2022 Volume 257 Issue 3 Pages 171-180
2022 Volume 257 Issue 3 Pages 171-180
A myeloid immune checkpoint, leukocyte immunoglobulin-like receptor (LILR) B4 (B4, also known as ILT3/CD85k in humans and gp49B in mice) is expressed on dendritic cells (DCs). However, a mode of regulation of DCs by B4/gp49B is not identified yet in relation to the ligand(s) as well as to the counteracting, activation-type receptor. Our recent identification of the physiological/pathological ligand for B4/gp49B as the fibronectin (FN) N-terminal 30-kDa domain poses the question of the relationship between B4/gp49B and a classical FN receptor/cellular activator, integrin, on DCs. Here we showed that FN is not constitutively tethered on the surface of bone marrow-derived cultured DCs (BMDCs) or splenic DCs, even though the FN receptor integrin and gp49B are co-expressed on these cells. Confocal laser scanning microscopic analysis, however, revealed weak correlation of fluorescent signals between gp49B and integrin β1, suggesting their partial co-localization on the BMDC surface even in the absence of FN. We found that the plating of BMDCs onto immobilized FN induced tyrosine phosphorylation of focal adhesion kinase (FAK) and spleen tyrosine kinase (Syk). In the absence of gp49B, while the FAK phosphorylation level was virtually unchanged, that of phosphorylation of Syk was markedly augmented. These results suggested that the immobilized FN induced a crosstalk between gp49B and integrin in terms of the intracellular signaling of BMDCs, in which gp49B suppressed the integrin-mediated pro-inflammatory cascade. Our observations may provide a clue for elucidating the mechanism of the therapeutic efficacy of B4/gp49B blocking in autoimmune disease and cancer.
Blocking of immune checkpoints, namely inhibitory receptors and their cognate ligands, between T cells and antigen-presenting cells is a powerful tool for augmenting anti-tumor immunity (Korman et al. 2006). In the context of maintaining tolerance, the checkpoint receptor-ligand interaction assures immune homeostasis so as not to damage self tissues. Dendritic cells (DCs) are particularly important as professional antigen-presenting cells regulating T cell activity by determining the threshold for initiation of an immune response. Examples of such immune checkpoint ligands on DCs are PD-L1/L2 and CD80/86, namely, the ligands for inhibitory receptors PD-1 and CTLA-4 on T cells, respectively (Pardoll 2012). Importantly, DCs are not only the cells providing the ligands for inhibitory receptors expressed on T cells but also those receiving the stimuli from the ligands expressed on other cells and surrounding tissues or plasma, and delivering an inhibitory signal into the cytoplasm. Such inhibitory receptors on DCs include immunoglobulin (Ig)-like receptors such as inhibitory Fc receptor for IgG, type B leukocyte Ig-like receptors (LILRBs), and C-type lectin-like receptors such as DC inhibitory receptor DCIR. Some inhibitory lectin-like receptors bind to carbohydrates. However, the whole spectrum of ligands for individual receptors has not been clarified (Kanazawa 2007; Kerscher et al. 2013).
LILRB4 (B4; also known as ILT3/CD85k in humans and gp49B in mice) is one of the DC-regulating, inhibitory Ig-like receptors. B4/gp49B was initially identified as an inhibitory receptor expressed on mast cells (Castells et al. 1994; Katz et al. 1996), and later it was found to be expressed on myeloid-lineage cells, such as uterine macrophages (Matsumoto et al. 2001), neutrophils (Zhou et al. 2003), conventional DCs (Kasai et al. 2008) and B-lineage cells, including marginal zone B cells (Fukao et al. 2014) and plasmablasts/plasma cells (Inui et al. 2016; Wong et al. 2019). B4/gp49B is also expressed on natural killer cells (Wang et al. 1997) and activated T cells (Sharma et al. 2021). In animal models, it was shown that anti-B4/gp49B antibody administration could release B4-suppressed anti-tumor immunity, and thus B4 is a potential target for immunotherapy for solid tumors (Sharma et al. 2021; Paavola et al. 2021) and acute myeloid leukemia (Deng et al. 2018). Therefore, understanding of the modes of function of B4/gp49B and its ligand(s) in immunity is important to understand how this receptor-ligand interaction attenuates T cells and other cells.
The ligand for B4/gp49B was initially reported to be integrin αvβ3 in mice (Castells et al. 2001), and recently to be activated lymphocyte cell adhesion molecule (ALCAM or CD166), an activation marker of T cells (Xu et al. 2018) and apolipoprotein E in humans (Deng et al. 2018). Importantly, the reported human B4 ligands seemed not to be shared by murine gp49B (Xu et al. 2018; Deng et al. 2018). We, however, recently found that human B4 and murine gp49B bind to fibronectin (FN) (Su et al. 2021). Furthermore, we discovered that the N-terminal 30 kDa domain of FN (FN30) contains the major binding site, and found that a 20-amino-acid stretch in FN type I repeat 2 within FN30 is the main target sequence. Blockade of FN30 binding to gp49B in vivo was effective in reducing IgG anti-double stranded DNA autoantibodies and in ameliorating glomerulonephritis in BXSB/Yaa lupus model mice, suggesting a role of B4/gp49B-FN in autoimmune pathogenicity (Su et al. 2021) in addition to its pathogenic role in tumors. These findings suggested that FN is an intrinsic pathophysiologic ligand common to both human and mouse B4/gp49B (Su et al. 2021). More recently, others also reported FN was a ligand for human B4 (Paavola et al. 2021).
Generally, the receptors for FN are known as a group of classical FN-binding integrins, such as α5β1, αvβ1 and αvβ3, some of which are expressed ubiquitously on cells including DCs, and αIIbβ3 expressed on platelets (Plow et al. 2000). Upon activation, these FN-binding integrins induce phosphorylation of focal adhesion kinase (FAK) by Src family kinase, which leads to canonical integrin signaling intracellularly connecting to the association with actin filaments and cell activation and dynamics such as adhesion and migration. In addition, integrin activation is known to accompany with induction of proinflammatory signaling via phosphorylation of spleen tyrosine kinase (Syk) also by Src family kinase. In general, tyrosine phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) by Src-family kinase recruits Syk and induces the Syk tyrosine phosphorylation that leads to the downstream activation signal (Coxon et al. 2017). Interestingly, it is suggested that Syk associates ITAM-independently with the cytoplasmic portion of integrin β chain (Mocsai et al. 2010), and Syk is now recognized as an essential component of integrin signaling in inflammation (Lin et al. 1995; Abram and Lowell 2007; Jakus et al. 2007; Mocsai et al. 2010; Antenucci et al. 2018). Thus, integrins can provoke both canonical FAK-mediated signaling and Syk-mediated proinflammatory signaling simultaneously. These integrins bind to the RGDS sequence (RGD motif) located in FN type III repeat 10, which is in the middle of a 220-250 kDa FN molecule (Plow et al. 2000; Pankov and Yamada 2002; Barczyk et al. 2010). On the other hand, the B4/gp49B ligand, FN30 is in the N terminal, thus being apart from the RGD motif.
Given that FN is the common ligand for both inhibitory receptor B4/gp49B and cell activating-type receptor integrins, we were interested in elucidating the relation between B4/gp49B- and integrin-mediated regulation in DCs. Our initial hypothesis was that a B4/gp49B-FN-integrin trimeric complex is a regulatory unit for DC activation (Fig. 1). At least for macrophages and macrophage cell line RAW264.7 cells, we in fact obtained a set of data suggesting the formation of such a trimeric complex (Itoi et al. 2022). Based on our hypothetical view, we analyzed FN and integrin expression profiles on bone marrow-derived cultured DCs (BMDCs), identifying several FN receptor integrins on the cells. However, FN was found to be absent on BMDCs on flow cytometry. In this study, we examined if FN-negative BMDCs could bind exogenous FN molecules through both B4/gp49B and integrins and if such FN-mediated simultaneous stimulation of integrin and gp49B could induce a signal cross-talk intracellularly. Our hypothetical idea of the association of FN, B4/gp49B, and integrin was supported also in BMDCs at least in our experimental settings. Moreover, such a trimeric complex was suggested as a regulatory unit for integrin-mediated pro-inflammatory signaling by Syk but not FAK-induced signaling in BMDCs, as described below.

Hypothetical structure of the B4/gp49B-FN-integrin trimeric complex.
Our hypothetical view of the trimeric complex on bone marrow-derived cultured dendritic cells (BMDCs) is shown. Fibronectin (FN) binding induces integrin activation leading to actin reorganization and a pro-inflammatory signal. Crosslinking of B4/gp49B via FN is supposed to induce an inhibitory signal. Spleen tyrosine kinase (Syk) is proposed to associate with the cytoplasmic portion of the integrin β chain and activated particularly after integrin aggregation (Antenucci et al. 2018), but this is not illustrated here.
FN30, fibronectin N-terminal 30-kDa domain; FNIII, fibronectin type III repeat; RGD, Arg-Gly-Asp, a major amino acid sequence for integrin binding; 2nd FN, double disulfide bonding to the 2nd fibronectin; ITIM, immunoreceptor tyrosine-based inhibitory motif; SFK, Src family kinase; FAK, focal adhesion kinase; circled P, phosphotyrosine(s).
FN from human plasma was purchased from Sigma-Aldrich (St. Louis, MO, USA). We used the following non-labeled or labeled antibodies: AlexaFluor546-conjugated anti-gp49 (#53584AF546) was from Santa Cruz Biotechnology (Dallas, TX, USA); Pacific Blue (PB)-labeled anti-mouse CD3ε (clone 145-2C11), PB-labeled anti-mouse CD19 (clone 6D5); FITC-labeled anti-mouse CD11c (clone N418), Allophycocyanin (APC)-labeled (clone 117310) or Peridinin-Chlorophyll-Protein Complex (PerCP)/Cyanin5.5-labeled anti-mouse I-A/I-E (M5/114.15.2) were from BioLegend (San Diego, CA, USA); Alexa 647-anti-mouse integrin β1 (MB1.2, Sigma-Aldrich); alexa647-anti-mouse integrin β3 (2C9.G2, BioLegend); PE-labeled anti-mouse integrin αv (RMV-7, BioLegend); PE-labeled anti-mouse integrin α5 (HMa5-1, BioLegend); Alexa488-labeled anti-FN30 made in house; non-labeled anti-FN module III (clone 14, Thermo Fisher Scientific, Waltham, MA, USA); Alexa488-labeled anti-rabbit IgG; anti-mouse H2-Kb/Db (28-8-6, BioLegend) and anti-mouse β2 microglobulin (S19.8, BD Bioscience, Franklin Lakes, NJ, USA) were labeled with Alexa Fluor 546 and 647 monoclonal antibody labeling kit (Invitrogen, Waltham, MA, USA), respectively. The following antibodies were from Cell Signaling (Danvers, MA, USA): ani-phosphorylated FAK (#3283), anti-FAK (#3285), anti-phosphorylated Syk (#2710), anti-Syk (#2712). Anti-FN30 mAbs (#5) were established in our laboratory. H1.1 (anti-gp49 IgG mAb, derived from Armenian hamsters) hybridoma cells were kindly provided by Dr. Wayne M. Yokoyama (Wang et al. 2000).
MiceC57BL/6 mice were purchased from CLEA Japan Inc. (Meguro, Tokyo, Japan). gp49B-deficient C57BL/6 mice were prepared as described previously (Kasai et al. 2008; Su et al. 2021). All mice were maintained and bred in the animal facility of the Institute of Development, Aging and Cancer, Tohoku University, under specific pathogen-free conditions, and used for experiments at 8-9-weeks old. All animal protocols were reviewed and approved by the Animal Studies Committee of Tohoku University.
Isolation and in vitro culture of cellsSingle cell suspensions were prepared from spleens and bone marrow after red blood cell depletion by ammonium chloride lysis as follows: splenocytes and bone marrow cells were prepared from mouse spleen samples and femur and tibial bones, respectively, and red blood cells were lysed with 155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM ethylenediaminetetraacetic acid (EDTA) solution. For preparing bone marrow-derived cultured dendritic cells (BMDCs), bone marrow cells were cultured in RPMI1640 (Sigma-Aldrich) containing heat-inactivated fetal bovine serum (FBS, Biowest, Nuaille, France), 1 mM sodium pyruvate, 50 µM 2-mercaptoethanol, 100 U/ml penicillin (Sigma-Aldrich), 100 µg/ml streptomycin (Sigma-Aldrich), and 20 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ, USA) at 37˚C in a humidified atmosphere containing 5% CO2. Culture medium was replaced at day 4. On day 6, floating and less-adherent cells were recovered after swirling the culture bottle and used for further experiments as BMDCs.
Flow cytometry and cell sortingCells were stained for 1 h on ice in phosphate-buffered saline (PBS) containing 2% FBS with fluorochrome-labeled antibodies. Prior to this, all samples were treated with non-conjugated anti-CD16/CD32 (clone 2.4G2) for 30 min to prevent non-specific antibody binding due to the Fcγ receptors. Samples were analyzed and sorted using FACSAria III (BD Biosciences). Data were collected using FACS Diva software and analyzed using FlowJo software (Tree Star).
Confocal laser-scanning microscopic analysisDistribution of surface proteins gp49 and integrin β1 was assessed by confocal microscopy. Cells were plated onto glass-bottom dishes [Matsunami; poly-lysine coated, dish diameter 35 mm, glass diameter 14 mmφ, glass thickness No.1S/1.5 (0.16-0.19 mm), and #D11131H]. Cells were fixed with 2% paraformaldehyde (PFA) for 1 h at room temperature. Unbound antibodies were removed by washing the cells three times with a washing solution [1% bovine serum albumin (BSA) in PBS]. The fixed cells were then stained with the antibodies of interest, i.e., AlexaFluor546-conjugated anti-gp49 (Santa Cruz #53584AF546), AlexaFluor647-conjugated anti-integrin β1 (Thermo Fisher Scientific MAB1997-AF647). After thorough washing, the cells were mounted in a medium comprising 35 g glycerol in 100 ml PBS and then examined at 2-D under Nikon A1 confocal microscope systems.
Evaluation of Pearson’s correlation coefficients between fluorescent signals of cell-surface moleculesCorrelation coefficient r between two sets of observed parameters, namely fluorescent signals of integrin and gp49 in this study, was evaluated as described for Pearson’s correlation analysis (Adler and Parmryd 2010). Briefly, each signal profile of a cell contour was analyzed with software with Nikon A1, the r value being calculated. The r values were obtained for over 5 cells randomly selected in the captured images and illustrated as a graph. Mean r values were interpreted according to the following criteria: r = −1, perfect negative linear relationship; −1 < r ≤ −0.70, strong negative linear relationship; −0.7 < r ≤ −0.4, negative relationship; −0.4 < r ≤ −0.2, weak negative linear relationship; −0.2 < r ≤ +0.20, no significant relationship; +0.2 < r ≤ +0.4, weak positive relationship; +0.4 < r ≤ +0.7, positive linear relationship; +0.7 < r < +1, strong positive linear relationship; and r = +1, perfect positive linear relationship. We considered r > +0.2 as a positive correlation.
Stimulation of BMDCs with fibronectin in vitro and western blottingFor preparation of immobilized FN, human FN solution was diluted to 10 µg/ml with PBS and added to a 96-well culture plate, and incubated for 2 h at room temperature, and the plate was washed twice with PBS before use. BMDCs were plated into the wells at 2.0 × 105 cells/well and incubated at 37˚C for different periods and recovered. Cells were lysed with RIPA lysis buffer [1% NP-40, 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate and proteinase inhibitors] containing Phospho Stop (Sigma-Aldrich), and the lysates were measured for their content of proteins by BCA method and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels (Wako Pure Chemicals, Osaka, Japan) under reducing conditions, transferred to PVDF membranes, and then immunoblotted in Can Get Signal Solution (TOYOBO, Osaka, Japan) and with different horseradish peroxidase (HRP)-labeled antibodies in Can Get Signal Solution 2. Detection of fluorescence was performed with Amersham ECL Prime (Cytiva, Marlborough, MA, USA) and ImageQuant LAS-4000 (Cytiva).
Statistical analysisStatistical analyses were carried out using GraphPad Prism® 6 (Version 6.0; GraphPad Software, San Diego, CA, USA) or R software (ver. 4.1.0, developed at Bell Laboratories, formerly AT&T, now Lucent Technologies). Data are presented as mean ± standard deviation (SD).
Our initial approach was to clarify the expression profiles of the subunits of major FN-binding integrins, namely α5, αv, β1 and β3, and FN and gp49B on the BMDC surface. We isolated bone marrow cells and cultured them in the presence of GM-CSF for 6 days to induce BMDCs. On day 6, the floating and less-adherent cells were collected by pipetting, sorted into lymphocytes and singlets, and then sort-purified to yield a population mainly comprising MHC class II-high CD11c+ cells and a lesser MHC class II-low cell population (Fig. 2A, B). We tentatively term this population as the class II-high population in this study. In parallel, analysis of non-floating adhered cells on day 6 revealed that the majority of them were MHC class II-low immature-type BMDCs compared to MHC class II-high, floating/less-adherent BMDCs (Fig. 2B). In this study, we tentatively denoted this population as the class II-low one. Microscopic observation showed cells rich in dendrites for the floating/less-adherent cell population, a typical feature of relatively matured BMDCs compared to that of the adherent, immature-type BMDCs (Fig. 2C). We also isolated primary DCs from splenocytes by sorting CD3–CD19–CD11chighMHC class IIhigh cells (Fig. 2D).
Flow cytometric analysis of BMDCs for gp49, integrin subunits, and FN30 and FN type III repeat 7-9 (FNIII7–9) (Fig. 1) expression revealed that MHC class II-high BMDCs were positive for surface gp49, integrins α5, αv, and β1 but negative for integrin β3, FN30, and FNIII (Fig. 3A-C). Although gp49 expression was not always robust, gp49B but not gp49A isoform expression was dominant using BMDCs prepared from gp49B-deficient mice (Fig. 3B). We concluded at this stage that gp49B and FN-binding integrins α5β1 and αvβ1 were expressed on our MHC class II-high BMDC preparation, but FN was not constitutively tethered to the BMDCs. Likewise, expression of the subunits of integrins α5, αv, β1 and β3 was found on our MHC class II-low BMDC preparation: the β1 expression was low, whereas β3 was weakly positive in contrast to on class II-high BMDCs. gp49B expression was evident on class II-low BMDCs like class II-high cells. The class II-low BMDCs were also negative for FN30 and FNIII1-7, indicating that the cells do not have tethered FN on their surface like the class II-high cells. Regarding splenic CD11c+ DCs, while integrin subunits αv, β1, and β3 were positive, we found that gp49, FN30, and FNIII expression were negative.

Gating strategies for bone marrow-derived cultured dendritic cells (BMDCs) and splenic CD11c+ dendritic cells (DCs) on flow cytometric analysis.
(A) Gating of CD11c+MHC-IIhigh BMDCs for flow cytometric analysis of the cell-surface expression of gp49, integrins, and FN. (B) CD11c+MHC-IIhigh BMDCs obtained by gating in (A) were further isolated as to their floating or less-adherent nature and denoted as CD11c+MHC-IIhigh BMDCs (left) for subsequent analysis. Remaining, adherent CD11c+MHC-IIhigh BMDCs obtained by gating in (A) were also detached and are denoted as CD11c+MHC-IIlow BMDCs (right). (C) Appearance of floating/less-adherent BMDCs on a glass coverslip (× 400 at the maximum magnification; left) and adherent BMDCs on a plastic dish (× 200 at the maximum magnification; right) under a light microscope. The arrow indicates a typical CD11c+MHC- IIhigh BMDC with many dendrites. (D) Gating strategies for splenic CD3–CD19–CD11c+MHC-IIhigh DCs on flow cytometric analysis of the cell-surface expression of gp49, integrins, and FN.
FSC, forward scatter; SSC, side scatter; A, area; H, height; PE, phycoerythrin. PE profile shows the basal level of further analysis.

Cell-surface expression profiles of gp49B, integrin α and β chains, and fibronectin (FN) on bone marrow-derived dendritic cells (BMDCs) and splenic dendritic cells (DCs).
(A) Murine BMDCs and splenic dendritic cells (DCs) were analyzed for their cell-surface expression of gp49, integrin α and β chains, FN N-terminal 30 kDa domain (FN30), and FN module III (FNIII) by flow cytometry. (B) gp49B but not the gp49A isoform is expressed on CD11c+MHC-IIhigh (left) and CD11c+MHC-IIlow cells (right). BMDCs prepared from wild-type and gp49B-knockout mice were analyzed for their cell-surface expression of gp49A/B by flow cytometry in order to determine the expression levels of gp49B. (C) Murine peritoneal macrophages were presented as a positive control and BMDCs were analyzed for their cell-surface expression of FN30 and FNIII by flow cytometry to confirm FN30/FNIII-negative nature of BMDCs.
Next, we examined the fluorescence signals of gp49B and integrin β1 on the surface of BMDCs by confocal microscopy. Firstly, we analyzed the signal correlation between MHC class I α chain and β2 microglobulin (β2m) as a positive control (Fig. 4A, upper), and found that it was reasonably high, which was judged as a positive linear relationship (Pearson’s correlation r = 0.594 ± 0.179, mean ± SD, n = 6) (Fig. 4B). Next, we tested the signals of gp49B and integrin β1. The fluorescent signal profiles were grossly ring-like for both gp49 and integrin β1 like MHC class I α and β2m, albeit their intensities were much lower (Fig. 4A, lower). The observed signals on cell contour lines were analyzed for correlation among the two fluorochromes. We observed a weak relationship for the Pearson’s correlation for gp49 and integrin β1, albeit it did not reach to a significant level set in advance (r = 0.194 ± 0.071, mean ± SD, n = 6) (Fig. 4B). It was unexpected that we observed a notable relation between integrin and gp49B even in the absence of FN in this experimental setting. This might indicate that gp49B and integrin molecules in part are close vicinity on the cell membrane. When FN could co-crosslink between gp49B and integrin such as by the cell adherence to FN-coated dish, the relation of gp49B and integrin would be more intimate. Collectively, these results supported a spatially close relationship, at least partly, between gp49B and integrin β1 on the surface of BMDCs even in the absence of their shared ligand, FN.
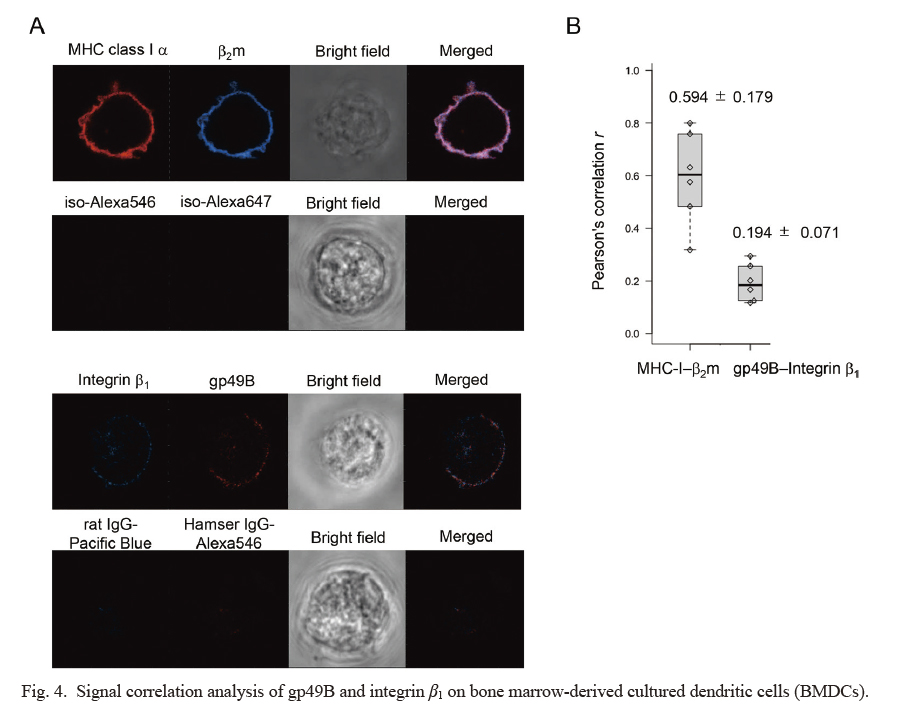
Signal correlation analysis of gp49B and integrin β1 on bone marrow-derived cultured dendritic cells (BMDCs).
(A) Top, positive control of correlation analysis. Cell-surface expression of MHC class I α chain and β2 microglobulin (β2m) on BMDCs is analyzed. Original purple fluorescence signal of β2m is converted to blue in this picture for discrimination from red fluorescence of MHC class I α. Second row, mouse IgG-Alexa546 and IgG-Alexa647 are similarly analyzed as negative controls. Third row, cell-surface gp49B and integrin β1 expression on BMDCs are analyzed by confocal laser-scanning microscopy. Bottom, rat IgG-Pacific Blue and anti-hamster IgG-Alexa546 are similarly analyzed as negative controls. Representative data of two independent experiments with similar results. (B) Signal correlation between MHC class I α chain and β2 microglobulin or gp49B and integrin β1 is analyzed for Pearson’s correlation. Correlation values are denoted as means ± SD (n = 6). In the box plot, the upper and lower whiskers denote the maximum and minimum values of the data set, respectively. Box indicates the area of mid-four values with a horizontal line of the mean of the mid two points.
We next examined if FAK phosphorylation would be induced by FN biding to integrins in BMDCs as an immediate downstream signal of ligand-activated integrin. We plated BMDCs onto an FN-coated culture plate, incubated them and prepared a cell lysate for western blot analysis. Such BMDC culture induced a substantial tyrosine phosphorylation of FAK in wild-type BMDCs (Fig. 5A). Interestingly, gp49B-deficient BMDCs also exhibited substantial FAK phosphorylation comparable to that of wild-type BMDCs (Fig. 5A), suggesting that FAK is not the direct target for potential gp49B-mediated regulation.
We next examined whether Syk phosphorylation was modulated by gp49B in BMDCs upon plating onto immobilized FN. Interestingly, while the phospho-Syk level increased upon FN-mediated stimulation of wild-type BMDCs, the level was further augmented in gp49B-deficient BMDCs (Fig. 5B), suggesting gp49B-mediated direct or indirect suppression of Syk phosphorylation in wild-type BMDCs. Overall, these results indicate that, while FN in an immobilized form induced immediate downstream FAK activation, the canonical FN-integrin-mediated signal was not affected by the presence or absence of gp49B. Instead, the FN-integrin-mediated pro-inflammatory activation of Syk was suppressed by gp49B, whose immunoreceptor tyrosine-based inhibitory motif (ITIM) could be phosphorylated by integrin-mediated Src family kinase activation in BMDCs as shown in Fig. 1.
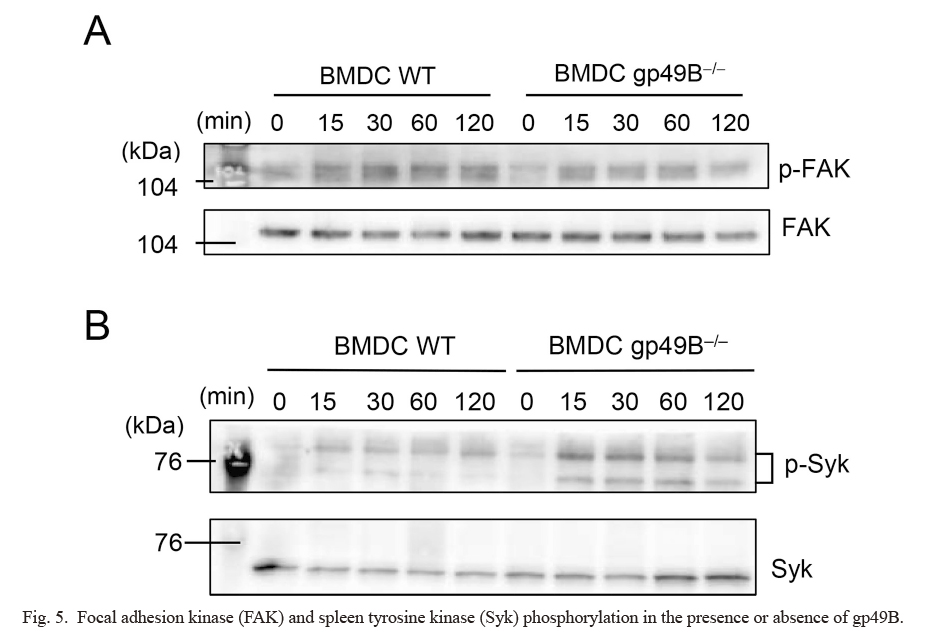
Focal adhesion kinase (FAK) and spleen tyrosine kinase (Syk) phosphorylation in the presence or absence of gp49B.
(A) Western blot analysis of tyrosine phosphorylated FAK after stimulation with plate-bound immobilized FN of BMDCs prepared from wild-type or gp49B-deficient mice. Phosphorylated FAK (upper) and total FAK (lower) are shown. Representative data from three independent experiments with similar results. (B) Western blot analysis of tyrosine phosphorylated Syk after stimulation with immobilized FN of wild-type or gp49B-deficient BMDCs. Phosphotyrosylated Syk (upper) and total Syk (lower) are shown. Representative data are shown for two independent experiments with similar results.
In this study, flow cytometric, confocal laser-scanning microscopic, and biochemical analyses of gp49B and integrin revealed that gp49B and integrin are substantially co-localized even in the absence of their shared ligand, FN, in BMDCs. Tentative formation of gp49B-FN-integrin trimeric complex was suggested upon cell plating onto immobilized FN. We found that a gp49B deficiency led to upregulated tyrosine phosphorylation of Syk, an immediate downstream signaling event coupled to FN-integrin activation, suggesting a novel regulatory role of gp49B as to the pro-inflammatory signal of integrin.
Biological analyses, including cell-protein and cell-cell binding assays, showed the ligand for gp49B was integrin αvβ3 (Castells et al. 2001). Also, interaction of αvβ3 with gp49B on bone marrow-derived mouse mast cells (BMMCs) was reported to inhibit antigen-induced immunoglobulin E-mediated cell activation. In biological assays, αvβ3-positive BY155.16 cells bound to gp49B-Fc protein in the presence of 0.1 mM Mn2+, whose binding was inhibited by antibodies either to αv or β3 or by a cyclic RGD peptide. In addition, gp49B and αvβ3 interacted in a cell-cell binding assay system, in which gp49B-positive BMMCs adhered to αvβ3-expressing BY155.16 cells in the presence of 0.1 mM Mn2+. The specificity of the adherence was confirmed by blocking with soluble gp49B-Fc and antibodies to αv and β3. In addition, a binding-inhibition assay with purified proteins showed that gp49B competed with vitronectin for binding to αvβ3. In contrast to these observations, we previously reported that the ligand for murine gp49B as well as human B4 is FN, and more specifically its N-terminal 30-kDa domain, FN30 (Su et al. 2021). We do not exclude the possibility that gp49B also shows affinity to integrin αvβ3. However, the observed binding between integrin αvβ3 and gp49B in the presence of 0.1 mM Mn2+ may include indirect bridging of integrin αvβ3 to gp49B via FN potentially derived from serum in the culture, namely the Mn2+-dependent formation of a trimeric complex. Meanwhile, our observation regarding a gp49B-FN-integrin triplet complex suggests that mast cells, macrophages, NK cells, and other gp49B- and integrin-positive cells could have a regulatory system in which gp49B suppresses the FN-integrin-mediated pro-inflammatory signal but not the canonical integrin signal, like BMDCs do, as shown in this study. From the viewpoint of clinical application, FN-integrin signaling is considered to be important as to tumor metastasis.
In general, integrins expressed on tumor cells contribute to tumor progression and metastasis by increasing tumor cell migration, invasion, proliferation, and survival. Integrin adhesion to the extracellular matrix provides the traction required for tumor cell invasion (Desgrosellier and Cheresh 2010). Integrin-mediated migration generally requires FAK and Src family kinase signaling. Clinical trials of anti-cancer antibodies are on-going, with FN-integrin α5β1 binding as the target (Desgrosellier and Cheresh 2010). FN is critical also for metastasis and immune evasion of tumors (Deng et al. 2018). The significance of integrin and B4 in tumors has also been documented (Colovai et al. 2007; de Goeje et al. 2015; Kumra and Reinhardt 2016; Sharma et al. 2021; Singh et al. 2021). Thus, the B4/gp49B-mediated suppression of FN-integrin-mediated proinflammatory activation shown in this study would be a novel therapeutic target for tumor progression and metastasis.
In conclusion, our current study indicates that our hypothetical view of a B4/gp49B-integrin signal crosstalk (Fig. 1) is taking place in BMDCs, as shown in this study and macrophages (Itoi et al. 2022). Downstream signaling by integrins is initiated by FAK recruitment and tyrosine phosphorylation by Src-family kinases, followed by actin filament reorganization. The activation signal also evokes the Syk-mediated pro-inflammatory signal. In this study, we found that Syk but not FAK is a target of potential B4/gp49B-mediated regulation. B4/gp49B could be a counter-regulator of the pro-inflammatory signal but not the canonical integrin-mediated signaling.
We thank W. M. Yokoyama (Washington University School of Medicine, St. Louis) for providing the hybridoma, and N. Halewood for the editorial assistance. Funding was provided by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (19H03484 to TT).
The authors declare no conflict of interest.