Abstract
The present case study was conducted on a 74-year-old man who visited our department due to a left renal and retroperitoneal tumor on computed tomography (CT). The patient was diagnosed with left renal cancer lymph node metastasis and was hospitalized a few weeks prior to surgery due to fever, malaise, and severe appetite loss. Biochemical laboratory findings at admission showed markedly high levels of inflammation. The cause of high inflammatory response was paraneoplastic syndrome. Tumor resection was considered necessary, and left nephrectomy and lymphadenectomy were performed; however, it did not improve the inflammatory response. After operation, positron emission tomography-CT revealed hyperaccumulation of 18F-fluorodeoxyglucose in the bone marrow throughout the body. Pathological examination of the resected specimen and bone marrow aspiration revealed the coexistence of idiopathic multicentric Castleman disease (CD) and renal cancer. Prednisolone and tocilizumab were administered for idiopathic multicentric CD and a tyrosine kinase inhibitor for renal cancer; however, they had poor therapeutic effect, and the patient died. CD is characterized by systemic symptoms due to the overproduction of interleukin-6. Treatment for idiopathic multicentric CD involves steroid and anti-interleukin-6 therapy. The diagnostic criteria for CD require the exclusion of malignant tumors although there are some cases in which CD and malignant tumors coexist. The prognosis for CD is relatively good; however, as in this case, the prognosis of CD coexisting with uncontrollable renal cancer is insufficient due to poor improvement in the inflammatory response.
Case Presentation
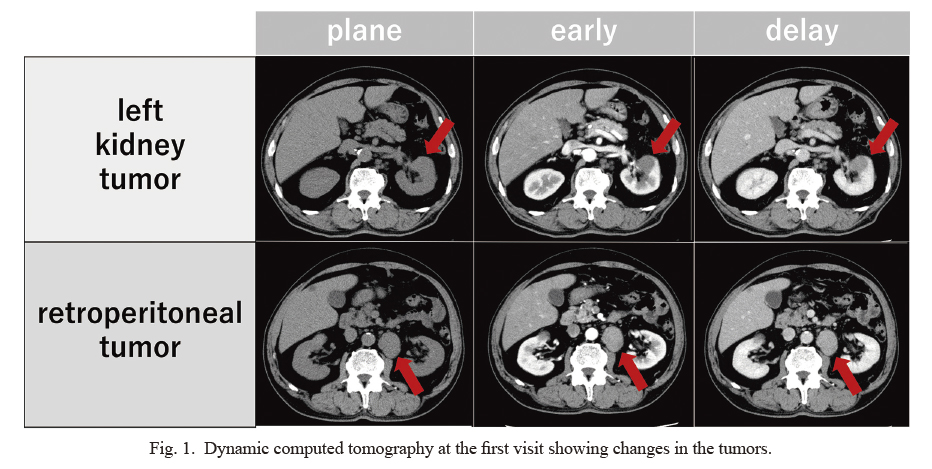
A 74-year-old man with a history of hypertension, diabetes, and asthma visited our department because of a left renal and retroperitoneal tumor on computed tomography (CT). Dynamic CT showed a left renal tumor with a major axis of 3 cm, a retroperitoneal tumor with a major axis of 4 cm in the left hilum of the left kidney, and some lymph nodes less than 1 cm around the retroperitoneal tumor (Fig. 1). The contrast pattern of the left renal tumor differed from that of clear cell renal cancer. The retroperitoneal tumor showed a contrast pattern similar to that of the left renal tumor. Based on the results of the biochemical laboratory and imaging findings, renal cancer and blood diseases, such as malignant lymphoma, were suspected. To diagnose the pathophysiological findings, CT-guided biopsy was performed on the retroperitoneal tumor. Furthermore, immunostaining of the biopsy specimens was positive for cytokeratin, which stains epithelial-derived tissue, and PAX8, which stains kidney-derived tissue. Based on these findings it was confirmed that the retroperitoneal tumor was a lymph node metastasis of renal cancer, and the patient was diagnosed with cT1aN1M0 left renal cancer.
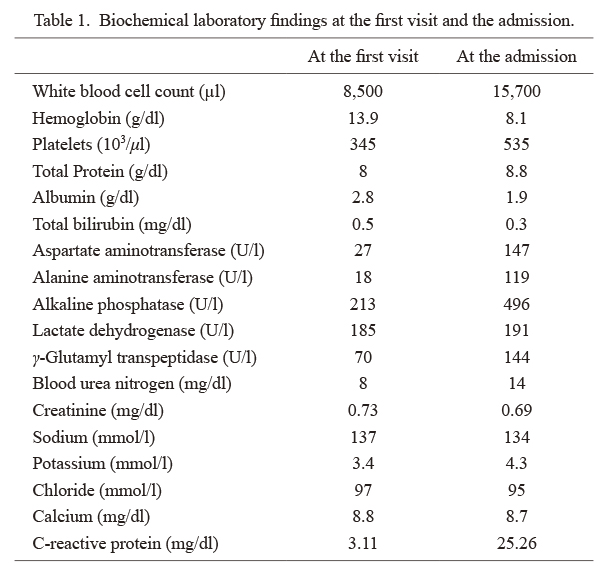
The patient was hospitalized a few weeks prior to surgery due to fever, malaise, and severe appetite loss. Biochemical laboratory findings at admission showed markedly high inflammation, anemia, and thrombocytosis (Table 1). However, CT imaging at admission did not reveal tumor growth or the origin of inflammation. We performed various clinical tests to determine the origin of fever; however, its origin remains unknown. Therefore, we hypothesized that a paraneoplastic syndrome of renal cancer could be the cause of inflammatory response.
The removal of primary tumor and lymph nodes was considered necessary for improving the hyperinflammatory condition. Therefore, the patient underwent left nephrectomy and lymphadenectomy; however, his general condition worsened during hospitalization. Macroscopic findings of renal tumors and lymph nodes are shown in Fig. 2a, b. The pathological and immunostaining findings did not show anything other than cytokeratin-positive and PAX8-positive cells to identify the histological type of renal cancer (Fig. 2c, d, e). The subtype of renal cell carcinoma could not be identified because of the poor differentiation. However, we observed marked infiltration of inflammatory cells around the tumor (Fig. 2c).
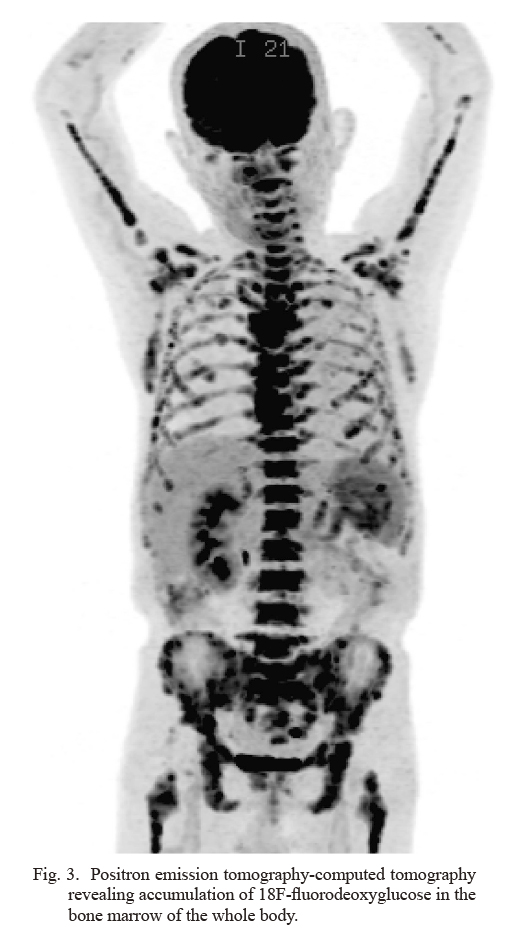
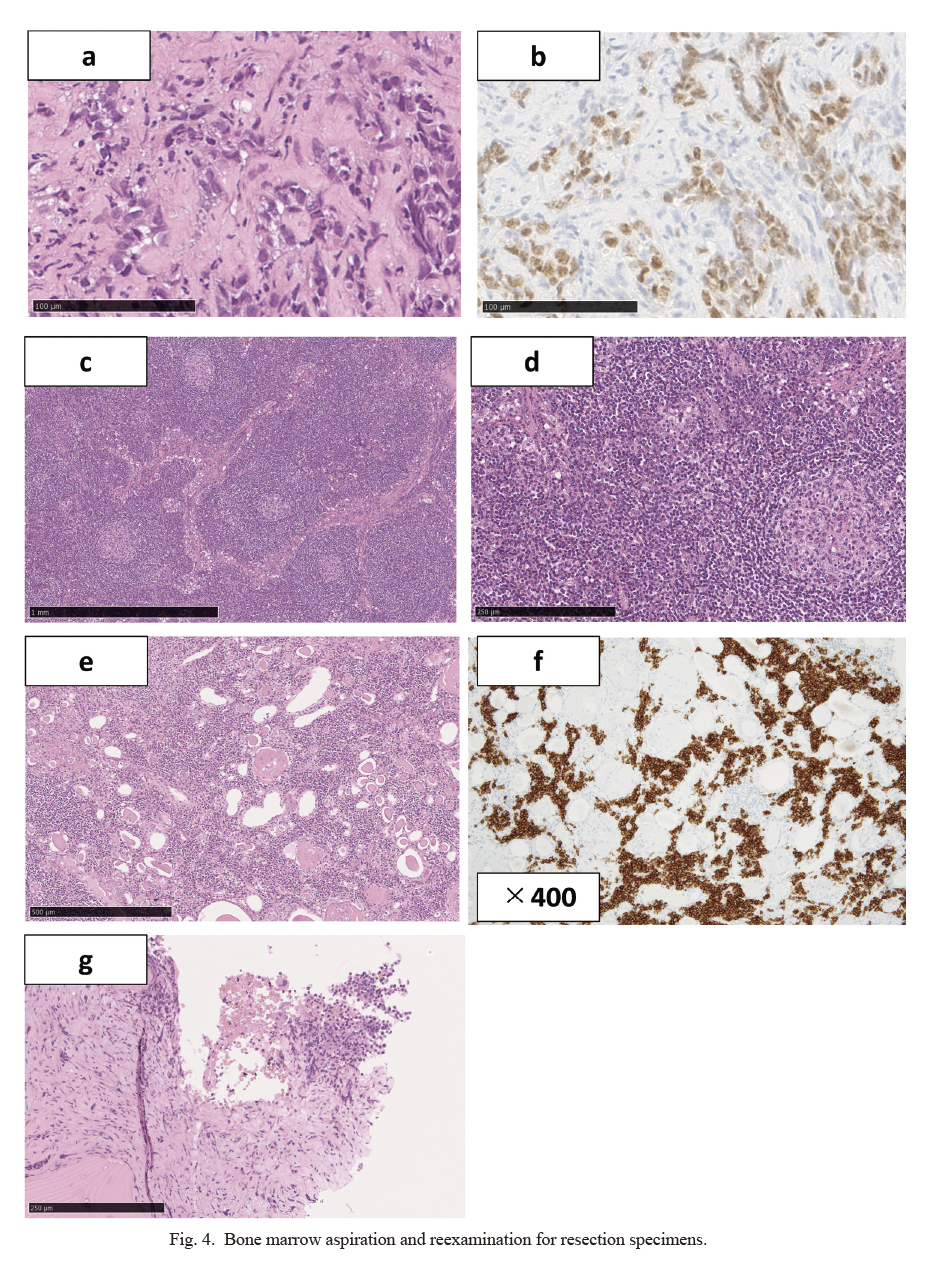
There was no improvement in inflammation after surgery. Positron emission tomography-CT assessing the inflammatory response revealed accumulation of 18F-fluorodeoxyglucose in the bone marrow of the whole body (Fig. 3). Since blood diseases were expected, we requested bone marrow aspiration and reexamined the coexistence of blood diseases in the excised specimen. Bone marrow aspiration specimens contained atypical cells that were PAX8-positive and bone marrow metastasis of renal cancer was observed (Fig. 4a, b). In addition, pathological reexamination of lymph nodes with advanced renal cancer revealed interfollicular enlargement and angiogenesis, atrophic germinal centers, concentric arrangement of the mantle layer, and numerous plasma cells between the enlarged follicles (Fig. 4c, d). Therefore, the patient was diagnosed with plasma cell-type CD. Furthermore, infiltration of plasma cells (Fig. 4e), which are CD38-positive cells (Fig. 4f), was observed in the normal tissue of the left kidney. In the bone marrow, plasma cells proliferating in a polyclonal manner were also observed, suggesting the involvement of CD (Fig. 4g). Therefore, the coexistence of renal cancer and CD in the left kidney, lymph nodes, and bone marrow was suggested. As a result of the negative report for human immunodeficiency virus (HIV) and the presence of CD in multiple regions, the patient was diagnosed with idiopathic multicentric CD. However, the general condition of the patient continued to get worsen due to a severe inflammatory reaction. Therefore, CD treatment should be performed before systemic therapy for advanced renal cancer to reduce the inflammatory reaction. Tocilizumab, an interleukin-6 (IL-6) receptor antibody, and prednisolone was administered to the patient. Although the C-reactive protein level temporarily improved, the patient’s condition worsened soon after. Considering the general condition of the patient, a tyrosine kinase inhibitor (TKI) was administered for the treatment of renal cancer; however, it had no effect. To make matters worse, tocilizumab was stopped owing to the appearance of its infusion reaction. Since the general condition of the patient did not improve after medications, supportive care was preferred. The patient died of renal cancer in a hospice near his home 3 weeks after discharge from our hospital.
The study protocol was reviewed and approved by the Ethics Committee of Tohoku University Hospital (approval number: 23423). Written informed consent was obtained from the patient’s family for publication of this case report and accompanying images.
Discussion
According to the diagnostic criteria for CD, it does not coexist with malignant tumors (Fajgenbaum et al. 2017). However, in reality, there are some reports, such as our case, on the coexistence of various types of cancer and CD.
CD encompasses several clinicopathologic disorders at the intersection of hematology, oncology, rheumatology, and virology, with overlapping histopathological and clinical characteristics (Fajgenbaum et al. 2017) . The cause is unknown, but it is believed to be associated with the development of autoimmune responses, paraneoplastic syndromes, and viruses. Various symptoms that can be explained by cytokine overproduction, such as IL-6 overproduction, appear, and cytokine storms cause multiple organ failure. CD can be diagnosed based on the presence of swollen lymph nodes and pathological findings.
There are two pathological findings of CD: hyaline-vascular type and plasma cell type. CD is classified according to lesion distribution. When the lesion is located in a single area, it is called unicentric CD. Unicentric CD is asymptomatic and has a mild inflammatory response. Moreover, unicentric CD is treated by surgical lymph node excision, which is curative in most patients (Fajgenbaum 2018). When lesions are present in multiple areas, they are called multicentric CD. Multicentric CD has strong systemic symptoms and inflammatory reactions (Fajgenbaum et al. 2017). Human herpesvirus 8 (HHV-8) associated multicentric CD is a subtype found primarily in patients with HIV. The treatment for HHV-8 associated multicentric CD is a combination therapy with anticancer and anti-HIV drugs. The prognosis for HHV-8 associated multicentric CD is relatively good, with a 3-year progression-free survival rate of 69% and a 3-year overall survival rate of 81% (Uldrick et al. 2014). In contrast, HIV-negative cases are known as idiopathic multicentric CD (Fajgenbaum et al. 2017). Treatment of idiopathic multicentric CD with prednisolone, tocilizumab, and the anti-IL-6 antibody siltuximab is common. In Japan, the 5-year survival rate of idiopathic multicentric CD is 91% (Kojima et al. 2008).
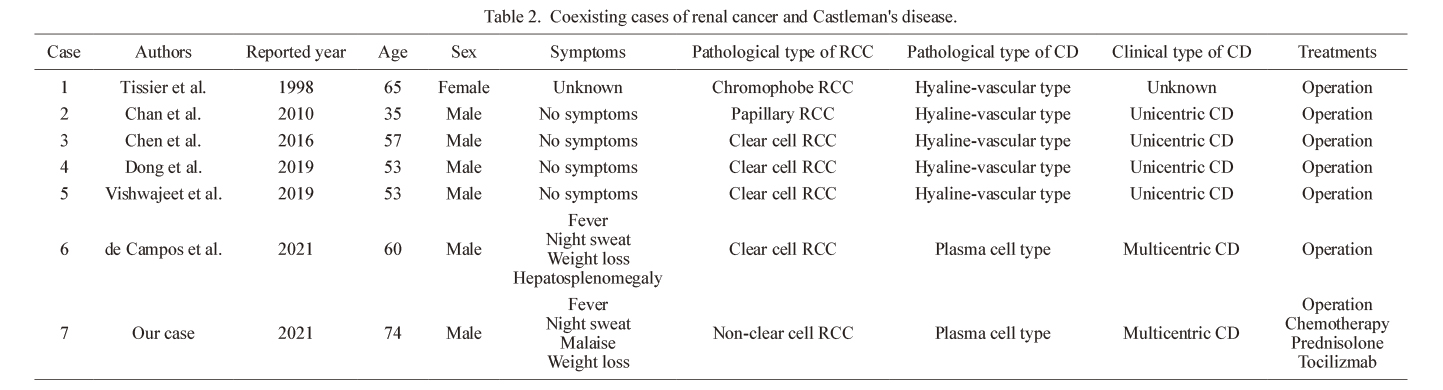
The following theories have been proposed to explain the coexistence of CD and cancer: paraneoplastic syndrome, tumor induction due to CD, precancerous condition of CD, unknown virus, gene mutation, and secondary carcinogenesis due to disease treatment (Liu et al. 2016). It is considered that renal cancer causes the release of various inflammatory cytokines, such as IL-6, and induces CD as a paraneoplastic syndrome. To date, seven cases have been reported, including our case, of coexisting renal cancer and CD (Table 2) (Tissier et al. 1998; Chan et al. 2010; Chen et al. 2016; Dong et al. 2019; Vishwajeet et al. 2019; de Campos et al. 2021). According to previous reports, complete surgical resection could be effective in patients with coexisting renal cancer and CD. However, cases of coexisting unresectable renal cancer and CD, such as our case, have not been previously reported.
Since our case was an idiopathic multicentric CD, prednisolone and tocilizumab were administered, and the inflammatory reaction was temporarily improved. Due to residual aggressive renal cancer, inflammation recurred soon after surgery. Treatment for residual renal cancer was necessary to suppress inflammation; therefore, we performed a systemic treatment. TKI therapy was initiated in consideration of the patient’s performance status. Although treatment with TKI was administered to the patient, it was unable to continue due to its poor effect on the patient’s general malaise. Consequently, best supportive care was preferred for the patient. The cause of the worsening prognosis was the progression of highly malignant renal cancer. However, the exacerbation of prognosis was also associated with Castleman’s disease in the background of highly inflammation might be caused by paraneoplastic syndrome.
We encountered a rare case of renal cancer coexisting with CD. CD should exclude malignant tumors as a diagnostic criterion and is a disease with a relatively good prognosis. However, as observed in this case, CD could coexist with unresectable and advanced malignant tumors and general inflammation due to CD could not be improved, resulting in a poor prognosis.
Author Contributions
All authors participated in the conception of this case report, helped draft the manuscript, and approved the manuscript for publication. In addition, Dr. Watanabe provided a histological illustration. All authors agree to be accountable for all aspects of this work.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Chan,
J.J.,
Loh,
A.H.,
Sim,
H.G.,
Tan,
M.H. &
Toh,
C.K.
(2010) Coexistence of unicentric Castleman’s Disease and locally advanced papillary renal cell carcinoma: more than a coincidental association? Ann. Acad. Med. Singap., 39, 584-585.
-
Chen,
S.,
Song,
L.,
Xie,
X.,
Han,
X. &
Cheng,
B.
(2016) A case of abdominal mesenteric Castleman’s disease with left renal cell carcinoma and stomach leiomyoma. Hell. J. Nucl. Med., 19, 285-288.
-
de Campos,
E.C.R.,
Junior,
M.G.A.,
Winheski,
M.R.,
Mehanna,
S.H.,
Cavalcanti,
M.S. &
Martins,
R.
(2021) Retroperitoneal castleman disease mimicking lymph node spread from clear renal cell carcinoma. A case report. Urol. Case Rep., 34, 101503.
-
Dong,
Y.,
Liu,
B.,
Ju,
G.,
Cai,
J.,
Zhang,
N. &
Wang,
L.H.
(2019) Concomitant perinephric Castleman disease and renal cell carcinoma initially suspected to be metastasis: a case report and 24 months follow up. Urol. Case Rep., 22, 70-72.
-
Fajgenbaum, D.C. (2018) Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease. Hematology Am. Soc. Hematol. Educ. Program, 2018, 318-325.
-
Fajgenbaum,
D.C.,
Uldrick,
T.S.,
Bagg,
A.,
Frank,
D.,
Wu,
D.,
Srkalovic,
G.,
Simpson,
D.,
Liu,
A.Y.,
Menke,
D.,
Chandrakasan,
S.,
Lechowicz,
M.J.,
Wong,
R.S.,
Pierson,
S.,
Paessler,
M.,
Rossi,
J.F.,
et al. (2017) International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood, 129, 1646-1657.
-
Kojima,
M.,
Nakamura,
N.,
Tsukamoto,
N.,
Otuski,
Y.,
Shimizu,
K.,
Itoh,
H.,
Kobayashi,
S.,
Kobayashi,
H.,
Murase,
T.,
Masawa,
N.,
Kashimura,
M. &
Nakamura,
S.
(2008) Clinical implications of idiopathic multicentric castleman disease among Japanese: a report of 28 cases. Int. J. Surg. Pathol., 16, 391-398.
-
Liu,
A.Y.,
Nabel,
C.S.,
Finkelman,
B.S.,
Ruth,
J.R.,
Kurzrock,
R.,
van Rhee,
F.,
Krymskaya,
V.P.,
Kelleher,
D.,
Rubenstein,
A.H. &
Fajgenbaum,
D.C.
(2016) Idiopathic multicentric Castleman’s disease: a systematic literature review. Lancet Haematol., 3, e163-175.
-
Tissier,
F.,
de Pinieux,
G.,
Thiounn,
N.,
Merran,
S.,
Gessain,
A.,
Tulliez,
M. &
Vieillefond,
A.
(1998) Castleman’s disease and chromophobe carcinoma of the kidney. An incidental association? Ann. Pathol., 18, 429-431.
-
Uldrick,
T.S.,
Polizzotto,
M.N.,
Aleman,
K.,
Wyvill,
K.M.,
Marshall,
V.,
Whitby,
D.,
Wang,
V.,
Pittaluga,
S.,
O’Mahony,
D.,
Steinberg,
S.M.,
Little,
R.F. &
Yarchoan,
R.
(2014) Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood, 124, 3544-3552.
-
Vishwajeet,
V.,
Kakkar,
N.,
Singhal,
P. &
Mandal,
A.K.
(2019) Synchronous retroperitoneal Castleman’s disease with clear cell renal cell carcinoma. BMJ Case Rep., 12, e230919.