2022 Volume 257 Issue 3 Pages 193-203
2022 Volume 257 Issue 3 Pages 193-203
Mongolia was listed among the 30 countries with a high tuberculosis burden in 2021. Approximately 10-11% of the tuberculosis cases are of children, which is higher than the global average (6.0%). As children are a vulnerable population, it is important to understand the current situation and prioritize the development of tuberculosis prevention strategies. However, only few studies have addressed childhood tuberculosis in Mongolia. Therefore, we aimed to describe the characteristics of childhood tuberculosis and to show its trends and estimates in Mongolia. We performed descriptive and trend analyses on secondary data from the National Center for Communicable Diseases from 2010 to 2020. A total of 4,242 childhood tuberculosis cases, compiled from nine districts of the capital city and 21 provinces, were analyzed. We found that tuberculosis occurred more frequently in school-age children, and 71.8% of the all cases were an extrapulmonary tuberculosis. Trend analysis revealed that childhood tuberculosis continuously increased with fluctuations from 2018 onwards. The central region, including the capital city of Ulaanbaatar, is the most tuberculosis-burdened. Childhood tuberculosis is estimated to increase in the central region and decrease in the others from 2021 to 2030. Our findings showed that the national childhood tuberculosis trend is increasing, although there are differences in the pattern between regions. Further studies are needed to identify the determinant factors of regional differences, and age-specific public health interventions, such as scale-up screening and preventive treatment, are in demand in high-prevalence areas.
Tuberculosis (TB), despite being a curable and preventable infectious disease, is still one of the leading causes of death worldwide. Until the coronavirus pandemic, TB was the leading cause of death from a single infectious agent (WHO, World Health Organization 2021). Approximately 7.6 million children under 15 years of age were infected with Mycobacterium tuberculosis (UNICEF 2018). The prevalence of childhood TB is much higher in low-income countries with persistent socioeconomic inequality, overcrowding, poor TB control programs, and a high prevalence of TB in adults (Millet et al. 2013). Childhood TB is a crucial public health problem as it reflects recent transmission and provides an accurate measure of the level of TB control. Further, infected children represent the main reservoir of probable future TB cases (Hamzaoui et al. 2014).
The WHO published a Roadmap for childhood TB in 2013 and the End TB Strategy 2030 in the following year. With these strategies, the WHO aims to reduce TB deaths in 2025 by 75% of those in 2015 (WHO 2013, 2014b). However, in 2015, 210,000 children aged under 15 years died due to TB (WHO 2016). In 2019, this number increased to 230,000, of which 80.0% were under 5 years of age (WHO 2020b). This indicates that the above aims are not likely to be achieved, and therefore, more efforts and actions are required. Apart from mortality, another concern is to reduce the prevalence of childhood TB. Globally, 67 million children are infected with TB; therefore, there is a potential risk of future development of the disease (WHO 2020a). Approximately only 20% of the children who are household contacts of bacteriologically confirmed cases of TB are on preventive treatment (Hamada et al. 2019). These facts indicate that children are a neglected group with regard to TB control. In 2018, the United Nations high-level meeting on TB aimed to develop strategies to treat 3.5 million children by 2022 (United Nations 2018); however, only 1.4 million were treated until 2021 (Chakaya et al. 2021). Global annual numbers of childhood TB cases were 1 million in 2017 (WHO 2018) and 1.1 million in 2020 (WHO 2021). These numbers suggest that the global TB goals are not likely to be achieved by using the current approaches and that immediate action is required.
Mongolia has a high population of youth, with one-third of the population is aged under 15 years (HDC, Health Development Center 2020). It is listed among the 30 high TB burden countries, with an incidence rate of 428/100,000 population, and the 30 high multidrug-resistant or rifampicin-resistant TB burden countries (rate of 6.5/100,000 population) (WHO 2021). Approximately 4,000 cases of TB are reported annually, of which approximately 11.0% are of childhood TB (HDC 2020). To reduce this high childhood TB burden and achieve the global goals detailed above, it is fundamental to improve the efforts to understand the current characteristics of childhood TB. Over the years, studies in Mongolia have been conducted with a focus on adult TB (Naranbat et al. 2009; Dobler et al. 2018), multidrug-resistant TB (Ganzaya et al. 2013; Narmandakh et al. 2020), and prevalence of TB among prisoners (Yanjindulam et al. 2012) and little is known about childhood TB. Understanding current trends and future estimates of TB will have a greater impact on planning, efficient allocation of available resources, and the development of specific strategies and public health interventions to control this disease better (Cole et al. 2020). However, Mongolia’s annual health indicators do not show trends or estimates of childhood TB in the country. This study aimed to describe the characteristics of childhood TB and to show the trends and estimates of this disease in Mongolia.
This was a retrospective descriptive study. In Mongolia, TB cases have to be reported and registered on the integrated electronic system along with paper-based reporting (official forms to report TB cases) to the National Center for Communicable Diseases (NCCD). NCCD summarizes collective reports from the TB surveillance and information system and paper-based reports from the nine districts of the capital city and the 21 provinces. Data quality and reports are verified and validated by the TB Surveillance and Research Department at NCCD. Data from patients with TB are reported to this department as part of routine public health surveillance. We used secondary data from the NCCD from 2010 to 2020 for this study. In addition, to calculate the childhood TB incidence rate ratio, we collected data on the child population (0-14 years old) from the publicly available dataset of the National Statistical Office of Mongolia for each year (MNSO, Mongolian National Statistical Office 2020).
Our dataset includes the annual number of provincial- and district-level cases, demographic information, and diagnostic information (i.e., forms of TB, percentages of organs affected with an extrapulmonary TB, sputum smear results and multidrug-resistance) of childhood TB. The dataset is a collective report and does not include individual information; therefore, this study was waived by an ethical review. The five diagnostic categories adopted by the Ministry of Health, Mongolia include 1) clinically active TB, 2) not clinically active TB, 3) bacteriologically confirmed TB, 4) presumptive TB, and 5) no TB exposure, not infected. In this study, we included data on clinically active TB only, because it is determined by clinical, bacteriologic, or radiographic evidence of current disease, and has to be reported mandatorily. Our study population consisted of children aged less than 15 years who were clinically diagnosed with pulmonary TB and extrapulmonary TB. Patients aged 15 years and older were excluded from the study.
Data analysisDescriptive analyses were conducted for demographic and clinical variables, including age, sex, TB type, sputum smear test, multidrug-resistant TB, and mortality. The time unit used in this study was years, and 11 data points were created. Annual changes were observed to analyze the trends. The distribution of childhood TB is also summarized by region. Results are presented as the sum, mean (± standard deviation), or percentage. Moreover, we calculated the childhood TB incidence rate by region, and the incidence rate ratio was calculated by comparing the incidence rate from 2010 to 2020 in the central region to that in the other regions. In the context of infectious diseases, exponential growth bias may lead to failure to understand the magnitude of childhood TB cases. Therefore, we estimated future childhood TB trends using exponential regression. The data were analyzed using R-studio version 1.0.136.
In the previous 11 years, 4,242 childhood TB cases were reported to NCCD, representing 9.9% of the total 42,970 TB cases in all ages. Fig. 1A shows the nationwide trend and the percentage of childhood TB cases. The trend fluctuated after the numbers peaked in 2016 (n = 516; 12.8%), decreased in the following year, and then steadily increased from 2018 to 2020. The highest number of childhood TB cases was reported in 2016 and the lowest in 2018 (n = 218; 8.4%). The number of cases resurged in 2020 and reached 11.9%. The average percentage of cases was 9.9% (± 1.5%) between 2010 and 2020. The trends in childhood TB types are shown in Fig. 1B. The percentage of pulmonary TB cases decreased from 49.2% (n = 189 in 2010) to 15.1% (n = 60 in 2020), while extrapulmonary TB cases increased from 50.8% (n = 195 in 2010) to 84.9% (n = 337 in 2020). Extrapulmonary TB became the dominant type of TB between 2014 and 2020, accounting for an average of 81.8% (± 4.8%) of all childhood TB cases. Among the childhood extrapulmonary TB cases from 2010 to 2020, the most common occurrence sites were hilar lymphadenopathy TB with an average of 55.7% (± 13.1%), followed by peripheral lymphadenopathy TB 21.3% (± 9.8%) and pleural TB 14.0% (± 4.2%). Table 1 shows the characteristics of childhood TB in Mongolia from 2010 to 2020. Two different age classifications of childhood TB were used by NCCD during the study period. Age group 1 consisted of three age groups (0-1, 2-7, 8-14 years) and was used from 2010 to 2013. There was a greater number of children aged 8-14 years (n = 668; 15.7%) and 2-7 years (n = 586; 13.8%) than children aged 0-1 year (n = 170; 4.0%) during this period. Age classification changed in 2014 to age group 2 (0-4; 5-14 years). The number of TB cases was higher among children aged 5-14 years (n = 1,932; 68.6%) than among children aged 0-4 years (n = 886; 31.4%) from 2014 to 2020.
The sex ratio of patients with childhood TB was approximately equal, with the total number of boys and girls being 2,111 (49.8%) and 2,131 (50.2%), respectively. More children were diagnosed with extrapulmonary TB (n = 3,044; 71.8%) than with pulmonary TB (n = 1,198; 28.2%). After 2013, the number of cases of pulmonary TB and extrapulmonary TB substantially changed and there was a decrease in the number of pulmonary TB cases (171 in 2013; 60 in 2020) and an increase in those of extrapulmonary TB (188 in 2013; 337 in 2020). Sputum smear tests were performed majorly on pulmonary TB cases, which accounted for 21.2% (n = 899) of all childhood TB cases; out of these, 88.5% (n = 796) tested positive. The sputum smear test coverage decreased from 49.2% (n = 189) in 2010 to 15.1% (n = 60) in 2020. Nonetheless, the percentage of sputum smear positive cases was high, with 92.1% (n = 174) in 2010 and 76.6% (n = 46) in 2020. A total of 131 multidrug-resistant TB cases were registered during the study period; only one case was reported in 2010, which increased to 14 cases in 2020. Mortality was reported in 16 cases over the previous 11 years.
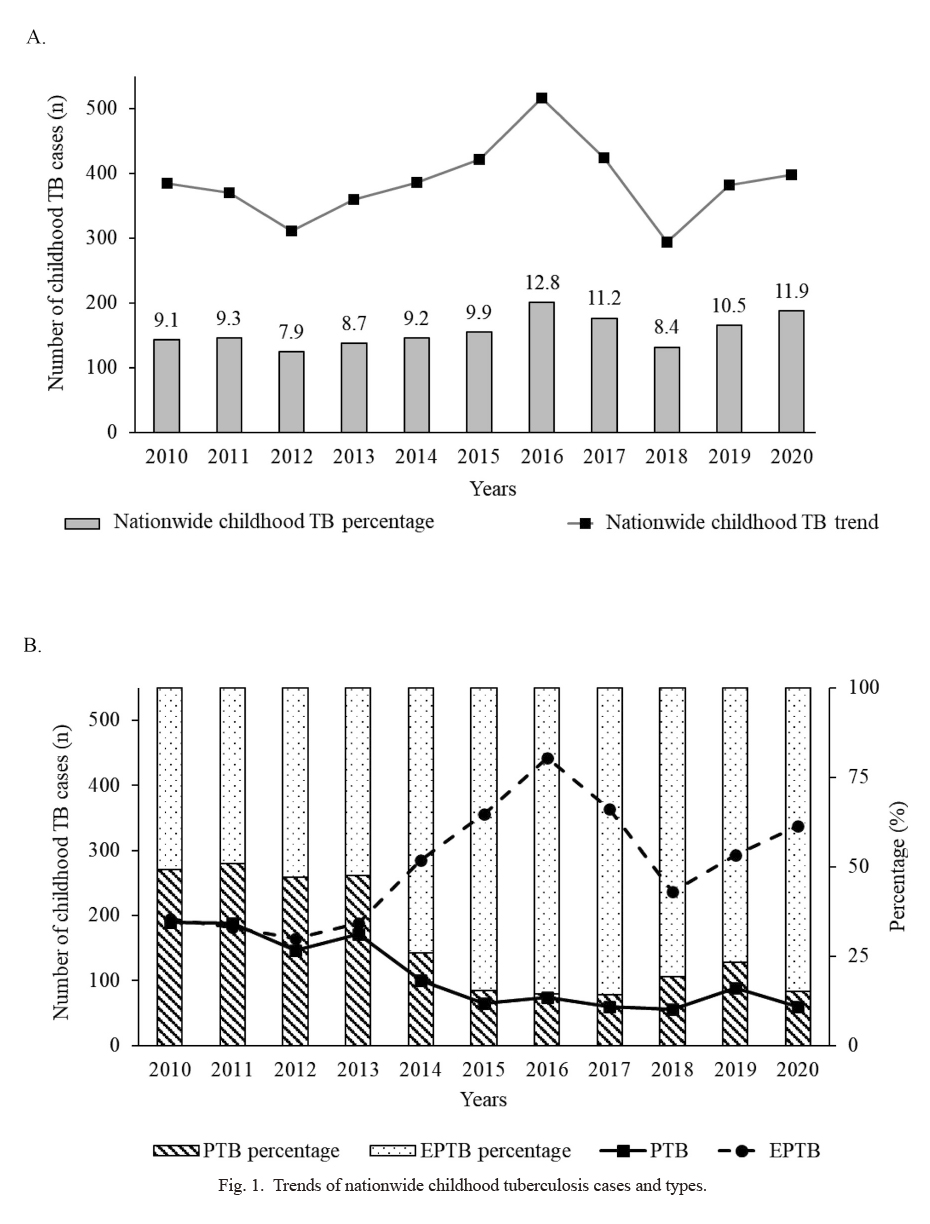
Trends of nationwide childhood tuberculosis cases and types.
(A) Nationwide tuberculosis (TB) trend 2010-2020. The percentage of childhood TB per year is shown in bar graphs. (B) Distribution of pulmonary tuberculosis (PTB) and extrapulmonary tuberculosis (EPTB) over 2010-2020. The bar graph indicates the percentages of PTB and EPTB. Line graphs show the actual number of children with PTB (solid lines) and EPTB (dashed line) in 2010-2020.

Characteristics of childhood tuberculosis in Mongolia, 2010- 2020.
n, number of childhood TB cases; (%), percentage of childhood TB cases; Age groups1, age classification 2010-2013; Age groups2, age classification 2014-2020. Note that the SS test data for 2014-2017 were not available.
TB, tuberculosis; PTB, pulmonary tuberculosis; EPTB, extrapulmonary tuberculosis; SS, sputum smear; MDR, multidrug-resistant.
Mongolia is divided into four geographical regions: Central, Northern, Western, and Eastern (MNSO 2018). All registered childhood TB cases by region from 2010 to 2020 are presented in Table 2. The central region accounted for the majority of childhood TB cases (n = 3,444; 81.2%). In the central region, the number of childhood TB cases in Ulaanbaatar was 2,839 (66.9%). Ulaanbaatar is divided into nine districts. Among the six districts in the urban area, Songinokhairkhan (n = 684; 16.1%), Bayanzurkh (n = 668; 15.7%), Bayangol (n = 504; 11.9%), and Chingeltei (n = 415; 9.8%) had a high number of childhood TB cases. Nalaikh (n = 68; 1.6%), Baganuur (n = 22; 0.5%), and Bagakhangai (n = 5; 0.1%), the three districts in the suburban area of Ulaanbaatar, cumulatively accounted for only 2.2% of all cases. The number of childhood TB cases varied between the provinces. In the central region, Darkhan-Uul (n = 198; 4.7%) and Selenge (n = 186; 4.4%) provinces had high numbers of childhood TB consistently throughout the study period. Even as the number of cases fluctuated between 2010 and 2020, Tuv province ranked third in the region during this period (n = 114; 2.7%).
In the northern region, the total number of childhood TB cases was over ten times lower (n = 333; 7.9%) than that of the central region. Khuvsgul province (n = 128; 3.0%) had the third-highest number of childhood TB cases overall and was the province in the northern region with the leading number of cases. Childhood TB cases in the remaining provinces of the northern region only accounted for 0.5-1.3% of the total number of cases. The overall proportion of childhood TB cases in the eastern region was 7.1% (n = 302) and each of the three provinces of this region accounted for more than 2.0% of the total number of cases. Notably, the number of cases in these provinces declined from 2011 to 2020. The western region had the least number of childhood TB cases, with a total of 163 cases (3.8%) between 2010 and 2020. Each of the provinces in this region accounted for only approximately 1.0% (0.4%-1.3%) of all cases. Khovd province recorded the highest number of cases in this region (n = 11) in 2017. Since 2018, all the provinces in the western region had up to five cases each year: the Govi-Altai province, in particular, had only one to two cases per year. Regional comparisons of childhood TB incidence rates are presented in Table 3. The ratio of the incidence rate in the central region to the other regions was 2.1 in 2010 and it increased to 4.6 in 2020.
The trends and estimates of childhood TB by region are shown in Fig. 2. The trend of childhood TB cases in the central region (Fig. 2A) showed fluctuations, with a peak in 2016 (n = 456), a drop to 239 cases in 2018, and a resurgence from 2018 to 2020. Further, the number of childhood TB cases in the central region are estimated to continue to increase in the future. In the other three regions (Northern, Eastern, and Western), a declining trend with fluctuations was observed between 2010 and 2020. The number of childhood TB cases in these regions is estimated to continuously decrease from 2021 to 2030 (Fig. 2B).

Childhood tuberculosis burden in different regions, 2010-2020.
a, Urban districts of Ulaanbaatar; b, Suburban districts of Ulaanbaatar. The numbers represent the number of cases each year.

Childhood tuberculosis incidence rate ratio in different regions, 2010-2020.
Incidence rate was estimated in different regions and expressed as the number of childhood tuberculosis cases per 100,000 children. The incidence rate ratio was calculated with the incidence rate of the central region as the numerator and the incidence rate of other regions as the denominator.
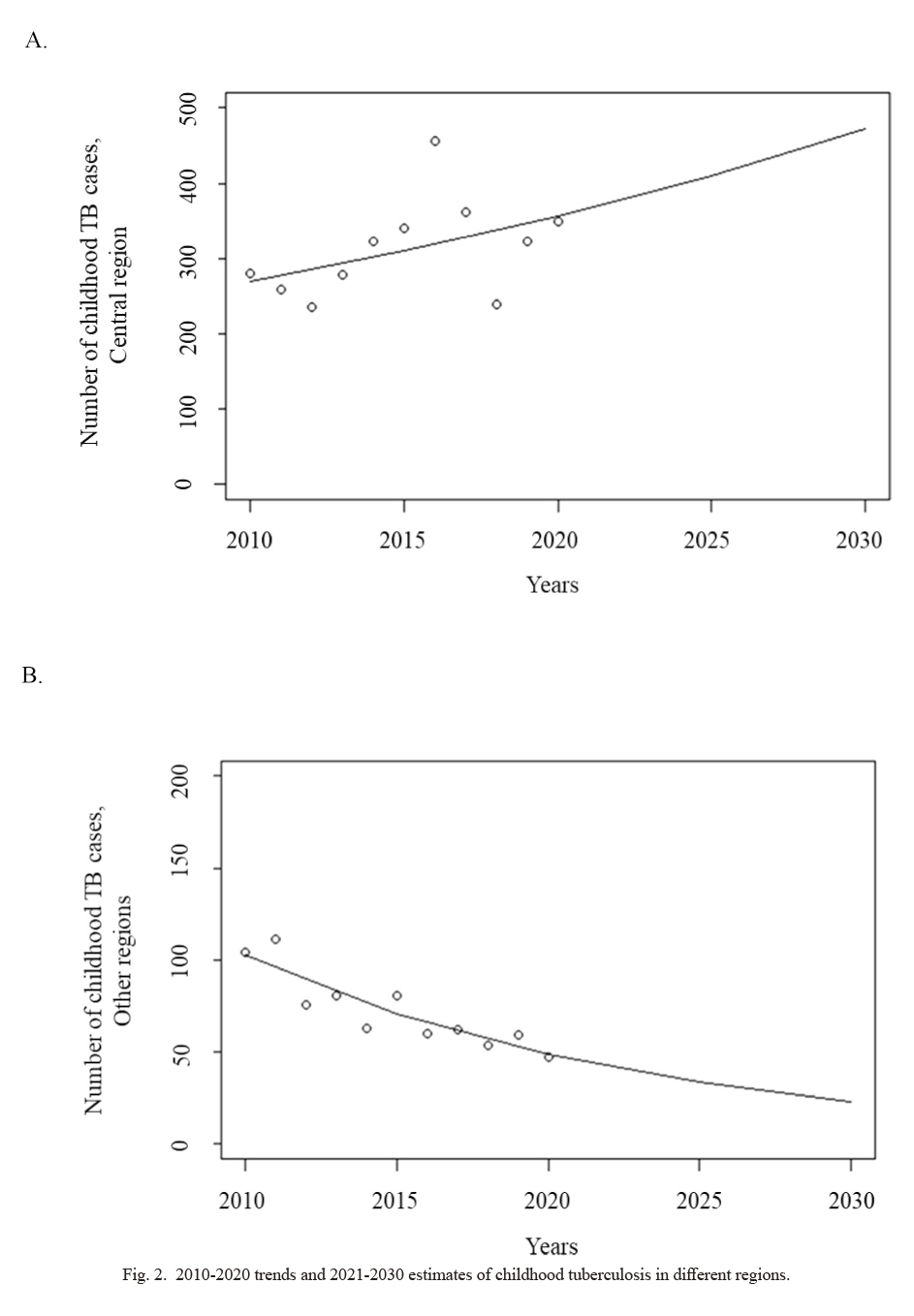
2010-2020 trends and 2021-2030 estimates of childhood tuberculosis in different regions.
(A) Trend and estimates of childhood tuberculosis (TB) in the central region. (B) Trend and estimates of childhood TB in other regions.
In the present study, we showed the characteristics, trends, and estimates of childhood TB in Mongolia. Our analysis revealed that the childhood TB percentage was higher among children aged 5-14 years (68.5% of the total childhood TB cases between 2014 and 2020) than among younger children. Childhood TB among this age group is frequent globally (53.0-75.0%) (Yerramsetti et al. 2022). This might be because children in this age group are more exposed to public places, as they attend school and participate in extracurricular activities, compared to younger children who mostly spend their time at kindergarten. Recently, a cross-sectional study investigated Mycobacterium tuberculosis infections using QuantiFERON-TB Gold screening test among 9,814 school-age children (6-13 years old) in Ulaanbaatar (Ganmaa et al. 2019a). According to this study, 9.6% of the participants were infected with Mycobacterium tuberculosis and 13.8% were later diagnosed with active TB, while 86.2% were diagnosed with latent TB. In addition, they found that increase in age and household pulmonary TB exposure were independent risk factors for developing childhood TB. However, a meta-analysis from the PROSPERO database (CRD42015015331) showed that TB diagnosis sensitivity is significantly different between children aged 0-4 years and 5-14 years; for instance, sputum smear test sensitivity is less than 1.0% in younger children (0-4 years old), and 14.0% in older children (5-14 years old) (Kunkel et al. 2016). Another study using the same cohort as the study by Ganmaa et al. (2019b) reported that 80.6% of patients with active TB had no physical symptoms; however, 96.9% of them had chest radiological abnormalities (Ganmaa et al. 2019b). Together, these studies and our results suggest that TB infection and illness are prevalent in this age group, and several cases may have not been detected at all.
Further, our study found that sputum smear test coverage and the percentage of sputum smear positive cases decreased in 2020 (n = 60 tests, 15.1% of the total number of cases in that year where 76.7% of cases were sputum smear positive) compared to 2010 (n = 189 tests, 49.2% of the total cases in that year where 92.1% of cases were sputum smear positive). Other studies have shown that sputum smear test coverage and positivity rate are universally low in children (average of 6.8% sputum smear positivity) compared to adults (average of 52.0% sputum smear positivity) (Mngomezulu et al. 2015; Kunkel et al. 2016). This lack of diagnostic sensitivity may be explained by several factors: children often cannot cough out sufficient sputum yet a concentration of 10,000 bacilli per ml of sputum is required for a smear-positive result (Piccini et al. 2014; PAHO 2018), and children have low bacillary concentrations compared to adults (Brent et al. 2017); thus, this may lead to under-diagnosis of TB among children aged 0-4 years than among children aged 5-14 years (Harries and Kumar 2018). Therefore, clinicians often rely on clinical symptoms and radiographic abnormalities rather than the sputum smear test for childhood TB cases (Thomas 2017). This might explain why the sputum smear test coverage decreased during the study period, which in turn explained the decreased number of pulmonary TB cases.
We also observed that the number of multidrug-resistant TB cases rapidly increased to 14 in 2013 and averaged 14.1 (± 5.0) cases per year thereafter. In this study, multidrug-resistant TB among children accounted for approximately 3.1% of all childhood TB cases (2010-2020). Globally, childhood multidrug-resistant TB is estimated to account for 3.3% of all childhood TB cases (Song et al. 2021), and this number varies between countries (WHO 2019; Mirzayev et al. 2021). However, we should consider the possibility that the increase in childhood multidrug-resistant TB cases in Mongolia might be due to improvements in diagnostic techniques. GeneXpert, a molecular biological test, was introduced in Mongolia (Rendell et al. 2017), and thus, the diagnostic capacity of multidrug-resistant TB increased during the study period. Additionally, urine-based and gastric lavage-based TB tests have been sporadically introduced in the country. However, these sensitive and patient-friendly tests are not routinely used in clinical practice.
We also found a major shift in the predominant TB type. The childhood extrapulmonary TB percentage increased from 50.8% to 84.9% between 2010 and 2020. Globally, extrapulmonary TB accounts for approximately 20%-30% of all childhood TB cases (WHO 2014b). It is believed that extrapulmonary TB frequently occurs in children because of the higher risk of lymphohematogenous spread compared to adults (Aygün et al. 2020). The WHO reported that 15% of all TB cases are extrapulmonary TB, ranging from 8% in the Western Pacific Region and 24% in the Eastern Mediterranean Region (WHO 2018). Several studies have investigated different types of childhood TB and found that 72.1% of the TB cases in China (Wang et al. 2020), 32.8% in Ethiopia (Ramos et al. 2019), 16% in Cambodia (Frieze et al. 2017), and 23% in Pakistan (Tahseen et al. 2020) are extrapulmonary TB. In the present study, we found that 71.8% of the total cases were extrapulmonary TB. Childhood TB, particularly extrapulmonary TB, is less symptomatic and thus difficult to diagnose (Devrim et al. 2014; Kritsaneepaiboon et al. 2017). According to the national guidelines, pulmonary TB diagnosis should be performed for a child with obvious signs and symptoms of pulmonary TB (chest radiographic changes, bacteriologic tests showing lung and airway involvement, etc.), and extrapulmonary TB diagnosis should be performed with 2-3 appropriate diagnostic tests consisting of bacteriological, pathological, and radiographic investigations in non-pulmonary TB suspects (MOH 2021). The high percentage of extrapulmonary TB in Mongolian children is probably observed due to the difficulties in diagnosing pulmonary TB, and lack of preventive treatment for latent cases that may potentially develop into active extrapulmonary TB. Possibilities of both over-diagnosing extrapulmonary TB and underdiagnosing pulmonary TB should be considered.
We observed that Songinokhairkhan, Bayanzurkh, and Chingeltei districts in Ulaanbaatar, and Darkhan-Uul and Selenge provinces tended to have a high number of childhood TB cases. This may be due to the high population density in these areas. TB has been found to be more prevalent in areas with high population density (Wang et al. 2019; Rao and Johnson 2021). Many studies have reported that population density is one of the factors that affect the prevalence of childhood TB (Kanchan et al. 2015; Wang et al. 2019; Wardani and Wahono 2020), because of the higher TB transmission rate, higher number of children in one classroom in schools, and socioeconomic disparities. Although Mongolia is a large country in terms of land and one of the countries with the least population density (2.1/km2), the population is unevenly distributed. For instance, 62.9% of the population lives in the central region, of which 75.5% live in Ulaanbaatar city. During the previous 11 years, the population density of Ulaanbaatar has constantly increased from 243.8/km2 in 2010 to 339.8/km2 in 2020 (MNSO 2020). All the urban districts of Ulaanbaatar city had a population density higher than 200.0/km2, while suburban districts, where fewer childhood TB cases were registered, had population densities ranging from 31.9 to 56.3/km2. The population densities of Darkhan-Uul (28.8/km2 in 2010 to 32.4/km2 in 2020) and Orkhon (113.7/km2 in 2010 to 134.7/km2 in 2020) also increased during this period (MNSO 2020). Correspondingly, our results demonstrated an increased incidence rate ratio in the central region.
Over the 11-year study period, we found that the number of childhood TB cases fluctuated and then increased after 2018. During the study period, childhood TB trends were unstable overall, and fewer fluctuations were observed in regions other than the central region. In the central region, the number of TB cases peaked in 2016 (n = 456). We assume that this occurred due to TB outbreaks at secondary schools in several districts of Ulaanbaatar city (Rahevar et al. 2021) and the additional TB cases found through the study conducted by Ganmaa et al. (2019b) between 2015 and 2016. In addition, our trend estimation analysis revealed that number of cases of childhood TB in the central region are likely to continuously increase in the future. Similarly, several countries, such as Nigeria, China, and Brazil, showed an increasing trend during the same study period (Daniel et al. 2015; Alves et al. 2020; Wang et al. 2020). While a decreasing trend was observed in most of the developed countries (Mor et al. 2013), the trend was often stable among foreign-born immigrant children in these countries (Cowger et al. 2019; Gafar et al. 2020). As mentioned above, we assume that regional differences in TB estimates may be due to the differences in population densities, number of contacts with TB, air pollution levels, and/or economic status. As we did not explore the independent factors that influence the regional differences in childhood TB cases, further studies are encouraged.
Finally, childhood TB remains an important public health issue in Mongolia. Even though Mongolia has achieved more than 98.0% coverage of the first dose of the Bacillus Calmette-Guérin (BCG) vaccine (HDC 2020), other preventive measures must be implemented. Several studies have shown that childhood TB contact tracing, identification of potential cases, and TB preventive treatment initiation effectively reduce the transmission of childhood TB (Detjen et al. 2013; WHO 2014a; Reuter et al. 2020). In recent years, the WHO (2020c) has recommended preventive treatment for latent TB because it has the potential to develop into active TB during the course of a patient’s lifetime. In 2021, the Mongolian government introduced a more elaborate preventive treatment strategy for latent TB and this is expected to have a greater impact on the prevention of this disease (MOH 2021). Household contact tracing should be improved in Mongolia in order to diagnose cases of latent TB. We suggest that household contact tracing should be focused on the communities and regions with a high number of TB cases. This could be an efficient, cost-saving, and human resource-friendly short-term strategy.
The main strength of this study is that it is the first to characterize the analyzed trends and estimate future trends of childhood TB in Mongolia. However, this study has some limitations. First, we used secondary data that depended on the availability of relevant parameters; therefore, TB treatment-related parameters could not be included. Second, our study did not use individual data; therefore, we were not able to show individual predictors of the disease.
In conclusion, our study helps to understand the characteristics, trends, and estimates of childhood TB in Mongolia. Childhood TB occurred most frequently among children aged 5-14 years, and extrapulmonary TB was predominant. In the central region, childhood TB showed an increasing trend and will continuously increase over the next 9 years. Our findings can be useful in highlighting the burden of TB in children aged 0-14 years and developing prevention strategies to protect them from this infection. We believe that region-specific and age-specific strategies based on child-focused communicable disease services such as preventive treatment for latent TB, household contact tracing, treatment follow-ups, and scale-up of screening of TB among school-age children could potentially improve the current TB scenario in Mongolia.
The authors declare no conflict of interest.