Abstract
Several studies have reported an association between sarcopenia and depression. Their results, however, are inconsistent, partly due to small sample sizes and lack of consideration of important confounders. The present study aimed to cross-sectionally examine this association in community-dwelling people in Japan. This study used baseline data from the Yuzawa cohort study (age ≥ 40 years), with the final analysis population comprising 2,466 participants. A self-administered questionnaire was used to elicit information related to sarcopenia, depressive symptoms, demographic characteristics, anthropometrics, disease history, and lifestyles. Sarcopenia was diagnosed using SARC-F, a validated questionnaire including components of Strength, Assistance in walking, Rising from a chair, Climbing stairs, and Falls. Depressive symptoms were assessed using the 11-item version of the Center for Epidemiologic Studies Depression Scale (CES-D). For depressive symptoms, prevalence ratios (PRs) were calculated, and odds ratio (ORs) were obtained using simple and multiple logistic regression analyses. Mean age of participants was 61.7 years (standard deviation = 11.8), and 10.5% and 34.7% had sarcopenia and depressive symptoms, respectively. Sarcopenic individuals had a significantly higher PR (2.00), unadjusted OR (3.67), and adjusted OR (4.96) compared to non-sarcopenic individuals, with an estimated adjusted PR of 2.7. There was a significant dose-dependent association between SARC-F scores and depressive symptoms in sarcopenic individuals (adjusted P for trend = 0.0028). In conclusion, sarcopenia and depressive symptoms were robustly associated in community-dwelling, middle-aged and older people in Japan. However, the direction of this association is unclear, and a future cohort study will be needed to determine causality.
Introduction
Depression is a common mental disorder that affects an estimated 4.4% of the global population according to World Health Organization. It is characterized by depressed mood, loss of pleasure, and hopelessness about the future, which could lead to suicide (World Health Organization 2017). Depressive symptoms are common in older people with long-term and disabling conditions. According to the US National Center for Health Statistics, proportions of adults who experience depressive symptoms are 18.4% among people aged 45-64 years and 18.4% among those aged ≥ 65 years (Villarroel and Terlizzi 2019). Depression also imposes a substantial economic burden. In Japan, the total cost related to dementia was approximately 11 billion US dollars in 2008, with direct medical costs of 1,570 million US dollars, depression-related suicide costs of 2,542 million US dollars, and workplace costs of 6,912 million US dollars (Okumura and Higuchi 2011).
There is scientific evidence that sarcopenia is a potential predictor of depression (Chang et al. 2017). Sarcopenia, which is defined as an age-related loss of skeletal muscle mass in addition to loss of muscle strength and/or reduced physical performance by the Asian Working Group for Sarcopenia, is associated with increased adverse outcomes including falls, functional decline, frailty, and mortality (Cruz-Jentoft and Sayer 2019). A meta-analysis of six studies in 2017 reported that sarcopenia was cross-sectionally associated with depression, with an adjusted odds ratio (OR) of 1.8 (Chang et al. 2017). Among those six studies, however, only three (Hsu et al. 2014; Cho et al. 2015; Byeon et al. 2016; de Souza Vasconcelos et al. 2016) were population-based studies [two of these studies (Cho et al. 2015; Byeon et al. 2016) used the same population]. Recently, three more population-based studies (Su et al. 2019; Szlejf et al. 2019; Yuenyongchaiwat and Boonsinsukh 2020) describing the impact of sarcopenia on depression have been reported. However, results of these studies were inconsistent, possibly due to small sample sizes or lack of consideration of important confounders, such as income and lifestyle factors.
In Japan, a population-based, cross-sectional study found no impact of sarcopenia on depression (Su et al. 2019), and the null association was suggested to be due to the small sample size (N = 310). However, a more recent study, also with a relatively small sample size (432 older adults), reported a possible association between sarcopenia and depressive symptoms (Hayashi et al. 2019). To resolve these conflicting results, a population-based study with a larger sample size will be needed.
We established a cohort study to explore risk factors of non-communicable chronic diseases in Japan (Yuzawa cohort study) in 2005. The Yuzawa cohort study was designed to collect detailed demographic and lifestyle information at baseline, so that these parameters can be statistically adjusted (Kabasawa et al. 2020). The present study aimed to determine whether there is an association between sarcopenia and depressive symptoms in middle-aged and older people based on data from the baseline survey of the Yuzawa cohort study.
Methods
Study design and participants
This cross-sectional study used baseline data from the Yuzawa cohort study (Kabasawa et al. 2020), a cohort study of all 5,560 residents aged ≥ 40 years in Yuzawa town (Niigata Prefecture, Japan). Self-administered questionnaires were hand-distributed to residents by a neighborhood association representative, with some questionnaires mailed subsequently. Of the residents, 3,449 (62.0%) agreed to answer the questionnaire and participate in the present study, and 950 were excluded due to missing values for items related to sarcopenia, depressive symptoms, or demographic/lifestyle. Of the 2,499 eligible participants of the baseline survey, 53 with extreme values (i.e., exceeding 3SD) of height, weight, or metabolic equivalent (MET) score were excluded as outliers. Thus, the final analysis population comprised 2,446 participants. The flow chart for participant selection is shown in Fig. 1. All participants provided written informed consent to participate in the study. The protocol of this study was approved by the Ethics Committee of Niigata University (No. 2019-0376).

The baseline survey of the Yuzawa cohort study was conducted in 2015 using self-administered questionnaire forms distributed to targeted individuals in Yuzawa town. The questionnaire asked information on sarcopenia-related items, depressive symptoms, demographic characteristics, anthropometrics, disease history, and lifestyle. Details of the Yuzawa cohort study has been published elsewhere (Kabasawa et al. 2020).
Assessment of sarcopenia
Sarcopenia was diagnosed using a 5-item, self-reported, simple questionnaire, which included components such as strength, assistance in walking, rising from a chair, climbing stairs, and falls (SARC-F) (Malmstrom and Morley 2013). Each component has a score of 0-2 points, with the total score ranging from 0 (best) to 10 (worst). A total score of ≥ 4 was defined as sarcopenia with a risk of adverse outcomes (Malmstrom and Morley 2013). A Japanese version of SARC-F was validated previously; the specificity was as high as 85.8% for men and 72.4% for women (Ida et al. 2017).
Assessment of depressive symptoms
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977). CES-D is a self-reported scale designed to measure depressive symptomatology during the previous week. We used the 11-item version of the CES-D, which consists of three categories (0, 1, 2). A total score of ≥ 7 was defined as having depressive symptoms (Yokoyama et al. 2008).
Data collection
Marital status was classified as married, never married, and others (divorced, separated, or bereaved); education levels as junior high school, high school, junior college, and university or higher; and occupation as sales/service/office work, professional/management, manual (security, farming/forestry/fishery, transportation, or labor services), jobless/housewives/others; and household income (in 10,000 yen per year) as 0-299, 300-599, 600-899, and ≥ 900. Self-reported height and weight were obtained, and body mass index (BMI) was calculated as weight divided by height squared. Total physical activity levels were estimated by calculating the MET score (MET-hours/day), which was obtained by multiplying the time spent on a given activity per day by its MET intensity. Smoking habit was classified as non-smoker, past smoker, < 20 cigarettes/day, and ≥ 20 cigarettes/day, and alcohol consumption as non- or rare-drinkers, 1-149, 150-299, 300-449, and ≥ 500 g (ethanol)/week. History of cancer, myocardial infarction, stroke, and diabetes was also obtained.
Statistical methods
Mean and standard deviation (SD) were calculated for continuous variables. We compared mean age and proportion of men and women between the 2,446 participants and 1,003 excluded residents, mean age between men and women, and prevalence of sarcopenia between men and women. Mean values and proportions between two groups were compared and tested using Student’s t-test and chi-square test, respectively. Participant characteristics were compared by the presence of sarcopenia (tested by Student’s t-test or chi-square test), and by the prevalence of and prevalence ratio (PR) and OR for depressive symptoms according to levels of covariate variables. The prevalence of and PRs and ORs for depressive symptoms were compared according to the presence of sarcopenia. To explore the dose-dependent association between SARC-F scores and depressive symptoms in sarcopenic individuals, ORs were calculated by classifying SARC-F scores into the following four groups: 4 (reference), 5, 6, and ≥ 7. Given that an OR may not be equal to the PR due to the relatively high prevalence of depressive symptoms in the present study, crude PRs were also calculated, and an adjusted PR value was estimated by multiplying the crude PR value by the ratio of adjusted/unadjusted ORs. In addition, subgroup analyses stratified by sex and age group (< 65 and ≥ 65 years) were conducted. Finally, the overall association between SARC-F scores and depressive symptoms was examined by calculating ORs with SARC-F scores divided into five groups, i.e., 0 (reference), 1, 2, 3, and ≥ 4 (sarcopenia). Unadjusted and adjusted ORs (and P for trend values) were calculated by simple and multiple logistic regression analyses. In multivariate analysis, ORs were adjusted for age, sex, BMI, marital status (dummy variable), education level, occupation (dummy variable), household income, total physical activity (continuous variable), smoking, alcohol consumption, and history of cancer, myocardial infarction, stroke, and diabetes as covariates.
Statistical analyses were performed using SAS statistical software (release 9.4, SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
First, we compared the characteristics of the 2,446 included and 1,003 excluded participants. Mean age was 61.7 years (SD = 11.8) for the former and 72.9 years (SD = 12.9) for the latter, showing a significant difference (11.2 years; P < 0.0001). The proportion of male participants was 1,259/2,446 (51.5%) for the former and 405/1,003 (40.4%) for the latter, also showing a significant difference (P < 0.0001).
Overall, mean age of male participants (61.8 years; SD = 11.6, N = 1,259) did not significantly differ from that of female participants (61.5 years; SD = 12.0, N = 1,187) (P = 0.4907). Among all participants, 848 (34.7%) had depressive symptoms and 257 (10.5%) had sarcopenia, including 114 (9.1%) men and 143 (12.1%) women (P = 0.0159). The prevalence of sarcopenia was 11/453 (2.4%) for those in their 40s, 41/556 (7.4%) for those in their 50s, 58/834 (7.0%) for those in their 60s, 70/432 (16.2%) for those in their 70s, and 77/171 (45.0%) for those in their 80s and older.
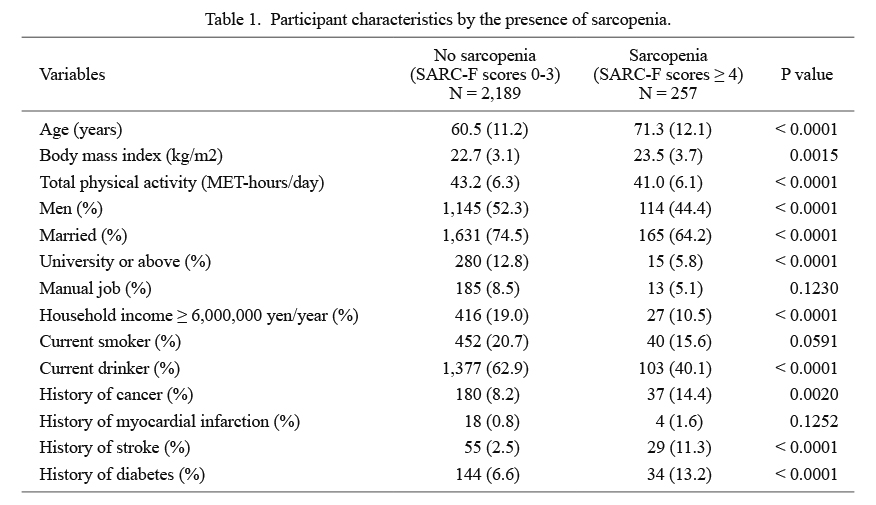
Participant characteristics in the non-sarcopenia and sarcopenia groups are summarized in Table 1. Sarcopenia was significantly associated with an older age, higher BMI, lower total physical activity level, female sex, unmarried status, lower education level, lower household income, less drinking, and history of cancer, stroke, and diabetes. The prevalence of and PRs and unadjusted ORs for depressive symptoms according to variables other than SARC-F scores are shown in Table 2. Depressive symptoms were significantly associated with sex, age, marital status, occupation, total physical activity, and smoking.
The prevalence of and PRs and ORs for depressive symptoms by sarcopenic status are shown in Table 3. Sarcopenic individuals had a higher PR (2.00), unadjusted OR (3.67), and adjusted OR (4.96) compared to non-sarcopenic individuals (reference). Based on these data, the adjusted PR was estimated to be 2.70 (2.00/3.67*4.96). The prevalence of and PRs and ORs for depressive symptoms according to SARC-F scores of sarcopenic individuals [i.e., SARC-F scores 4 (reference), 5, 6, and ≥ 7] are also shown in Table 3. There was a dose-dependent association between SARC-F scores and depressive symptoms in the overall population, males, and those aged ≥ 65 years.
The prevalence of and ORs for depressive symptoms according to SARC-F scores [i.e., 0 (reference), 1, 2, 3, and ≥ 4] are shown in Table 4. There was a dose-dependent association between SARC-F scores and depressive symptoms (P for trend < 0.0001).
Discussion
The present study found a robust association between sarcopenia and depressive symptoms in middle-aged and older people, regardless of sex and age. Specifically, SARC-F scores were dose-dependently associated with the prevalence of depressive symptoms. Our results were comparable to those reported in the literature. Among six population-based cross-sectional studies that reported ORs for depression/depressive symptoms adjusted for potential confounders of sarcopenia (Hsu et al. 2014; Byeon et al. 2016; de Souza Vasconcelos et al. 2016; Hayashi et al. 2019; Szlejf et al. 2019; Yuenyongchaiwat and Boonsinsukh 2020) (Table 5), Hsu et al. (2014) reported an OR of 2.25 for Geriatric-Depression-Scale (GDS)-diagnosed depressive symptoms in 353 Taiwanese men aged ≥ 65 years (mean, 82.7 years); de Souza Vasconcelos et al. (2016) reported an OR of 1.53 for GDS-diagnosed depressive symptoms in 1,374 Brazilian adults aged ≥ 65 years (mean, 73.4 years); Szlejf et al. (2019) reported an OR of 2.23 for Clinical-Interview-Scheduled-Revised (CIS-R)-diagnosed depressive symptoms in 5,927 Brazil adults aged ≥ 55 years (mean, 62.7 years); Hayashi et al. (2019) reported an OR of 2.38 for GDS-diagnosed depressive symptoms in 432 Japanese older adults (mean, 73 years); and Yuenyongchaiwat and Boonsinsukh (2020) reported an OR of 2.09 for GDS-diagnosed depressive symptoms in 330 Thai adults aged ≥ 60 years (mean, 66.9 years). Meanwhile, Byeon et al. (2016) reported no association between sarcopenia and depression or depressive symptoms in 7,364 Korean adults aged ≥ 20 years. Since that study targeted a relatively younger population (mean age, 49.5 years) compared to the other five studies (mean age range, 62.7-82.7 years) (Hsu et al. 2014; de Souza Vasconcelos et al. 2016; Hayashi et al. 2019; Szlejf et al. 2019; Yuenyongchaiwat and Boonsinsukh 2020), differences in age might explain this discrepancy.
The results obtained in the previous studies were not adjusted for important confounders of depression, namely, socioeconomic status (SES) including education, income, and occupation (Hinata et al. 2021). Only education was adjusted for in four of the six studies (Hsu et al. 2014; Hayashi et al. 2019; Szlejf et al. 2019; Yuenyongchaiwat and Boonsinsukh 2020). The present study calculated ORs adjusted for all SES variables as well as lifestyle factors. After adjusting for these potential confounders, our results showed that sarcopenic individuals are 2.7 times more likely to have depressive symptoms. Moreover, a dose-dependent association between SARC-F scores and depressive symptoms was observed, suggesting that sarcopenia is an important correlate of depression.
The association between sarcopenia and depressive symptoms was more robust in men than in women in the present study. Previous studies reported similar findings (Kim et al. 2016; Ida et al. 2018), where the association between sarcopenia and suicidal ideation was observed in men but not in women. The reason for these findings is unclear but might involve sex differences. Sex differences in sarcopenia, such as a more prominent age-related decrease in muscle mass, muscle strength, and physical function in men than in women, have been reported, despite the fact that the prevalence of sarcopenia is higher in women due to lower absolute muscle mass (Anderson et al. 2017). Differences in endogenous sex hormone levels may account for the association between sarcopenia and depressive symptoms, although the underlying mechanism is not well-understood (Morssinkhof et al. 2020). Further studies will be needed to clarify the mechanism underlying sex differences in the association between sarcopenia and depressive symptoms.
The prevalence of depressive symptoms was as high as 34.7% in the present study. The prevalence of CES-D-diagnosed depressive symptoms in representative Japanese adults was reported to be 30.1%, which is significantly higher than that reported for Western counterparts (≤ 20%) (Furihata et al. 2011). This may be explained by cultural features specific to Japan, where people suppress the expression of positive affect, possibly resulting in an overestimation of the prevalence of depressive symptoms (Iwata et al. 1995).
With respect to the mechanism underlying the association between sarcopenia and depressive symptoms, the involvement of chronic inflammation, oxidative stress, and neurotrophins has been suggested (Pasco et al. 2015). A number of studies have found chronic inflammation to be a common cause of sarcopenia (Pan et al. 2021) and depressive symptoms (Ng et al. 2018). For example, levels of inflammatory markers such as interleukin-6, tumor necrosis factor-α, and C-reactive protein are increased in individuals with both sarcopenia (Pan et al. 2021) and depression (Ng et al. 2018). Neurotrophins, which are released not only from the brain but also from skeletal muscle, are also a possible cause of both sarcopenia and depressive symptoms (Pasco et al. 2015). On the other hand, from psychological and behavioral perspectives, the association between sarcopenia and depressive symptoms might be bidirectional, i.e., sarcopenia increases the risk of depressive symptoms, while long-term depressive status increases the risk of sarcopenia. Future studies will be needed to clarify the causality of the association.
In the present study, sarcopenic individuals had a higher BMI compared to non-sarcopenic individuals (Table 1). The association between sarcopenic obesity and depression has been previously reported (Hamer et al. 2015; Lee et al. 2021), and visceral adipose tissue was suggested to play a role through obesity-related endocrine hormones (Lee et al. 2021). Thus, sarcopenia might be associated with an adverse, obesity-related trait, which might also explain the robust association between sarcopenia and depressive symptoms.
The robust association between sarcopenia and depressive symptoms was observed even after adjusting for lifestyle factors and other important confounders. However, this study has some limitations worth noting. First, the study used a cross-sectional design and thus could not determine the causality of the association, which may provide clues for the prevention of depression by controlling sarcopenia, and vice versa. A cohort study will be needed to address these issues. Second, we used questionnaire-based tools to evaluate sarcopenia and depression, and no direct diagnostic methods were employed; this may have caused misclassification. Third, although the specificity of SARC-F was reported to be acceptably high in a Japanese population, its sensitivity was reported to be low (men, 14.6% and women, 33.3%); as a result, its positive predictive value was low (men, 33.3 and women, 17.3) (Ida et al. 2017). Despite this, data from the study by Ida et al. (2017) showed that SARC-F-diagnosed sarcopenic individuals had a lower skeletal mass compared to undiagnosed individuals. Nonetheless, our results should be confirmed in studies using a direct method for diagnosing sarcopenia. Fourth, 2,111 of 5,560 (38%) Yuzawa residents did not participate in the present study, suggesting that selection bias may have occurred and influenced the results. Individuals having serious depressive symptoms may have been less likely to participate in the present study. Fifth, the proportion of excluded participants due to missing data was higher among older participants (≥ 65 years) compared to those aged < 65 years (Fig. 1). Thus, selection bias may have occurred in the former group, resulting in an underestimation of ORs. Sixth, as the present study was conducted in Yuzawa town, a local, rural area in Japan, our results may not be generalizable to urban areas, where the prevalence of sarcopenia or depressive symptoms may differ. Finally, ORs apparently overestimated real PRs due to the high prevalence (34.7% overall) of depressive symptoms. This should be considered in the interpretation of the ORs. Future studies to address these limitations are warranted.
In conclusion, there was a robust association between sarcopenia and depressive symptoms in community-dwelling, middle-aged and older people in Japan after adjusting for major confounders. However, the direction of this association is unclear, and the causality of the association needs to be determined in a future cohort study.
Acknowledgments
We thank all study participants and institutions in Niigata Prefecture and Yuzawa town for their cooperation. We also thank all members of the cohort study group for their continued support. This study was supported by funding from Niigata Prefecture and partly by the 2021 basic fund of Niigata University.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Anderson,
L.J.,
Liu,
H. &
Garcia,
J.M.
(2017) Sex differences in muscle wasting. Adv. Exp. Med. Biol., 1043, 153-197.
-
Byeon,
C.H.,
Kang,
K.Y.,
Kang,
S.H.,
Kim,
H.K. &
Bae,
E.J.
(2016) Sarcopenia is not associated with depression in Korean adults: results from the 2010-2011 Korean National Health and Nutrition Examination Survey. Korean J. Fam. Med., 37, 37-43.
-
Chang,
K.V.,
Hsu,
T.H.,
Wu,
W.T.,
Huang,
K.C. &
Han,
D.S.
(2017) Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing, 46, 738-746.
-
Cho,
Y.,
Shin,
S.Y. &
Shin,
M.J.
(2015) Sarcopenic obesity is associated with lower indicators of psychological health and quality of life in Koreans. Nutr. Res., 35, 384-392.
-
Cruz-Jentoft,
A.J. &
Sayer,
A.A.
(2019) Sarcopenia. Lancet, 393, 2636-2646.
-
de Souza Vasconcelos,
K.S.,
Domingues Dias,
J.M.,
de Carvalho Bastone,
A.,
Alvarenga Vieira,
R.,
de Souza Andrade,
A.C.,
Rodrigues Perracini,
M.,
Oliveira Guerra,
R. &
Corrêa Dias,
R.
(2016) Handgrip strength cutoff points to identify mobility limitation in community-dwelling older people and associated factors. J. Nutr. Health Aging, 20, 306-315.
-
Furihata,
R.,
Uchiyama,
M.,
Takahashi,
S.,
Konno,
C.,
Suzuki,
M.,
Osaki,
K.,
Kaneita,
Y. &
Ohida,
T.
(2011) Self-help behaviors for sleep and depression: a Japanese nationwide general population survey. J. Affect. Disord., 130, 75-82.
-
Hamer,
M.,
Batty,
G.D. &
Kivimaki,
M.
(2015) Sarcopenic obesity and risk of new onset depressive symptoms in older adults: English Longitudinal Study of Ageing. Int. J. Obes. (Lond.), 39, 1717-1720.
-
Hayashi,
T.,
Umegaki,
H.,
Makino,
T.,
Cheng,
X.W.,
Shimada,
H. &
Kuzuya,
M.
(2019) Association between sarcopenia and depressive mood in urban-dwelling older adults: a cross-sectional study. Geriatr. Gerontol. Int., 19, 508-512.
-
Hinata,
A.,
Kabasawa,
K.,
Watanabe,
Y.,
Kitamura,
K.,
Ito,
Y.,
Takachi,
R.,
Tsugane,
S.,
Tanaka,
J.,
Sasaki,
A.,
Narita,
I. &
Nakamura,
K.
(2021) Education, household income, and depressive symptoms in middle-aged and older Japanese adults. BMC Public Health, 21, 2120.
-
Hsu,
Y.H.,
Liang,
C.K.,
Chou,
M.Y.,
Liao,
M.C.,
Lin,
Y.T.,
Chen,
L.K. &
Lo,
Y.K.
(2014) Association of cognitive impairment, depressive symptoms and sarcopenia among healthy older men in the veterans retirement community in southern Taiwan: a cross-sectional study. Geriatr. Gerontol. Int., 14 Suppl 1, 102-108.
-
Ida,
S.,
Murata,
K.,
Nakadachi,
D.,
Ishihara,
Y.,
Imataka,
K.,
Uchida,
A.,
Monguchi,
K.,
Kaneko,
R.,
Fujiwara,
R. &
Takahashi,
H.
(2017) Development of a Japanese version of the SARC-F for diabetic patients: an examination of reliability and validity. Aging Clin. Exp. Res., 29, 935-942.
-
Ida,
S.,
Murata,
K.,
Nakai,
M.,
Ito,
S.,
Malmstrom,
T.K.,
Ishihara,
Y.,
Imataka,
K.,
Uchida,
A.,
Monguchi,
K.,
Kaneko,
R.,
Fujiwara,
R. &
Takahashi,
H.
(2018) Relationship between sarcopenia and depression in older patients with diabetes: an investigation using the Japanese version of SARC-F. Geriatr. Gerontol. Int., 18, 1318-1322.
-
Iwata,
N.,
Roberts,
C.R. &
Kawakami,
N.
(1995) Japan-U.S. comparison of responses to depression scale items among adult workers. Psychiatry Res., 58, 237-245.
-
Kabasawa,
K.,
Tanaka,
J.,
Nakamura,
K.,
Ito,
Y.,
Yoshida,
K.,
Takachi,
R.,
Sawada,
N.,
Tsugane,
S. &
Narita,
I.
(2020) Study design and baseline profiles of participants in the Uonuma CKD cohort study in Niigata, Japan. J. Epidemiol., 30, 170-176.
-
Kim,
J.H.,
Kim,
D.H. &
Park,
Y.S.
(2016) Body composition, sarcopenia, and suicidal ideation in elderly Koreans: Hallym aging study. J. Korean Med. Sci., 31, 604-610.
-
Lee,
J.I.,
Busler,
J.N.,
Millett,
C.E.,
Principe,
J.L.,
Levin,
L.L.,
Corrigan,
A. &
Burdick,
K.E.
(2021) Association between visceral adipose tissue and major depressive disorder across the lifespan: a scoping review. Bipolar Disord. doi: 10.1111/bdi.13130 [Epub ahead of print].
-
Malmstrom,
T.K. &
Morley,
J.E.
(2013) SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc., 14, 531-532.
-
Morssinkhof,
M.W.L.,
van Wylick,
D.W.,
Priester-Vink,
S.,
van der Werf,
Y.D.,
den Heijer,
M.,
van den Heuvel,
O.A. &
Broekman,
B.F.P.
(2020) Associations between sex hormones, sleep problems and depression: a systematic review. Neurosci. Biobehav. Rev., 118, 669-680.
-
Ng,
A.,
Tam,
W.W.,
Zhang,
M.W.,
Ho,
C.S.,
Husain,
S.F.,
McIntyre,
R.S. &
Ho,
R.C.
(2018) IL-1beta, IL-6, TNF- alpha and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci. Rep., 8, 12050.
-
Okumura,
Y. &
Higuchi,
T.
(2011) Cost of depression among adults in Japan. Prim. Care Companion CNS Disord., 13, PCC.10m01082.
-
Pan,
L.,
Xie,
W.,
Fu,
X.,
Lu,
W.,
Jin,
H.,
Lai,
J.,
Zhang,
A.,
Yu,
Y.,
Li,
Y. &
Xiao,
W.
(2021) Inflammation and sarcopenia: a focus on circulating inflammatory cytokines. Exp. Gerontol., 154, 111544.
-
Pasco,
J.A.,
Williams,
L.J.,
Jacka,
F.N.,
Stupka,
N.,
Brennan-Olsen,
S.L.,
Holloway,
K.L. &
Berk,
M.
(2015) Sarcopenia and the common mental disorders: a potential regulatory role of skeletal muscle on brain function? Curr. Osteoporos. Rep., 13, 351-357.
-
Radloff,
L.S.
(1977) The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas., 1, 385-401.
-
Su,
Y.,
Hirayama,
K.,
Han,
T.F.,
Izutsu,
M. &
Yuki,
M.
(2019) Sarcopenia prevalence and risk factors among Japanese community dwelling older adults living in a snow-covered city according to EWGSOP2. J. Clin. Med., 8, 291.
-
Szlejf,
C.,
Suemoto,
C.K.,
Brunoni,
A.R.,
Viana,
M.C.,
Moreno,
A.B.,
Matos,
S.M.A.,
Lotufo,
P.A. &
Bensenor,
I.M.
(2019) Depression is associated with sarcopenia due to low muscle strength: results from the ELSA-Brasil study. J. Am. Med. Dir. Assoc., 20, 1641-1646.
-
Villarroel,
M. A.,
& Terlizzi, E. P. (2019) Key findings Data from the National Health Interview Survey (NCHS Data Brief No. 379), U.S. Department of Health & Human Services.
https://www.cdc.gov/nchs/data/databriefs/db379-H.pdf [Accessed: October 10, 2021].
-
World Health Organization
(2017) Depression and Other Common Mental Disorders: Global Health Estimates, World Health Organization.
https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf?sequence=1&isAllowed=y[Accessed: October 10, 2021].
-
Yokoyama,
E.,
Kaneita,
Y.,
Saito,
Y.,
Uchiyama,
M.,
Matsuzaki,
Y.,
Tamaki,
T.,
Munezawa,
T. &
Ohida,
T.
(2008) Cut-off point for the 11-item shorter form of the CES-D depression scale. Nihon Univ. J. Med., 50, 123-132.
-
Yuenyongchaiwat,
K. &
Boonsinsukh,
R.
(2020) Sarcopenia and its relationships with depression, cognition, and physical activity in Thai community-dwelling older adults. Curr. Gerontol. Geriatr. Res., 2020, 8041489.