Abstract
The fifth wave of the coronavirus disease 2019 (COVID-19) pandemic caused by delta variant infection depleted medical resources, and the Japanese government announced glucocorticoid use for outpatients. An appropriate outpatient-glucocorticoid treatment for COVID-19 has not been established; therefore, we created treatment manuals with indications for glucocorticoid administration in a care facility adequately equipped to manage patients with mild to moderate COVID-19. Thirty-eight patients (24 males, 14 females; mean age 40.5 ± 11.8 years) were treated with glucocorticoids from August 1 to October 1, 2021 [COVID-19 staging, mild (n = 1), moderate I (n = 19), and moderate II (n = 18)]. Patients were treated with 6.6 mg/day d.i.v. or 6 mg/day p.o. dexamethasone, or 20-30 mg/day p.o. prednisolone. The median (25th-75th percentile) number of days from the date of onset to glucocorticoid administration was 8.0 days (7.0-11.25 days). While 24 patients were hospitalized, the condition of 14 improved without hospitalization. The median number of days from glucocorticoid administration to hospitalization was 1.0 day (range, 1.0-1.0 day). In the non-hospitalized patients, the median number of days of glucocorticoid administration was 5.0 days (5.0-5.25 days). The mean number of days from glucocorticoid administration to discharge from the care facility for non-hospitalized patients was 8.4 ± 3.3 days. The adverse reactions among non-hospitalized patients included insomnia (n = 1) and mild liver dysfunction (n = 3). The present method of glucocorticoid administration can be safely used for patients with COVID-19 in care facilities.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the worldwide outbreak of coronavirus disease 2019 (COVID-19). The Japanese guidelines for COVID-19 treatment recommend hospitalization for patients with pneumonia due to SARS-CoV-2 (Clinical Practice Guidance Review Committee 2021c). However, during the fifth wave of the COVID-19 pandemic in Japan, i.e., from July to September 2021 (Fig. 1), there was scarcity of hospital beds as COVID-19 cases were rapidly increasing in number and advancing in severity. Not only patients with pneumonia but also those with hypoxia could not be accommodated.
Miyagi Prefecture is located in the northeast of Japan and has experienced large earthquakes over several decades. Tohoku University Hospital and the government of Miyagi Prefecture have been closely collaborating, especially since the Great East Japan Earthquake that occurred on March 11, 2011 (Akaishi et al. 2021). After the outbreak of COVID-19, Tohoku University and Miyagi Prefecture jointly established a few care facilities for the isolation of patients with COVID-19 who were not indicated for administration to the hospital. The doctors at Tohoku University Hospital have been in charge of calling these facilities. While symptomatic COVID-19 patients were admitted to one care facility that could provide additional medical services, such as prescription medicines, the asymptomatic patients with positive polymerase chain reaction test result and those with minor symptoms were moved to other care facilities. The doctors visited the medical care facility three times a week to examine patients exhibiting symptoms since October 2020.
In the fifth wave of COVID-19 pandemic, the SARS-CoV-2 delta variant (B.1.617.2) became dominant. The positivity rate of the SARS-CoV-2 delta variant (B.1.617.2) during this period was 90.2%-100% (Miyagi Prefecture Government 2021). Patients whose condition rapidly deteriorated were managed until they could be moved to a hospital. A robust clinical management system with oxygen support, drip infusion, and provision to administer glucocorticoid therapy had to be established since there was no antiviral drug that could be administered to outpatients. In September 2021, the Japanese government, recognizing the depleted medical resources, announced the use of glucocorticoids for outpatients presenting with hypoxia (Ministry of Health, Labour and Welfare 2021). However, there were many COVID-19 outpatients without hypoxia but with high fever and physical exhaustion, and there have been few reports on the efficacy of glucocorticoids in patients with mild to moderate COVID-19 who were not hospitalized.
This study aimed to report the medical management of COVID-19 cases at a care facility during the delta variant outbreak and to discuss the safety and efficacy of glucocorticoids administered to outpatients under limited conditions.
Materials and Methods
Eligibility criteria for patients
The medical records of Tohoku University Hospital and the care facility were accessed to select patients who were treated with oral or intravenous glucocorticoids from August 1 to October 1, 2021.
Classification of the severity of COVID-19 and treatments recommended in Japan
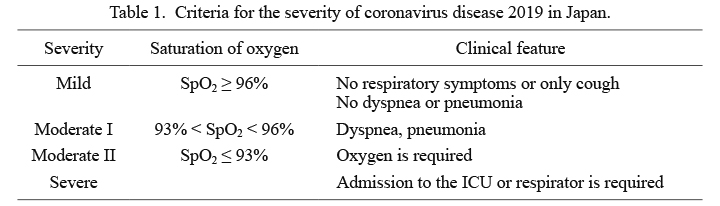
The Ministry of Health, Labour and Welfare in Japan announced and revised the treatment guidelines for COVID-19 in March 2020. The latest guidelines as of January 20, 2022, the 6.1 edition, was announced on December 28, 2021 (Clinical Practice Guidance Review Committee 2021c). In the guidelines, the severity of COVID-19 was classified as follows: mild, no pneumonia and no hypoxia [oxygen saturation of peripheral artery (SpO2) ≥ 96%]; moderate I, pneumonia with or without hypoxia (93% < SpO2 < 96%); moderate II, pneumonia and hypoxia (SpO2 ≤ 93%); and severe, ventilator requirement (Table 1).
The recommended treatment strategies for patients with mild COVID-19 during the abovementioned period in Japan (Clinical Practice Guidance Review Committee 2021a, b) were restricted to observation and symptomatic therapy when necessary. Only patients with aggravated risk factors received a neutralizing antibody therapy (casirivimab/imdevimab). Remdesivir was recommended for patients with moderate I to severe COVID-19, dexamethasone (DEX) was for patients with moderate II or severe COVID-19, and both of them could be used for hospitalized patients. Therefore, most patients in the care facility with mild and moderate I COVID-19 symptoms were treated with acetaminophen, expectorant drugs, and antitussive agents. Traditional Japanese (kampo) medicine was also used.
Medical treatment system established at the care facility
Since October 2, 2020, Tohoku University Hospital has collaborated with the government of Miyagi Prefecture and provided medical services to patients with COVID-19 through a care facility managed by Miyagi Prefecture. Doctors prescribe medicines to patients in the facility using mobile tablet terminals connected to the medical records unit of Tohoku University Hospital. Since January 2021, we have performed chest radiography and blood tests to adequately diagnose and treat patients with COVID-19 at the facility, along with doctor visits. To the best of our knowledge, there were no other care facilities in Japan that provided chest radiography and blood sampling tests along with doctor visits. When the number of patients with severe symptoms started rising in July 2021, Tohoku University Hospital sent doctors for consultation every day and established a 24-h system to monitor oxygen saturation of blood and pulse rate. An electrocardiogram, a portable ultrasonic inspection device, oxygen compressor, and drip infusion systems were also provided when necessary. All medical information from patients in the care facilities were recorded in individual electronic files that were originally created by the staff of Tohoku University and Miyagi Prefecture.
Standardized treatment strategies for patients with COVID-19 in the care facility
An action card and manuals were created for standardized treatment and shared them with doctors and para-medical staff (Fig. 2A, B). Patients with a cough score of ≤ 4 on the numeral rating scale (NRS) (0, no symptoms to 10, maximum level of symptoms) were prescribed over-the-counter medicines. If the NRS score for cough was ≥ 5, chest X-ray and blood examination were performed. An anticoagulant was administered when the level of D-dimer was over 0.5 μg/mL. When the patients had pneumonia and an SpO2 level of ≥ 96%, proper medications were prescribed. When the SpO2 level was ≤ 95%, the patient was provided with a 24-h SpO2 and pulse monitoring system. The 24-h monitoring system consisted of a pulse oximeter that was attached to the patient’s finger (Rad-97® with Radius PPG™, Masimo Japan, Tokyo, Japan) and a central managing system (CORD for Iris, TAKAHASI KOUGEI Laboratory Co. Ltd., Tokyo, Japan) that compiled the biometric information obtained from the pulse oximeter. If the SpO2 level was 94-95% and high fever and severe cough persisted for ≥ 7 days from onset, prednisolone was administered at a dose of 30 mg/day for 14 days after onset. If the SpO2 level was ≤ 93%, oxygen inhalation was started and coordination for hospitalization was requested at the COVID-19 Coordination Division of the government of Miyagi Prefecture. Patients (without diabetes and those who were not pregnant) who could not be admitted on the same day were administered DEX at a dose of 6 mg/day orally or 6.6 mg/day by drip infusion for 14 days after onset. Blood pressure and blood sugar levels were measured once a day to monitor the side effects of glucocorticoids. After completion of glucocorticoid therapy, patients were called for follow-up within a week to check for recurrence.

Patients with COVID-19 were discharged after 10 days from the onset of symptoms and after at least 72 h post symptom resolution, as per the guidelines of the Ministry of Health, Labour and Welfare. Observations that qualified a patient for discharge were defined as follows: body temperature < 37°C and an NRS score ≤ 2 for respiratory symptoms (e.g., cough, sputum, or dyspnea). Although the body temperature of the patients who received glucocorticoids decreased, discharge decisions were dependent on these criteria after the administration of glucocorticoids was completed.
Ethical statement
This retrospective observational study was approved by the Tohoku University Ethics Committee (Institutional Review Board number: 2021-1-447). All procedures were carried out following the current version of the Declaration of Helsinki, revised in 2013. Informed consent was obtained in an opt-out manner.
Statistical analysis
Distributions of normally distributed numeric variables are described as mean and standard deviation (SD), and non-normally distributed numeric variables are described as median and interquartile range (25th-75th percentiles). Student’s t-test or the Mann-Whitney U-test was used to compare the two groups according to the distribution patterns of the evaluated variables. The prevalence of categorical variables between the two groups was compared using the chi-square test. In each analysis, p-value of less than 0.05 was considered statistically significant. All analyses were performed using Statistical Package for the Social Sciences version 21 (IBM Japan, Tokyo, Japan).
Results
Characteristics of the patients
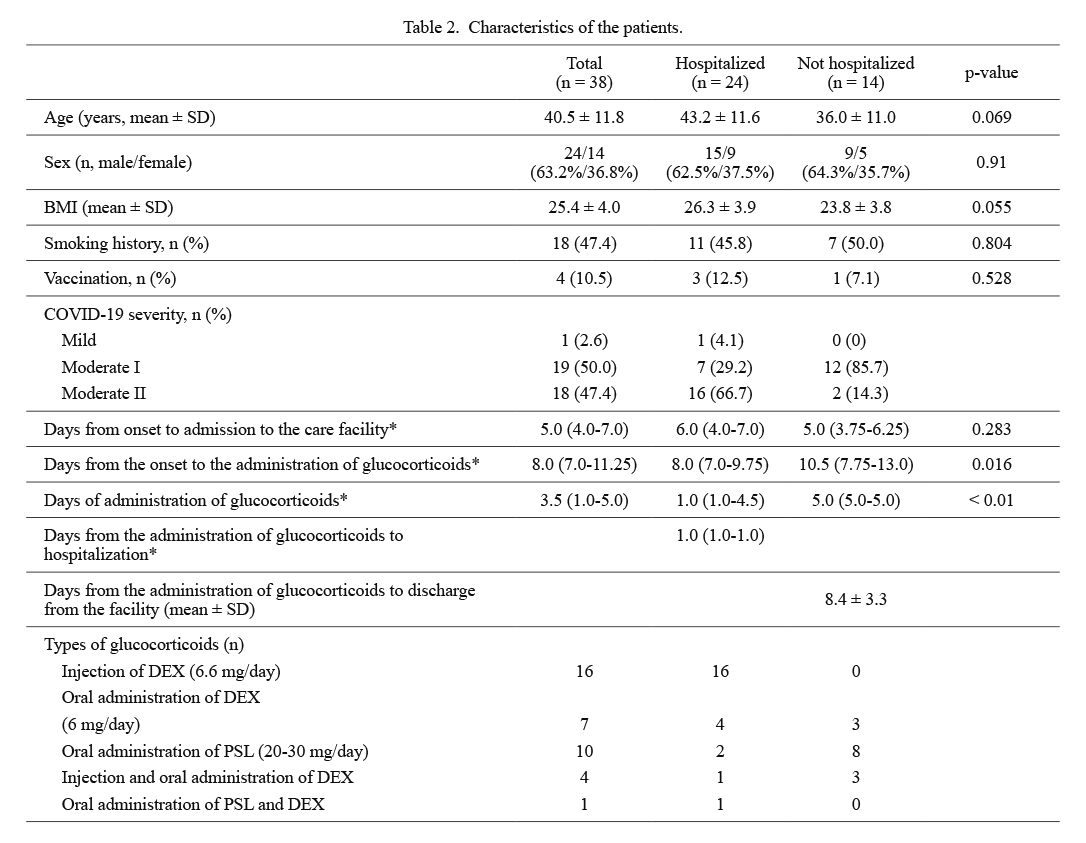
Table 2 shows the characteristics of patients who received glucocorticoids. Of the 38 patients (24 males and 14 females), 24 were hospitalized and 14 were not. Seventeen hospitalized patients received oxygen, while only two among the non-hospitalized patients required oxygen. Hospitalized patients tended to be older than non-hospitalized patients and had a higher body mass index (BMI). A patient with mild COVID-19 had to be admitted to the hospital due to persistent cough and depressive state. There were no differences in sex, smoking history, vaccination rate, and days from the onset of symptoms to care facility admission between hospitalized and non-hospitalized patients. Hospitalized patients received glucocorticoids significantly earlier than non-hospitalized patients [8.0 (7.0-9.75) vs. 10.5 (7.75-13.0) days from onset, p = 0.016]. Patients who were indicated for hospitalization could be admitted to the hospitals within 1.0 (1.0-1.0) day after glucocorticoid administration. The duration of glucocorticoid therapy was significantly shorter for hospitalized patients than for non-hospitalized patients [1.0 (1.0-4.5) vs. 5.0 (5.0-5.0), p < 0.01]. Non-hospitalized patients could be discharged from the facility 8.4 ± 3.3 days after administration of glucocorticoids. The number of patients who received an infusion of DEX was higher among hospitalized patients. None of the patients died while under care at the facility or after hospitalization.
Table 3 shows the results of the blood tests of patients with COVID-19 who were administered glucocorticoids. The levels of aspartate aminotransferase, creatine kinase, C-reactive protein (CRP), and D-dimer were significantly higher in hospitalized patients than in non-hospitalized patients.
Among non-hospitalized patients, four had adverse reactions: mild insomnia (n = 1) and mild liver dysfunction (n = 3). Insomnia improved with 5 mg of zolpidem tartrate. There were no patients whose blood pressure and blood sugar, measured once a day, exceeded 150/100 mmHg and 200 mg/dL, respectively.
Presentation of the cases
Subsequently, we present the clinical courses of the two non-hospitalized patients whom we could follow-up through a second examination at the facility after improvements in their symptoms.
Case 1: A 23-year-old male presented with fever (38.0°C) and tested positive for COVID-19 on day 1. The patient had a constant high fever of 39-40°C and was admitted to the care facility on day 6. He had smoked 20 cigarettes per day for the past 3 years and had pneumonia when he was a junior high school student. He had not been vaccinated against SARS-CoV-2. His height was 171 cm, body weight was 60 kg, and BMI was 20.5 kg/m2. His body temperature was 38.2°C with an SpO2 of 97%, blood pressure of 123/75 mmHg, and pulse rate of 106 beats per min, and the NRS scores were 6 and 4 for cough and nausea, respectively, on admission day.
The patient underwent chest radiography on the day of admission, which showed pneumonia shadows in the middle right lung (Fig. 3A). Blood tests showed inflammatory changes with a white blood cell count of 2,720/μL and CRP of 4.07 mg/L, thrombogenesis tendency with D-dimer of 0.56 μg/mL, and decreased platelet count of 11.0 × 104/μL. He was diagnosed with moderate I COVID-19 stage. We prescribed 7.5 g/day of gokoto and shosaikotokakikyosekko, which are kampo medicines for cough and sore throat; 60 mg/day of edoxaban tosilate hydrate; 2 mg/day of tulobuterol; and 500 mg of acetaminophen for fever. Since his SpO2 level decreased to 93% on day 7, he was fitted with a 24-h monitoring device. As the monitoring device showed that the SpO2 level stayed over 94%, O2 supplementation was not provided. However, high fever (38-40°C) and cough (NRS score, 6-8) continued, and the patient was prescribed 6 mg/day of DEX on day 8 for 5 days. After the administration of DEX, the NRS scores for cough and fever decreased, and the SpO2 level increased (Fig. 4). The chest radiography on day 14 showed an improvement in the pneumonia shadow (Fig. 3B). He was discharged on day 16.
Case 2: A 27-year-old male presented with fever (38-39°C), cough, sputum, general malaise, and headache. The patient tested positive for COVID-19 on day 2. The examination carried out in the hospital revealed mild pneumonia and, therefore, no need for hospitalization. He was admitted to the care facility on day 7. There was no history of smoking, but he had Kasabach-Merritt syndrome until the age of 12 and underwent surgery for pneumothorax when he was 21 years old. He had not been vaccinated against SARS-CoV-2. His height was 172 cm, body weight was 61 kg, and BMI was 20.6 kg/m2. On the day of admission, his body temperature was 37.3°C, SpO2 level was 98%, blood pressure was 106/80 mmHg, and pulse rate was 88 beats per min. The NRS scores were 6 for cough, 6 for dyspnea, 4 for chest pain, 3 for general malaise, 3 for runny nose, 3 for nasal obstruction, 8 for sore throat, and 3 for headache.
The patient underwent chest radiography at the facility on day 10, which revealed infiltrative shadows in the bilateral lower lobes (Fig. 5A). Blood tests showed no abnormal findings: white blood cell count of 4,040/μL and CRP of 0.77 mg/L. He was diagnosed with moderate I COVID-19. We prescribed 7.5 g/day of kakkonto and shosaikotokakikyosekko, which are kampo medications for common cold and fever, respectively; 30 mg/day of dimemorfan phosphate; and 750 mg/day of L-carbocisteine. The NRS score for cough and body temperature gradually decreased on day 16 but increased again on day 18. On day 21, the NRS score for cough was elevated to 5, body temperature rose to 37.1°C, pulse rate was 114 beats per min, SpO2 level was 98%; therefore, the airway inflammation was considered to be prolonged and 20 mg/day of prednisolone was prescribed. After 5-day administration of prednisolone, NRS scores for fever and cough decreased (Fig. 6). Chest radiography on day 28 showed an improved pneumonia shadow and increased lung volume (Fig. 5B). The patient was discharged on day 34.

Discussion
In this study, we reported the medical management and clinical course of patients with COVID-19 who received glucocorticoids at a care facility during the outbreak of the delta variant infection. In Miyagi Prefecture, any patients whose condition worsened rapidly and required oxygen and glucocorticoids could be hospitalized almost the next day. Since no patient died while under care at the facility and after hospitalization, our method could be useful for the management of patients admitted at the right time.
There were only mild adverse reactions among non-hospitalized patients, and high level of blood pressure or blood sugar was not observed. These results demonstrate the safety of the presented method for non-hospitalized patients with COVID-19.
We presented the cases of two patients who were administered glucocorticoids. Both patients were in the moderate I COVID-19 stage. The Case 1 patient was administered 6 mg/day DEX due to the tendency toward hypoxia and thrombosis. His fever, cough NRS, and SpO2 level improved dramatically after the administration of DEX. Meanwhile, the Case 2 patient was administered 20 mg/day of prednisolone as his oxygenation was adequate, body temperature elevation was mild, and all blood test results were within the normal range. The NRS score for cough also gradually improved, but the duration from the time of glucocorticoid administration to the day of discharge was 8 days for Case 1 and 13 days for Case 2. These results suggest that the higher the dose of glucocorticoids, the faster the symptoms improved, and that DEX was more effective against COVID-19 than prednisolone. However, in some cases symptoms improved rapidly even with 20 mg/day of prednisolone, indicating that the response to type and dose of glucocorticoids varies from case to case. The Japanese guidelines for the treatment of COVID-19 followed in the present study (Clinical Practice Guidance Review Committee 2021a, b) stipulated that DEX should only be used in patients who require oxygen. Therefore, prednisolone was prescribed if the patient did not need oxygen, severe cough did not improve, or the patient’s general condition tended to deteriorate. The indications for prednisolone in Japanese medical insurance included bronchial asthma, asthmatic bronchitis, and improvement of general condition due to severe wasting disease. During the delta variant pandemic, many patients had nausea, vomiting, and diarrhea in addition to severe respiratory symptoms. Prednisolone was administered with the aim of improving these symptoms, which may have been effective in COVID-19 patients who did not need oxygen. DEX is considered to have no benefit in patients with mild to moderate COVID-19 (RECOVERY Collaborative Group et al. 2021). However, Case 1 results proved that DEX could be useful for outpatients with moderate I COVID-19 with a hypoxic tendency if the timing of the medication is carefully considered.
Glucocorticoids for pneumonia have been suggested in clinical practice, but their efficacy has been debated. A previous systematic review and meta-analysis indicated that glucocorticoids for severe infectious pneumonia are safe and effective (Stern et al. 2017; Jiang et al. 2019; Huang et al. 2019). For COVID-19 pneumonia, the efficacy of glucocorticoids for hospitalized patients has been well investigated (Sarma et al. 2020; RECOVERY Collaborative Group et al. 2021; Ferreto et al. 2021; Yu et al. 2021), and they have been shown to reduce mortality and risk of progression to mechanical ventilation in patients with severe COVID-19 (Sarma et al. 2020). However, there is little evidence on the efficacy and safety of glucocorticoids in outpatients with mild to moderate COVID-19. A comparative analysis conducted by Szente Fonseca et al. (2020) reported that the use of hydroxychloroquine, prednisone, or both for SARS-CoV-2-positive outpatients aged ≥ 40 years significantly reduced hospitalization risk by 50-60%. They administered drugs expected to have antiviral effects, such as hydroxychloroquine, at the first doctor visit, and prednisone (1 mg/kg qd × 5 days, maximum 80 mg/day) was administered on day 6 from symptom onset. Early administration of glucocorticoids without concomitant antiviral medication may promote viral proliferation and cause severe COVID-19. In the hospital setting, anti-inflammatory drugs can be used in combination with antiviral drugs, but this was difficult to achieve in the outpatient setting during the present study. However, we had been forced to prescribe glucocorticoids in the outpatient setting due to the scarcity of hospital beds. A retrospective cohort study of inpatients with COVID-19 treated with systemic glucocorticoids in a Japanese hospital demonstrated that the patients in the successfully treated group were administered glucocorticoids for a median of 7 days after the onset of symptoms, and the administration was stopped 13 days after the onset of symptoms. In contrast, in the rebound group, glucocorticoids were started in a median of 5 days after symptom onset and administered for a median of 5 days (Imai et al. 2021). Based on this report, we started drug administration after 7 days of onset and continued until at least 14 days of onset in the care facility. This report was for hospitalized patients, but from our experience, it may also be applicable to outpatients.
A retrospective study by Shionoya et al. (2021) reported that the respiratory status of patients with COVID-19 having pneumonia could deteriorate when the administration of glucocorticoids preceded the administration of antiviral drugs. The rates of intubation, admission to the intensive care unit, and extracorporeal membrane oxygenation induction were significantly higher in the steroids-first group than in the antiviral drugs-first group. In the steroids-first group, the day of administration from the onset was 5.6 ± 2.4 days compared with 9.7 ± 5.6 days in the antiviral drugs-first group. As faster administration of glucocorticoids may cause worsening of the disease, if the day of administration from the onset in the steroids-first group was longer than 7 days, the results may have been different. Evidence on the use of glucocorticoids for COVID-19 still needs to be accumulated. Some studies are currently underway (Saiz-Rodríguez et al. 2020; Les Bujanda et al. 2021), and their results are expected to be reported.
On December 24, 2021, the Ministry of Health, Labour and Welfare granted special approval to molnupiravir, the first oral RNA polymerase inhibitor in Japan that can be used for patients with mild to moderate I COVID-19. Molnupiravir can be used only for patients at risk of aggravation, and its efficacy in Japanese patients requires further studies. As molnupiravir or other oral antiviral drugs are used more widely, the use of glucocorticoids in the outpatient setting will decrease. However, for patients whose symptoms worsen despite the use of antivirals, the present study may be useful as a reference for glucocorticoid therapy.
Clinical trials have been conducted for patients with mild to moderate COVID-19 using RNA polymerase inhibitors, protease inhibitors, antiparasitic drugs, and kampo medicines in Japan (Takayama et al. 2021; Clinical Practice Guidance Review Committee 2021c). The results from these trials are awaited.
The limitations of this study are the small number of patients and the observational nature of the study. We were unable to confirm whether the patients admitted to the hospital developed a severe stage of COVID-19. More evidence is needed, especially from randomized controlled studies, to confirm the efficacy of glucocorticoids in patients with COVID-19.
The presented method for administering glucocorticoids is safe and useful for patients with COVID-19 at the care facility. This may be adapted to other forms of COVID-19 pandemic or new respiratory infectious diseases.
Acknowledgments
We thank the COVID-19 Coordination Division of the government of Miyagi Prefecture. We would also like to thank Editage (https://www.editage.com) for English language editing. This work was supported by JSPS KAKENHI Grant Number 21K10367, Grant-in-Aid for Scientific Research (C). This study was also supported by the Department of Kampo and Integrative Medicine at the Tohoku University School of Medicine.
Conflict of Interest
A.K., M.O., S.T., and T.I. belong to the Department of Kampo and Integrative Medicine at the Tohoku University School of Medicine. The department received a grant from Tsumura, a Japanese manufacturer of kampo medicine; however, this grant was used according to the Tohoku University rules. Potential conflicts of interest were addressed by the Tohoku University Benefit Reciprocity Committee and appropriately managed. The other authors declare no conflict of interest.
References
-
Akaishi,
T.,
Morino,
K.,
Maruyama,
Y.,
Ishibashi,
S.,
Takayama,
S.,
Abe,
M.,
Kanno,
T.,
Tadano,
Y. &
Ishii,
T.
(2021) Restoration of clean water supply and toilet hygiene reduces infectious diseases in post-disaster evacuation shelters: a multicenter observational study. Heliyon, 7, e07044.
-
Clinical Practice Guidance Review Committee
(2021a) Guidance for medical treatment for COVID-19. ver. 5.2.
https://www.mhlw.go.jp/content/000815065.pdf [Accessed: December 28, 2021] (in Japanese).
-
Clinical Practice Guidance Review Committee
(2021b) Guidance for medical treatment for COVID-19. ver. 5.3.
https://www.mhlw.go.jp/content/000825966.pdf [Accessed: December 28, 2021] (in Japanese).
-
Clinical Practice Guidance Review Committee
(2021c) Guidance for medical treatment for COVID-19. ver. 6.1.
https://www.mhlw.go.jp/content/000875183.pdf [Accessed: January 20, 2022] (in Japanese).
-
Ferreto,
L.E.D.,
Bortoloti,
D.S.,
Fortes,
P.C.N.,
Follador,
F.,
Arruda,
G.,
Ximenez,
J.P. &
Wendt,
G.W.
(2021) Dexamethasone for treating SARS-CoV-2 infection: a systematic review and meta-analysis. Sao Paulo Med. J., 139, 657-661.
-
Huang,
J.,
Guo,
J.,
Li,
H.,
Huang,
W. &
Zhang,
T.
(2019) Efficacy and safety of adjunctive corticosteroids therapy for patients with severe community-acquired pneumonia: a systematic review and meta-analysis. Medicine (Baltimore), 98, e14636.
-
Imai,
R.,
Ro,
S.,
Tomishima,
Y. &
Nishimura,
N.
(2021) Steroid resistance and rebound phenomena in patients with COVID-19. Respir. Investig., 59, 608-613.
-
Jiang,
S.,
Liu,
T.,
Hu,
Y.,
Li,
R.,
Di,
X.,
Jin,
X.,
Wang,
Y. &
Wang,
K.
(2019) Efficacy and safety of glucocorticoids in the treatment of severe community-acquired pneumonia: a meta-analysis. Medicine (Baltimore), 98, e16239.
-
Les Bujanda,
I.,
Loureiro-Amigo,
J.,
Bastons,
F.C.,
Guerra,
I.E.,
Sanchez,
J.A.,
Murgadella-Sancho,
A.,
Rey,
R.G.,
Lopez,
J.L. &
Alvarez,
J.S.
(2021) Treatment of COVID-19 pneumonia with glucocorticoids (CORTIVID): a structured summary of a study protocol for a randomised controlled trial. Trials, 22, 43.
-
Ministry of Health, Labour and Welfare
(2021) Cooperation for priority use of dexamethasone for patients in need until stable supply of dexamethasone is available.
https://www.mhlw.go.jp/content/000834106.pdf [Accessed: February 5, 2022] (in Japanese).
-
Miyagi Prefecture Government
(2021) Test results of L452R mutant strain.
https://www.pref.miyagi.jp/documents/647/20211102.pdf [Accessed: November 15, 2021] (in Japanese).
-
RECOVERY Collaborative Group; Horby,
P.,
Lim,
W.S.,
Emberson,
J.R.,
Mafham,
M.,
Bell,
J.L.,
Linsell,
L.,
Staplin,
N.,
Brightling,
C.,
Ustianowski,
A.,
Elmahi,
E.,
Prudon,
B.,
Green,
C.,
Felton,
T.,
Chadwick,
D.,
et al.
(2021) Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med., 384, 693-704.
-
Saiz-Rodríguez,
M.,
Pena,
T.,
Lazaro,
L.,
Gonzalez,
A.,
Martinez,
A.,
Cordero,
J.A.,
Vicente,
J.T.,
Richard,
F.,
Coma,
M.J.,
de Frutos,
M.,
Labrador,
J. &
Pueyo,
A.
(2020) Outpatient treatment of COVID-19 with steroids in the phase of mild pneumonia without the need for admission as an opportunity to modify the course of the disease: a structured summary of a randomised controlled trial. Trials, 21, 632.
-
Sarma,
P.,
Bhattacharyya,
A.,
Kaur,
H.,
Prajapat,
M.,
Prakash,
A.,
Kumar,
S.,
Bansal,
S.,
Kirubakaran,
R.,
Reddy,
D.H.,
Muktesh,
G.,
Kaushal,
K.,
Sharma,
S.,
Shekhar,
N.,
Avti,
P.,
Thota,
P.,
et al.(2020) Efficacy and safety of steroid therapy in COVID-19: a rapid systematic review and meta-analysis. Indian J. Pharmacol., 52, 535-550.
-
Shionoya,
Y.,
Taniguchi,
T.,
Kasai,
H.,
Sakuma,
N.,
Imai,
S.,
Shikano,
K.,
Takayanagi,
S.,
Yahaba,
M.,
Nakada,
T.A.,
Igari,
H.,
Sakao,
S. &
Suzuki,
T.
(2021) Possibility of deterioration of respiratory status when steroids precede antiviral drugs in patients with COVID-19 pneumonia: a retrospective study. PLoS One, 16, e0256977.
-
Stern,
A.,
Skalsky,
K.,
Avni,
T.,
Carrara,
E.,
Leibovici,
L. &
Paul,
M.
(2017) Corticosteroids for pneumonia. Cochrane Database Syst. Rev., 12, CD007720.
-
Szente Fonseca,
S.N.,
de Queiroz Sousa,
A.,
Wolkoff,
A.G.,
Moreira,
M.S.,
Pinto,
B.C.,
Valente Takeda,
C.F.,
Reboucas,
E.,
Vasconcellos Abdon,
A.P.,
Nascimento,
A.L.A. &
Risch,
H.A.
(2020) Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: comparative analysis. Travel Med. Infect. Dis., 38, 101906.
-
Takayama,
S.,
Kashima,
M.,
Namiki,
T.,
Ito,
T.,
Ono,
R.,
Arita,
R.,
Saito,
N.,
Nakae,
H.,
Irie,
Y.,
Kobayashi,
S.,
Yoshino,
T.,
Ishigami,
T.,
Tanaka,
K.,
Nogami,
T.,
Minakawa,
S.,
et al. (2021) Conventional and Kampo medicine in the treatment of mild to moderate COVID-19: a multicenter, retrospective observational study protocol by the Integrative Management in Japan for Epidemic Disease (IMJEDI study-Observation). Tradit. Kampo Med., 8, 106-110.
-
Yu,
G.Q.,
Jiang,
Z.H.,
Yang,
Z.B.,
Jiang,
S.Q. &
Quan,
X.Q.
(2021) The effect of glucocorticoids on mortality in severe COVID-19 patients: evidence from 13 studies involving 6612 cases. Medicine (Baltimore), 100, e27373.