
B.,
Ghadipasha,
M.,
Mahmoudiasl,
G.R.,
Piryaei,
A.,
Aliaghaei,
A.,
Abdi,
S.,
Abbaszadeh,
H.A.,
Abdollahifar,
M.A. &
Forozesh,
M.
(2021) COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis, 26, 415-430.
-
Ng,
D.L.,
Al Hosani,
F.,
Keating,
M.K.,
Gerber,
S.I.,
Jones,
T.L.,
Metcalfe,
M.G.,
Tong,
S.,
Tao,
Y.,
Alami,
N.N.,
Haynes,
L.M.,
Mutei,
M.A.,
Abdel-Wareth,
L.,
Uyeki,
T.M.,
Swerdlow,
D.L.,
Barakat,
M.,
et al. (2016) Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am. J. Pathol., 186, 652-658.
-
Tan,
Y.X.,
Tan,
T.H.,
Lee,
M.J.,
Tham,
P.Y.,
Gunalan,
V.,
Druce,
J.,
Birch,
C.,
Catton,
M.,
Fu,
N.Y.,
Yu,
V.C. &
Tan,
Y.J.
(2007) Induction of apoptosis by the severe acute respiratory syndrome coronavirus 7a protein is dependent on its interaction with the Bcl-XL protein. J. Virol., 81, 6346-6355.
-
Tian,
S.,
Xiong,
Y.,
Liu,
H.,
Niu,
L.,
Guo,
J.,
Liao,
M. &
Xiao,
S.Y.
(2020) Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol., 33, 1007-1014.
-
Tomashefski,
J.F. Jr.
(2000) Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med., 21, 435-466.
-
van der Hoek,
L.,
Pyrc,
K.,
Jebbink,
M.F.,
Vermeulen-Oost,
W.,
Berkhout,
R.J.,
Wolthers,
K.C.,
Wertheim-van Dillen,
P.M.,
Kaandorp,
J.,
Spaargaren,
J. &
Berkhout,
B.
(2004) Identification of a new human coronavirus. Nat. Med., 10, 368-373.
-
Xu,
Z.,
Shi,
L.,
Wang,
Y.,
Zhang,
J.,
Huang,
L.,
Zhang,
C.,
Liu,
S.,
Zhao,
P.,
Liu,
H.,
Zhu,
L.,
Tai,
Y.,
Bai,
C.,
Gao,
T.,
Song,
J.,
Xia,
P.,
et al. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med., 8, 420-422.
-
Ye,
Z.,
Wong,
C.K.,
Li,
P. &
Xie,
Y.
(2008) A SARS-CoV protein, ORF-6, induces caspase-3 mediated, ER stress and JNK-dependent apoptosis. Biochim. Biophys. Acta, 1780, 1383-1387.
-
Zhang,
Y.,
Zheng,
L.,
Liu,
L.,
Zhao,
M.,
Xiao,
J. &
Zhao,
Q.
(2020) Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int., 40, 2095-2103.
-
Zhou,
M.,
Zhang,
X. &
Qu,
J.
(2020) Coronavirus disease 2019 (COVID-19): a clinical update. Front. Med., 14, 126-135.