2022 Volume 257 Issue 3 Pages 181-191
2022 Volume 257 Issue 3 Pages 181-191
Tumor necrosis factor-α-induced protein-8 like-2 (TIPE2) as a novel negative immune regulator plays an important role in several human diseases. However, its influences in cervical cancer and preeclampsia (PE) remain unclear. This study aims to explore the important role of TIPE2 in cervical cancer and PE via regulating cell invasion. TIPE2 expression in the cervical cancer tissues or the placenta of PE patients was detected. Human cervical cancer cell lines and trophoblasts were transfected with adenovirus expressing human TIPE2 and green fluorescent protein (GFP) (Ad-TIPE2), or the control adenovirus expressing GFP (Ad-GFP). Xenograft models were also constructed on nude mice, aiming to clarify how TIPE2 affects in vivo growth of cervical cancer cells. TIPE2 was down-regulated in the tumor tissues or placenta of patients with cervical cancer or PE. As a result, CaSKi and Hela cells in the Ad-TIPE2 group had decreased migration and invasion, with significant up-regulations of TIPE2 and E-cadherin, but down-regulations of β-catenin and N-cadherin. Ad-TIPE2 decreased the volume and weight of xenograft tumors in the nude mice, with the down-regulation of Ki67. The quantity of cells (HTR8/SVneo and JEG3 cells) transfected with Ad-TIPE2 had increased, with up-regulations of TIPE2, matrix metalloproteinase (MMP)-2 and MMP-9. TIPE2 overexpression could reduce the invasion and migration of cervical cancer cells via inhibiting the epithelial-mesenchymal transition (EMT) process, and promote trophocyte invasion via upregulating the expression of MMPs, and it may be used as a potential therapeutic target for cervical cancer and PE.
Cervical cancer is a well-known frequent gynecological malignant tumor, only secondary to the breast cancer worldwide (Lertkhachonsuk et al. 2013). In recent years, cervical cancer has witnessed gradual increases in the prevalence and mortality rate, tremendously threatening the physical and mental health of women (Ma et al. 2020). Tumor metastasis is credited as most important process that affects the tumor deterioration of patients or even induces the majority of cancer-related death, thus naturally, overcoming metastasis has become the top priority of tumor therapy (Hisamatsu et al. 2012). Generally, the main characteristic of cervical cancer is local spread, and in some cases, patients may have a poorer prognosis due to the early invasion and metastasis, with a 5-year-survival rate of 30% to 50% (Wu et al. 2018). Hence, to search for the mechanism of invasion and metastasis is of great significance for improving the survival and quality of patients with cervical cancer.
Preeclampsia (PE) is not only an idiopathic, systemic disease but also a multi-systemic damage during pregnancy, classically manifested as new-onset hypertension and proteinuria (Murphy et al. 2015). Currently, oxidative stress, abnormal trophoblast invasion, immune responses alterations and anti-angiogenesis are associated with the onset of PE, although the pathogen and pathogenesis of PE are not exactly clear (Sircar et al. 2015; El-Sayed 2017). Especially, the inadequate invasion of trophoblasts, leading to the superficial implantation of placenta and dysfunction in the remodeling of spinal artery of uterus, plays a key role in the PE development (Chaiworapongsa et al. 2014; Wu et al. 2015). Trophoblasts, similar to the highly invasive cancer cells, can sustain the physiological function of placenta by expressing the similar genes to modulate the invasive ability of cells (Hansson et al. 2014). Thus, to investigate the biological molecular characteristics of trophoblasts and their invasion ability might be helpful for uncovering the possible PE mechanism.
Tumor necrosis factor-α-induced protein 8-like 2 (TIPE2), a member of tumor necrosis factor α-inducible protein 8 (TNFAIP8) family, is a new regulator in immune system playing pivotal roles in sustaining the immune balance and regulating the tumor progression (Zhang et al. 2011; Li et al. 2014a). According to the published literature, TIPE2 was discovered to implicate in the development of various types of tumors, such as human non-small cell lung cancer (Li et al. 2015), prostatic cancer (Lu et al. 2016) and osteosarcoma (Deng et al. 2015). Additionally, Zhang et al. (2016) reported that TIPE2 could inhibit the growth and metastasis of breast cancer cells via suppressing the activity of AKT and p38 signaling pathway. Also, TIPE2, as per the work of Liu et al. (2020), could inhibit the epithelial-mesenchymal transition (EMT) process by blocking the nuclear translocation of β-catenin, thereby suppressing the malignant features of endometrial cells. Of note, TIPE2 can suppress inflammation by switching arginine metabolism from nitric oxide synthase to arginase (Lou et al. 2014). Lim and Lappas (2019) demonstrated that in the mesometrium, TIPE2 was down-regulated in the advanced stage of pregnancy, which would be further decreased during the delivery, and at the same time, the loss of TIPE2 in the primary mesometrium and amnioblast deteriorated the inflammatory responses. Nevertheless, there remain no evidence suggesting the role of TIPE2 in the cervical cancer and trophoblasts.
Thus, in this study, we explored the mechanism of TIPE2-mediated cell invasion in the development of cervical cancer and PE, aiming to provide novel insights and effective targets for the treatments of cervical cancer and PE.
This study had been approved by the ethical committee of our hospital, and all patients signed the written informed consents prior to the study. This study complied to the Guide for the Care and Use of Laboratory Animals (Carbone 2012), and all animal-related experiments had been approved by the Ethical Board for the Laboratory Animals of our hospital.
SubjectsA total of 55 patients, aged at an average of 47.6 ± 9.3 years old [mean ± standard deviation (SD)] with cervical cancer, who underwent the surgical resection for the treatment of cervical cancer between January 2020 and December 2020 in this hospital, were subjected to the collection of specimens, including the resected cervical cancer tissues and the corresponding tumor-adjacent normal tissues. All patients were confirmed by the pathological test and they claimed no history of chemotherapy, radiotherapy or biotherapy or other kinds of adjuvant therapy before surgery. Besides, a total of 35 PE patients who chose the Caesarean section in the Department of Gynecology and Obstetrics at our hospital were also selected at the same period, with an average age of 29.3 ± 4.2 years old, and 40 pregnant women who had normal delivery in this hospital were selected as the Normal group, with an average age of 28.9 ± 3.9 years old. All subjects had single birth, with no history of hypertension, diabetes mellitus, renal diseases, endocrine disorders, abnormal pregnancy or delivery, smoking or alcohol addiction, or medication, and during the pregnancy, no subjects had the infectious diseases. The clinical characteristics of PE patients were presented in Table 1. No significant differences in the maternal age and antepartum body mass index (BMI) were observed between the PE group and Normal group (all P > 0.05). In the PE group, the gestation age at delivery was earlier, and the birth weights were less than those in the Normal group (all P < 0.05). Systolic and diastolic blood pressures were significantly higher in the PE group (all P < 0.05). Fresh, septic placental tissues were collected, from which tissue blocks were collected from the different sites, including the fetal and maternal surface of placenta, and placed at −80°C for later use after the blood removed by washes in the normal saline.
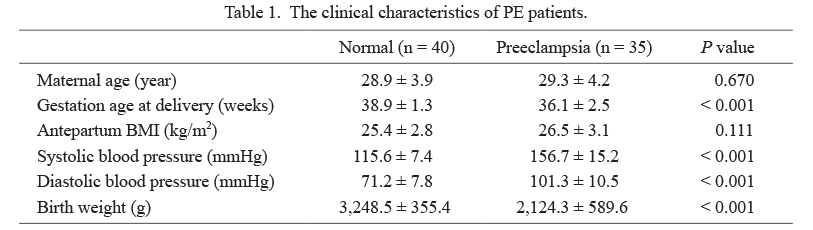
The clinical characteristics of PE patients.
PE, preeclampsia; BMI, body mass index.
Collected cervical cancer tissues and tumor-adjacent normal tissues, after being fixed, dehydrated, cleared and embedded into the paraffin, were sliced into sections with the thickness of 4 μm. Prior to the immunohistochemistry, sections were placed at 60°C for 3 h, de-paraffinized and hydrated. Then, they were heated in the sodium citrate buffer at 94°C for antigen retrieval and incubated in 3% H2O2 for 15 min. Next, sections were incubated with working solution of goat serum for 15 min at room temperature, with the primary anti-TIPE2 antibody (1:100, PA5-38711; Thermo Fisher Scientific, Carlsbad, CA, USA) at 4°C overnight, followed by washes in phosphate-buffered saline (PBS), and with the working solution of horseradish peroxidase (HRP)-conjugated secondary antibodies (1:1,000, ab6728, Abcam, Cambridge, UK) at 37°C for 15 min. Residual working solution of secondary antibody was removed by washes in PBS. Sections were then incubated with the horseradish peroxidase-labeled streptomycin-avidin working solution at 37°C for 15 min and then mounted after the color development by using the diaminobenzidine (DAB) kit (Gene Tech, Shanghai, China). TIPE2 expression levels were evaluated according to the staining intensity and the positive staining area (Liu et al. 2020). The staining intensity was divided into four grades: − (score 0), + (score 1), ++ (score 2) and +++ (score 3). The positive staining area was also classified into four categories (given as a percentage of the whole section): − (< 1%, score 0), + (1-33%, score 1), ++ (34%-66%, score 2), +++ (67-100%, score 3). The sum of staining intensity and positive staining area scores was used as the final TIPE2 staining score (no expression, total score 0; weak expression, total score 1 and 2; moderate expression, total score 3 and 4; strong expression, total score 5 and 6).
Cell cultureIn this part, normal human cervical epithelial cells (HCerEpiC) and human cervical cancer cell lines (including Hela, BT-B, C33A, CaSKi and SiHa) were all provided by the Shanghai Cellbank of Chinese Academy of Sciences (CAS) (Shanghai, China), while human villous trophoblasts (HTR8/SVneo and JEG3) were provided by American Type Culture Collection (ATCC, Manassas, VA, USA). For cell culture, RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Rockville, MD, USA) was used, while in the environment, temperature and CO2 were set at 37°C and 5%, with saturated humidity. When cell confluence reached about 80% to 90%, cells were digested in 0.25% trypsin and passed. Cells in the logarithmic phase were chosen for following experiments.
Cell grouping and transfectionHuman cervical cancer cells (CaSKi and Hela) and trophoblasts (HTR8/SVneo and JEG3) were grouped into the Mock group (Cells were not transfected), Ad-GFP group (Cells were transfected by the adenovirus of Ad-GFP) and Ad-TIPE2 group (Cells were transfected by recombinant adenovirus of Ad-TIPE2). The recombinant adenovirus of Ad-TIPE2 (containing the genes encoding the human TIPE2 and green fluorescent protein) and control adenovirus of Ad-GFP (only containing the gene encoding the green fluorescent protein) were constructed as previously described (Zhu et al. 2016). Cells were seeded on a 96-well plate, and, after incubation overnight, medium was refreshed. Then, adenovirus was added into the complete medium to transfect the cells as per multiplicity of infection = 10, and cells were harvested for later use after 48 h.
Transwell assayMatrigel in volume of 100 μL was coated on the surface of the upper chamber of Transwell (Costar, Cambridge, MA, USA) and placed at 37°C with 5% CO2 overnight. Cells in logarithmic phase were rinsed in PBS and digested in trypsin, followed by centrifugation at 1,000 rpm for 5 min and cell counting. Cells (2 × 104) were placed in the upper chambers of Transwell, and in the lower chambers, 500 μL of complete medium supplemented with 0.05% PBS was added, with caution to prevent the formation of bubbles in the medium of lower chambers and the interspace between the chamber bottom and membrane. Chambers were then placed at 37°C with 5% CO2 for cell culture for 24 h, and the medium was then discarded. Chambers were rinsed in PBS three times, and cells failing to pass through the membrane were removed by using a cotton swab. Cells in the lower chamber were stained in 0.1% crystal violet (Wako Chemicals USA Inc., Richmond, VA, USA) for 0.5 h at room temperature, rinsed in the running water, dried at room temperature and observed under the microscope. This experiment was repeated three times.
Wound healing testAfter cell density adjusted to 4 × 105 cells/mL, the cell suspension in a volume of 200 μL was seeded in a 6-well plate for culture until the confluence of monolayer cells. A clean, septic tip was used to draw a line vertical to the plate, ensuring the consistency of width. Then, with the medium being removed, cells were rinsed in the PBS three times to remove the debris and placed in the serum-free medium to be observed and photographed under the inverted microscope. Thereafter, cells were cultured for 48 h and, after two washes in PBS, and cells were observed and photographed by using the inverted microscope (OLYMPUS, Tokyo, Japan) to calculate the reunion rate of wounds. This part was repeated three times.
Western blottingThe total proteins were extracted from cells by using the RIPA Protein Lysis Buffer, and according to the instruction of bicinchoninic acid (BCA) kit (Thermo Fisher Scientific, Waltham, MA, USA), the protein concentration was determined. Then, proteins were subjected to the 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the Polyvinylidene Fluoride (PVDF) membrane (Millipore, Billerica, MA, USA) by 30 mA currency for 120 min. On the membrane, the unoccupied sites were blocked in non-fat milk for 1 h, and the proteins on the membrane were probed by being incubated with the primary antibodies (Abcam) including anti-TIPE2 (1:1,000, ab110389), β-catenin (1:1,000, ab68183), E-cadherin (1:10,000, ab40772), N-cadherin (1:5,000, ab76011), matrix metalloproteinase (MMP)-2 (1:1,000, ab92536), MMP-9 (1:1,000, ab76003) and β-actin (1:1,000, ab8226) at 4°C overnight, followed by 3 washes in tris-buffered saline with 0.1% Tween® 20 detergent (TBST) (15 min/wash). The resulting immunoblots were detected by incubation with the horseradish peroxidase-labeled secondary antibodies (1:2000, ab6728, Abcam) at room temperature for 1 h, followed by 3 washes in TBST. Final immunoblots were developed by being incubated with chemiluminescence (ECL) reagent (Amersham Biosciences, Little Chalfont, UK) and the bands were developed in a gel imager. The intensity of band was analyzed by using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA), and with the intensity of β-actin as loading control, the relative expression of target protein was expressed by the ratio of the intensity of target band to that of β-actin.
Construction of xenograft models on nude miceA total of 18 BALB/c mice aged at 4 weeks old and weighed 12-18 g were provided by Shanghai Laboratory Animal Center, CAS (Shanghai, China). Human cervical cancer cells, CaSKi, in the 4th to 6th generation in the logarithmic phase, were digested in trypsin and divided into the Mock group, Ad-GFP group and Ad-TIPE2 group and centrifuged at 1,000 r/min for 5 min, with the medium being discarded. Cells were then suspended in the PBS buffer and counted, and accordingly, cell density was adjusted to 2 × 106/mL. Single cell suspension in the volume of 200 μL was inoculated into the right-handed subcutaneous tissues on the back, with 6 mice in each group. Every four days, the length (L) and width (W) of tumor were measured and recorded to calculate the tumor volume by using the following formula: V = L × W2/2. The tumor growth curve was drawn. After nude mice fed for 21 days, they were decapitalized by cervical dislocation to harvest the complete tumor tissue to measure the weight of tumor. The expression of Ki67 in the xenograft tumor tissue was determined via the immunohistochemistry staining.
Statistical analysisAll data were subjected to the statistical analysis by using the SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). Measurement data were expressed by the form of mean ± SD and compared between either two groups by using t test, or among groups by using the one-way analysis of variance (One-way ANOVA), followed by the Tukey’s post hoc test for pairwise comparison. The relationship between TIPE2 expression and clinicopathological features was analyzed using a Mann-Whitney unpaired test. P < 0.05 suggested that the difference had statistical significance.
Immunohistochemistry staining was performed to detect the expression profiles of TIPE2 in the cervical cancer tissues, and the results indicated that the expression of TIPE2 in the cervical cancer tissues was much lower than that in the tumor-adjacent tissues (P < 0.05, Fig. 1A, B). The relationship of TIPE2 expression with clinicopathological characteristics of cervical cancer patients were examined (Table 2). The TIPE2 expression was closely associated with histological grades (P = 0.006), International Federation of Gynecology and Obstetrics (FIGO) stage (P = 0.024), lymph nodes metastasis (P = 0.017), and Human papillomavirus (HPV) infection (P < 0.001), but showed no significant associations regarding the age and histological type (all P > 0.05). Besides, western blotting was carried out to measure the protein expression of TIPE2 in different cervical cancer cell lines. As a result, cervical cancer cells (BT-B, Hela, SiHa, CaSKi and C33A) presented with the significant down-regulation of TIPE2 protein expression as compared to the normal cervical epithelial cells (HCerEpiC) (P < 0.05, Fig. 1C, D), while the lowest expression was observed in CaSKi and Hela cells. Thus, CaSKi and Hela cells were adopted for the subsequent experiments. Moreover, western blotting also showed that the protein expression of TIPE2 in the placenta of PE patients was down-regulated evidently (P < 0.05, Fig. 1E, F).

Expression of tumor necrosis factor-α-induced protein-8 like-2 (TIPE2) in the cervical cancer tissues and preeclampsia (PE) placenta tissues.
(A, B) The expression of TIPE2 in the cervical cancer tissues was much lower than that in the tumor-adjacent tissues detected by immunohistochemistry staining. (C, D) The cervical cancer cells (BT-B, Hela, SiHa, CaSKi and C33A) presented with the significant down-regulation of TIPE2 protein expression as compared to the normal cervical epithelial cells (HCerEpiC) detected by western blotting; *P < 0.05 vs. HCerEpiC cells. (E, F) The protein expression of TIPE2 in the placenta of PE patients detected by western blotting was down-regulated.
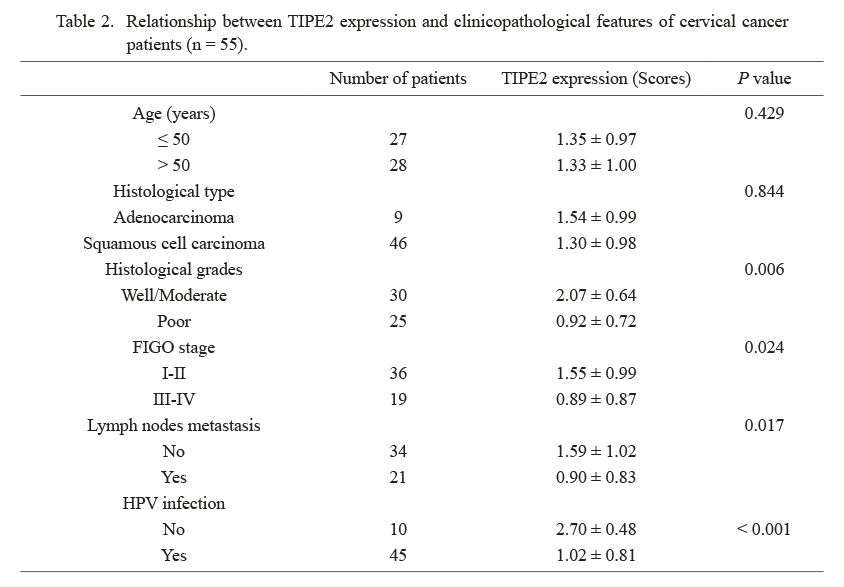
Relationship between TIPE2 expression and clinicopathological features of cervical cancer patients (n = 55).
FIGO, International Federation of Gynecology and Obstetrics; HPV, Human papillomavirus.
Wound healing test and Transwell assay were conducted to determine the invasion and migration abilities of cervical cancer cells (Fig. 2). By comparison with the Mock group, cervical cancer cells (CaSKi and Hela) in the Ad-TIPE2 group presented sharp decreases in the cell quantity of invasion (all P < 0.05), while those in the Ad-GFP group showed no significant variations concerning the cell invasion and migration (all P > 0.05).

Overexression of TIPE2 suppresses cervical cancer cells (CaSKi and Hela) migration and invasion which detected by wound healing test and Transwell assay.
(A, B) The invasion and migration of CaSKi (A) and Hela (B) cells detected by Transwell assay and wound healing test. (C, D) Comparison of the invasion cell number and wound closure of CaSKi cells (C) and Hela cells (D) among the groups. *P < 0.05 vs. the Mock group.
Western blotting was considered to measure the expression of TIPE2 and β-catenin pathway-related proteins in the cervical cancer cells, while the results in Fig. 3 showed that CaSKi and Hela cells in the Ad-TIPE2 group manifested sharp up-regulations of TIPE2 and E-cadherin but down-regulations of β-catenin and N-cadherin, as compared with the Mock group (all P < 0.05).

TIPE2 overexpression could up-regulate E-cadherin and down-regulate β-catenin and N-cadherin expressions in the cervical cancer cells.
(A, B) The expression of TIPE2 and β-catenin signaling pathway-related proteins in CaSKi cells (A) and Hela cells (B) detected by western blotting. *P < 0.05 vs. the Mock group.
The volume of implanted tumor in the nude mice of Ad-TIPE2 group was much lower than that in the Mock group, with a retard in the growth of tumor (all P < 0.05, Fig. 4). After experiment, nude mice were decapitalized to harvest the tumors for measuring the weight, and as a result, the weight of xenograft tumor in the Ad-TIPE2 group decreased significantly as compared to that in the Mock group (P < 0.05). Besides, immunohistochemistry staining used to detect the expression of Ki67 in the xenograft tumors demonstrated that the expression of Ki67 in the xenograft tumors of Ad-TIPE2 group decreased significantly when comparing to that of Mock group (P < 0.05).
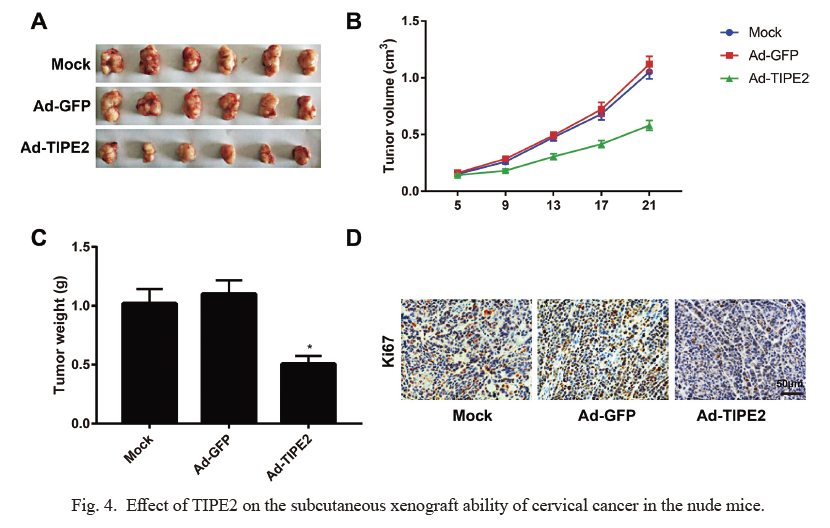
Effect of TIPE2 on the subcutaneous xenograft ability of cervical cancer in the nude mice.
(A) Specimens of xenograft tumors in nude mice. (B) The tumor volume of nude mice in the Ad-TIPE2 were reduced when compared with the Mock group. (C) The tumor weight was dramatically lighter in Ad-TIPE2 group than in Mock group. (D) Immunohistochemistry demonstrated that the proliferation marker Ki-67 was significantly reduced in the Ad-TIPE2 when compared with the Mock group. *P < 0.05 vs. the Mock group.
Transwell assay was conducted to detect the invasion ability of HTR8/SVneo cells and JEG3 cells (Fig. 5). As a consequence, the cell number of invasion in the Ad-TIPE2 group increased evidently (P < 0.05), while no significant difference was shown in comparison of the invasion cell number between the Ad-GFP group and Mock group (P > 0.05).

TIPE2 overexpression could reduce the invasion ability of HTR8/SVneo and JEG3 cells detected by Transwell assay.
(A) The invasion and migration of HTR8/SVneo and JEG3 cells detected by Transwell assay. (B, C) Comparison of the invasion cell number of HTR8/SVneo cells (B) and JEG3 cells (C) among the groups. *P < 0.05 vs. the Mock group.
Western blotting was employed to determine the expression of TIPE2 and MMPs in the trophoblasts of different groups. As seen from Fig. 6, the HTR8/SVneo cells and JEG3 cells in the Ad-TIPE2 group exhibited the significant up-regulations of TIPE2, MMP-2 and MMP-9 (P < 0.05), but no significant difference was observed as compared with the Mock group (P > 0.05).

Overexpression TIPE2 could enhance the expression of matrix metalloproteinase (MMP)-2 and MMP-9 in HTR8/SVneo and JEG3 cells.
The protein expression of TIPE2, MMP-2 and MMP-9 in the HTR8/SVneo cells (A) and JEG3 (B) determined by western blotting.
First of all, we noted that TIPE2 was down-regulated in the specimens of cervical cancer and PE placenta. Consistently, increasing evidence has demonstrated the down-regulation or even depletion of TIPE2 in multiple tumors, like esophageal carcinoma (Zhu et al. 2018), lung cancer (Liu et al. 2015) and breast cancer (Wang et al. 2017b). In addition, TIPE2 expression was also revealed to be associated with histological grades, FIGO stage, lymph nodes metastasis, and HPV infection. Similarly, Sun et al. (2021) found that TIPE2 expression was negatively related to tumor size, TNM stage and metastasis of lymph nodes in pancreatic ductal adenocarcinoma (PDAC). This might reveal the involvement of TIPE2 in the development of cervical cancer. Moreover, down-regulation of TIPE2 protein expression was found in the decidua of patients with missed abortion by Sun et al. (2017), which demonstrated a positive correlation with the serum levels of IL-10. Considering the above, TIPE2 may affect the malignant features of cervical cancer and PE.
In light of this finding, the cervical cancer cells were selected for the in vitro transfection, and as a consequence, TIPE2 overexpression could weaken the invasion and migration abilities of cervical cancer cells, with up-regulation of E-cadherin and down-regulations of β-catenin and N-cadherin. In agreement with the findings in the gastric cancer, Yin et al. (2017) observed that TIPE2 could up-regulate the expression of E-cadherin but down-regulate the expression of N-cadherin and vimentin, thereby reversing the EMT process and inhibiting the invasion and metastasis of gastric cancer cells. Therefore, TIPE2 overexpression, possibly via delaying the EMT process, was able to inhibit the migration and invasion of cervical cancer cells. As reported, EMT refers to a biological process of transformation of epithelial cells into the mesenchymal cells, which endows the epithelial cells with the ability of invasion and metastasis, and represents the major source where the epithelium-derived tumors gain the phenotype of invasion (Tiwari et al. 2012; Santamaria et al. 2017; Liu et al. 2018). Current evidence has shown that EMT is usually concomitant with the silencing or down-regulation of E-cadherin, an indicator of epithelium, and the re-expression or up-regulation of Vimentin and N-cadherin, the indicators of mesenchymal cells (Zhang and Weinberg 2018; Liao and Yang 2020). As for β-catenin, it is a key molecule regulating the Wnt signal pathway, and plays a crucial role in EMT process (Jiang et al. 2007; Anastas and Moon 2013). After Wnt/β-catenin pathway activated, the stimulatioin of Wnt ligand can facilitate the accumulation of β-catenin in cytoplasm and entry of β-catenin into the nucleus, where it could bind to TCF/LEF1 to activate the downstream gene of β-catenin/TCF/LEF1, thus being involved in the cell cycle to promote the survival, migration and invasion (Rao and Kuhl 2010; Zhang and Yan 2016). Coincidently, Liu et al. (2020) found that TIPE2, via binding to β-catenin, can suppress the nuclear translocation of β-catenin to inhibit the EMT process and decrease the migration and invasion abilities of endometrial cells. Liu et al. (2016) demonstrated that in human glioma cell lines, TIPE2 was also down-regulated, while TIPE2 overexpression could reduce the level of β-catenin and inhibit the EMT process under the hypoxic environment, thus blocking the migration and invasion of glioma cells. Based on these data, it is reasonable to hypothesize that TIPE2, possibly by suppressing the activity of β-catenin, can reduce the expression of EMT-related proteins to block the invasion and migration of cervical cancer cells.
As reported, the HTR-8/SVneo cell line is an immortalized trophoblast cell line, which has been reported to exhibit a number of similar characteristics to those of parental trophoblast cells (Zuo et al. 2014), while JEG-3 is a human choriocarcinoma cell line and currently taken as a promising model for trophoblasts (Ikeda et al. 2012). In order to further uncover the mechanism of TIPE2 in PE, the HTR8/SVneo and JEG3 cells were selected to perform transfection, and as a result, TIPE2 overexpression was found to enhance the invasion abilities of HTR8/SVneo and JET3 cells, with up-regulations of MMP-2 and MMP-9. As we know, MMPs are the major modulators for the degradation of extracellular matrix and play key roles in the invasion of trophoblasts (Li et al. 2014b). For example, increasing the activity of MMP-2 and MMP-9 has been confirmed by the previous study to enhance the migration and invasion of trophoblasts (Zhang et al. 2018). Taken together, TIPE2 may be involved in the progression of PE through promoting the invasion of trophoblasts by affecting the expression of MMPs. However, under the normal pregnancy, Th2 immune responses dominate in the matrix, while the Th1 responses that enhance the inflammation are inhibited (Yamaguchi et al. 2009). The risk of PE may be increased due to the increases in the cytokines and quantity of Th1 cells in matrix and/or reductions in the quantity of Th2 cells and cytokines (Dong et al. 2005; Wang et al. 2017a). More importantly, TIPE2 possesses the significant negative regulation of T cell activation (Zhang et al. 2015). Therefore, TIPE2 overexpression may alter the orientation of immune responses of Th1/Th2 in pregnancy, thereby making the immune response skewing to the Th2 and enhancing the trophoblast invasion to improve the superficial implantation of placenta (Sun et al. 2017). It is widely accepted that PE is caused by the interaction of both genetic and environmental factors (Janani and Changaee 2017). Due to the complicated pathogenesis of preeclampsia, the findings of our current results are far from explaining the pathogenesis of PE. Hence, more in-depth studies are needed to explore the TIPE2 function and its biological mechanism in PE in the future.
Our work also demonstrated that TIPE2 could inhibit the invasion of cervical cancer cells to promote the invasion of trophoblasts. It is generally believed that trophoblasts and tumor cells share similar biological characteristics, including proliferation, apoptosis, migration, and invasion (Huang et al. 2019). As such, trophoblasts cannot be defined as normal cells. Furthermore, trophoblasts function in spiral artery remodeling, which is a key event during PE progression (Rahat et al. 2014). Previous studies have shown that trophoblasts invasion, though similar to tumor cells, was strictly regulated by the cascade reactions of paracrine and autocrine factors and the condition of matrix, which differed from tumor cells (von Rango 2008; Mendes et al. 2019). This may be the reason why TIPE2 showed the different effect on the cervical cancer cells and trophoblasts.
However, there was certain limitation in this study. For example, due to resource limitations, squamous intraepithelial lesions (SIL) were not collected to investigate the correlation between TIPE2 expression and cervical malignant transformation. Therefore, the further exploration should be conducted when fund and time permit.
Together, our findings showed that TIPE2 was down-regulated in the cervical cancer and PE placenta. Moreover, TIPE2 overexpression could not only reduce the invasion and migration of cervical cancer cells via inhibiting the EMT process, but also promote the trophoblasts invasion via upregulating the expression of MMPs.
The authors declare no conflict of interest.