2022 年 257 巻 4 号 p. 273-281
2022 年 257 巻 4 号 p. 273-281
At the end of 2019, the world met with the coronavirus disease 2019 (COVID-19), which will affect all humanity. Later in the course, the genetic variants of SARS-CoV-2 have emerged, bringing new questions and concerns. This study investigated differences between patients infected with the B.1.1.7 (UK variant) and the B.1.617.2 (Delta variant) regarding patient complaints, intensive care unit (ICU) admission and stay time, intubation, severe disease, mortality rates, and laboratory parameters. Hospitalized 205 patients infected with B.1.1.7 and 207 patients infected with B.1.617.2 were included in the study. Laboratory parameters, admission complaints, and the percent saturation of oxygen in the blood (SpO2) were recorded on the same day as the diagnosis and clinical findings during their follow-up. Cough and fever were more common complaints in the B.1.1.7 infected group, whereas tiredness, joint pain, and gastrointestinal complaints were more frequent in patients infected with B.1.617.2. The B.1.617.2 infected group had higher severe disease, acute coronary syndrome (ACS), mortality rates, neutrophil, troponin, and ferritin levels. In conclusion, patients infected with B.1.617.2 had a higher risk of intubation, ACS, and mortality rates. Cough and fever were more common in B.1.1.7 infected group, whereas tiredness was more frequent in B.1.617.2 infected group. Vaccination with at least one dose of Pfizer-BioNTech or CoronaVac is independently associated with a decreased mortality risk caused by two variants (Odds Ratio 0.4).
At the end of 2019, the world met with the coronavirus disease 2019 (COVID-19), which will affect all humanity. The causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is highly transmissible and mortal. The effects of the COVID-19 were not only on health but on the following processes which disrupted the supply-demand balance and production, causing the world economy to be shaken (Sarfraz et al. 2022).
At the beginning of the pandemic, the whole world seemed helpless. SARS-CoV-2 has proven to be more contagious than other types of coronavirus, even in asymptomatic persons (Johansson et al. 2021), and is considered a concern for public health. The COVID-19 pandemic has led to the overloading of clinics and intensive care units, thus causing health systems to collapse and healthcare workers to be exhausted (Naldi et al. 2021). Later in the pandemic, the genetic variants of SARS-CoV-2 emerged and circulated the world. About a year later, on December 14, 2020, the United Kingdom (UK) Government was notified of the emergence of a SARS-CoV-2 variant that was under investigation (Wise 2020) and was later identified as a lineage of B.1.1.7. (UK variant). India has experienced a surge in cases of COVID-19 since late March 2021, causing thousands of deaths reported in early May 2021 (ECDC 2021). The B.1.617.2 (Delta variant) has now been detected across the globe (Bolze et al. 2022).
Concerns about its transmissibility, pathogenicity and the variant’s effect on vaccine efficacy have followed. Recent studies reported a possible association between B.1.617.2 and severe COVID-19, and an increased viral load with prolonged viral shedding in respiratory samples (Ong et al. 2021). The recognition of diseases is undoubtedly crucial in preventive medicine and treatment. At the pandemic’s beginning, the scientific world was desperately looking for a cure. Treatments including azithromycin and hydroxychloroquine have been proven successful (Gautret et al. 2020). However, there were no significant results in other studies (Cavalcanti et al. 2020; Skipper et al. 2020). Proper use of masks, social distancing, and hygiene still have a very important place in reducing transmission. However, medical and conservative treatment and vaccination are vital to control the disease in the ongoing life. The present study investigated differences between the patients infected with B.1.1.7 and the B.1.617.2 variants regarding patient complaints, hospital stay time, intensive care unit (ICU) admission and stay time, intubation, severe disease, mortality rates, and laboratory differences.
The data of patients admitted to a tertiary city hospital in Turkey between May 2021 and November 2021 with complaints compatible with COVID-19 were retrospectively investigated. Out of them hospitalized, 205 patients infected with laboratory-confirmed B.1.1.7 variants and 207 patients infected with B.1.617.2 variants were included in the study (Fig. 1). The diagnosis was made by real-time reverse-transcription polymerase chain reaction (PCR) of nasopharyngeal swab samples. Variant analysis was performed in the Microbiology PCR laboratory of the hospital where the study was conducted. Variants were identified using sequencing and based on the spike (S) gene status. Patients who were not hospitalized, those with a test result other than B.1.1.7 and B.1.617.2, and those whose required information could not be obtained were excluded from the study (Fig. 1). Two patient groups were compared by examining the laboratory parameters, admission complaints, and the percent saturation of oxygen in the blood (SpO2) obtained on the same day as the diagnosis and clinical findings during their follow-up. The patients with one of the criteria comprising oxygen saturation below 93% in room air, C-reactive protein (CRP) value above 50 mg/L, D-dimer value above 1 μg FEU/ml, ferritin level above 500 ng/mL, lymphocyte value below 500 103/μL, and severe involvement on computed tomography were hospitalized. Severe illness is defined as suspected respiratory infection symptoms in addition to any of the following indicators: shortness of breath, respiratory rate above 30 breaths/min, oxygen saturation at rest below 93%, and PaO2/FiO2 level below 300 mmHg (1 mmHg = 0.133 kPa).
CRP and D-dimer were examined using the particle-enhanced immunoturbidometric essay. High-sensitive troponin-T and ferritin were assayed using the sandwich principle. Leukocytes were measured using fluorescent flow cytometry; erythrocytes and platelets were measured using the impedance method. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), ferritin-to-lymphocyte ratio (FLR), and C-reactive protein (CRP)-to-lymphocyte ratio (CLR) values were calculated by dividing neutrophil, platelet, ferritin, and CRP levels to the lymphocyte count, respectively. Ferritin-to-D-dimer ratio (FDR) and ferritin-to-CRP ratio (FCR) were obtained by dividing ferritin by D-dimer and CRP, respectively. CRP-to-D-dimer ratio (CDR) and lymphocyte-to-monocyte ratio (LMR) were obtained by dividing CRP to D-dimer and lymphocyte to monocyte levels, respectively.
The ethics committee approval was obtained from the Clinical Research Ethics Committee of a tertiary city hospital in Turkey. All statistical analyses were carried out using SPSS 25.0 software. The Kolmogorov-Smirnov test was performed to examine the normality of the data. Continuous variables were given in terms of mean ± standard deviation (SD) and median values (min-max) while the categorical variables were given in terms of frequency and percent. The Student t-test were used for parametric assumptions and Mann Whitney U test for non-parametric assumptions compared the independent groups. Multivariate binary logistic regression was used to identify independent predictors associated with in-hospital mortality. The receiver operating characteristic (ROC) curve analysis was performed for optimal cut-off values to predict Delta variant. In addition, a p-value less than 0.05 was set as the statistical significance level.

Flowchart of the study population selection including exclusion criteria.
The mean age was determined to be 54 ± 13 years in the B.1.1.7 infected group and 59 ± 16 years in the B.1.617.2 group (Table 1). The sex distribution in the B.1.1.7 infected group was 106 (51.7%) women and 99 (48.3%) men, while it was 109 (52.7%) women and 98 (47.3%) men in the B.1.617.2 group. Patients admitted with cough and fever were higher in the B.1.1.7 group whereas tiredness, joint pain, and gastrointestinal system (GIS) symptoms (mainly diarrhea) were higher in the B.1.617.2 infected group. Severe disease, acute coronary syndrome (ACS), intubation and mortality rates, clinic and ICU stay time rates were higher whereas SpO2 levels at first admission remained lower in the B.1.617.2 infected group. Hypertension, coronary artery disease, and malignancy rates were higher in the B.1.617.2 group. The rate of patients vaccinated with at least one dose of Pfizer-BioNTech, or CoronaVac (status vaccinated or not) remained lower in the B.1.1.7 group.
Patients infected with B.1.617.2 variant had higher levels of neutrophil, red cell distribution width (RDW), CRP, troponin-T, ferritin, NLR, PLR, FDR, CDR, FLR, and CLR (Table 2). More than half of the intubated patients (54.5%), 49% of patients with severe disease, and 42.6% of patients admitted to the ICU died. Among survivors, 53.9% of patients were vaccinated with at least one dose of Pfizer-BioNTech or CoronaVac. Twelve percent of patients admitted with cough complaints and 26.2% of tiredness died (Table 3). Binary logistic regression analyses were carried out to predict the risk of in-hospital mortality for the B.1.1.7 and B.1.617.2 variant groups (Table 4). Patients who had ACS, aged above 65 years, ICU admission, higher PDW levels, lower lymphocyte levels had increased risk of mortality, whereas male sex, higher SpO2 levels at admission, shortness of breath, joint pain, lower FDR and mean platelet volume (MPV) levels, more elevated platelet and monocyte levels were found to be associated with a decreased risk of mortality. Optimal cut-off values calculated by the ROC analysis and the ROC curves are presented in Fig. 2. The areas under the curve (AUC) of ferritin, CDR, FLR, and CLR were found as 0.86, 0.77, 0.86, and 0.90, respectively. The optimal cut-off values for variables were as follows: ferritin ≥ 210 (72% sensitivity and specificity), CDR ≥ 51.6 (73% sensitivity and 72% specificity), FLR ≥ 134.5 (80% sensitivity and 78% specificity), and CLR ≥ 19.3 (84% sensitivity and 83% specificity). p-values were < 0.001 for all (Table 5).
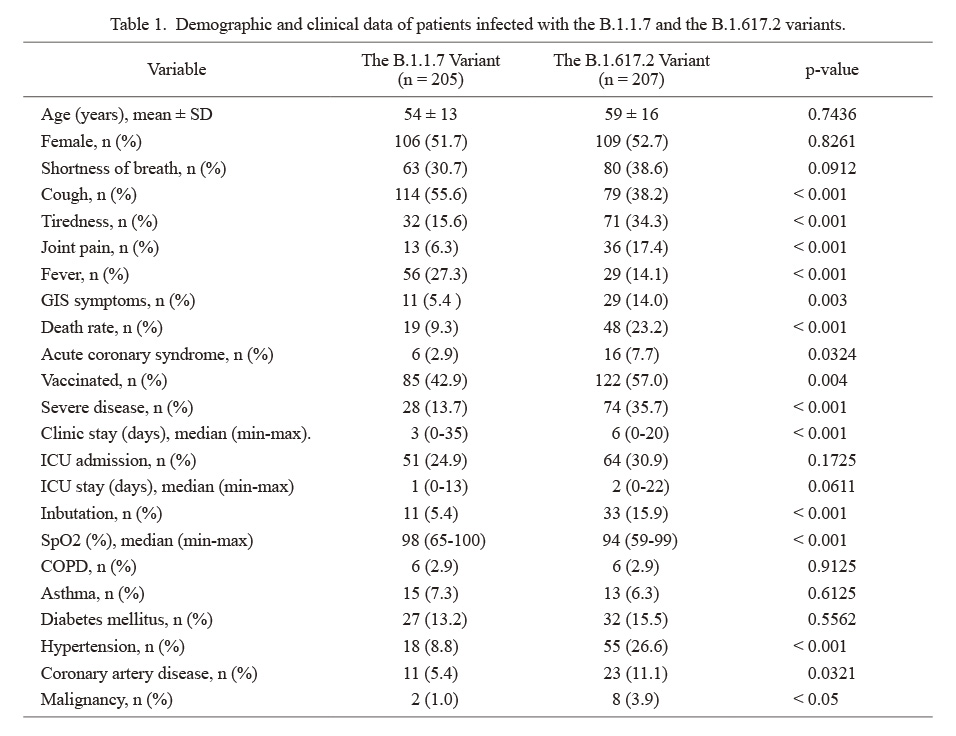
Demographic and clinical data of patients infected with the B.1.1.7 and the B.1.617.2 variants.
GIS, gastrointestinal system; ICU, intensive care unit; SpO2, the percent saturation of oxygen in the blood; COPD, chronic obstructive pulmonary disease.
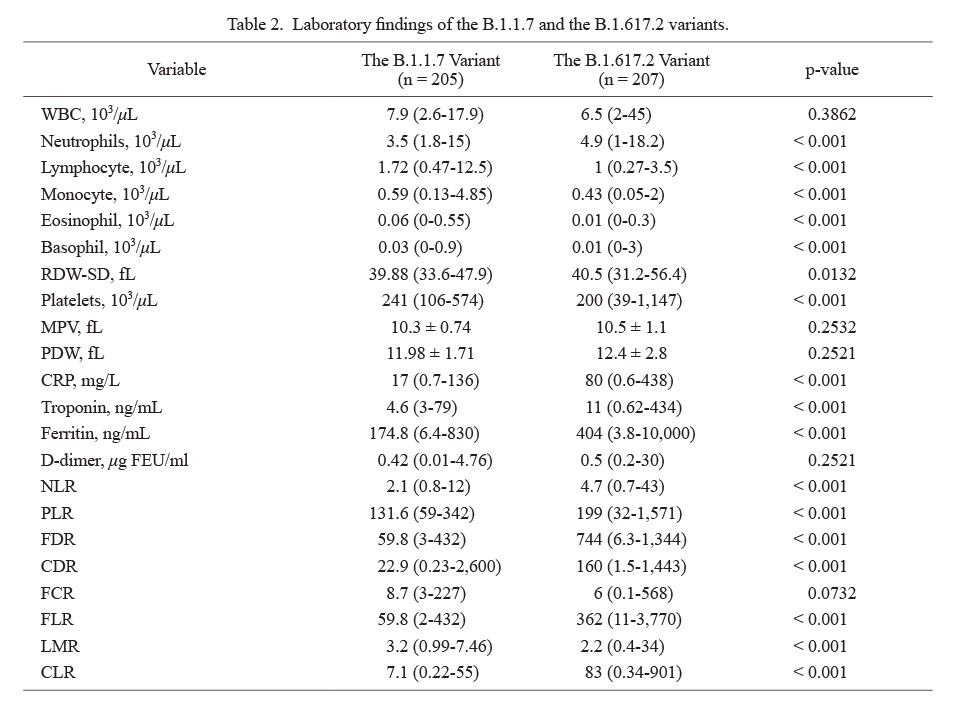
Laboratory findings of the B.1.1.7 and the B.1.617.2 variants.
Normally distributed variables were presented as mean ± SD while skew distributed ones presented as median (min-max).
WBC, White blood cell count; RDW-SD, red blood cell distribution width standard deviation; MPV, mean platelet volume; PDW, platelet distribution width; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; FDR, ferritin-to-D-dimer ratio; CDR, CRP-to-D-dimer ratio; FCR, ferritin-to-CRP ratio; FLR, ferritin-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; CLR, CRP-to-lymphocyte ratio.

The results of the study regarding in-hospital mortality.
ICU, intensive care unit; COPD, chronic obstructive pulmonry disease; GIS, gastrointestinal system.
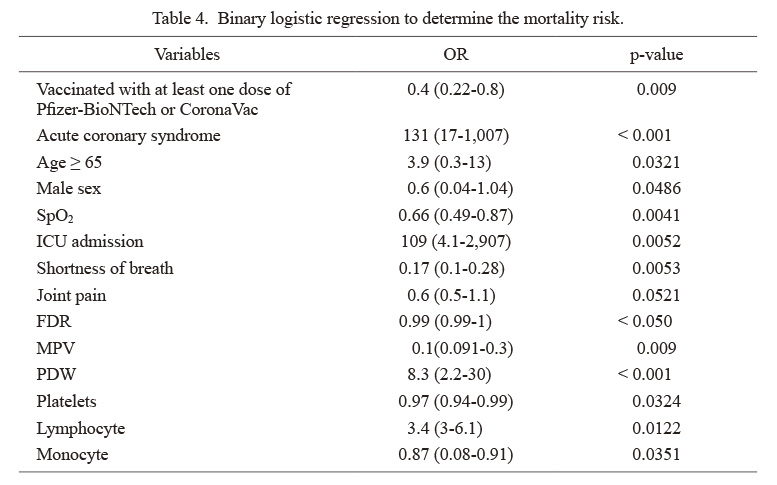
Binary logistic regression to determine the mortality risk.
Data in parentheses show 95% confidence interval.
OR, odds ratio; SpO2, the percent saturation of oxygen in the blood; ICU, intensive care unit; FDR, ferritin-to-D-dimer ratio; MPV, mean platelet volume; PDW, platelet distribution width.

Receiver operating characteristic (ROC) curve comparing the prediction of B.1.617.2 variant variables for ferritin, C-reactive protein (CRP)-to-D-dimer ratio (CDR), ferritin-to-lymphocyte ratio (FLR) and CRP-to-lymphocyte ratio (CLR).

Receiver operating characteristic (ROC) curve analysis to predict Delta variant.
AUC, Area under the ROC Curve; CDR, C-reactive protein (CRP)-to-D-dimer ratio; FLR, ferritin-to-lymphocyte ratio; CLR, CRP-to-lymphocyte ratio.
COVID-19 has been a concern for the whole world since it was defined. Detection of new variants in the COVID-19 disease brought new questions and concerns. Better recognition of newly detected variants will undoubtedly help in the fight against the disease. Fever and cough were reported frequent complaints among COVID-19 patients (Qin et al. 2021). The findings of the present study add to our knowledge about differences between the B.1.617.2 and B.1.1.7 variants. In the present study, patients infected with B.1.617.2 variant had suffered more frequently from tiredness, however, cough and fever were more common complaints among the B.1.1.7 infected group. As time passed, the scientific world started to recognize the COVID-19 disease better and learned that it does not only affect the respiratory tract but many other organ systems, including the neurologic system (Attia et al. 2021; Williamson et al. 2022), cardiovascular system (Guo et al. 2020) and the GIS (Sukharani et al. 2021). In the present study, GIS symptoms were more common in the B.1.617.2 infected group. Even though it did not reach statistical significance, the B.1.617.2 infected group had higher rates of shortness of breath. Patients admitted with shortness of breath had a decreased risk of mortality (OR 0.17). We do not know the exact reason for the reduced risk of mortality, but dyspnea is a serious symptom, and the lower risk of mortality in these patients might be due to their earlier hospital admission and prompt treatment.
COVID-19 is a heterogeneous disease with different complaints and clinical presentations. The SpO2 level is undoubtedly a vital parameter that indicates well-being. In the present study, the B.1.1.7 infected group had a higher median SpO2 at admission than the B.1.617.2 group associated with a decreased risk of mortality (OR 0.66). The B.1.617.2 variant was reported to be more mortal than other variants (Venkatraja et al. 2021). In the present study, severe disease, intubation rates, acute coronary syndrome, and death rates were higher in patients infected with B.1.617.2. Higher mortality might be due to cardiovascular involvement in which the B.1.617.2 group had more frequent CAD and hypertension rates. In the light of these findings, physicians should be careful that patients infected with the B.1.617.2 variant may worsen due to cardiac events.
In the fight against COVID-19, as well as the clinical presentation, laboratory parameters also can guide physicians in both treatment and follow-up of the patients. The present study aimed to investigate the laboratory differences between patients infected with B.1.617.2 and B.1.1.7 variants. The CRP is an important marker that is reported to be significantly increased in the initial phases of the infection for severe COVID-19, and high levels are associated with a poor prognosis (Wang et al. 2020). In the present study, CRP levels were higher in the B.1.617.2 infected group, which might be due to a heavier inflammation derived by the B.1.617.2 variant. Ferritin and neutrophil levels were also higher in the B.1.617.2 group and a cut-off value above 210 ng/mL for ferritin may predict B.1.617.2. Some patients with COVID-19 develop thrombocytopenia, a severe pro-inflammatory state which can be challenging to manage. The difficulty is choosing the appropriate anticoagulant while thrombocytopenia on the other side. In a study from China, Wang et al. (2020) reported myocardial injury and increased mortality risk among COVID-19 patients with lower platelet counts. In the present study, the B.1.1.7 variants had a higher platelet count, which was associated with a decreased risk of mortality (OR 0.97).
A dominant role for monocyte-macrophage activation in the development of immunopathology of COVID-19 was reported previously (Gomez-Rial et al. 2020). Reduced rates of activated monocytes were observed in patients with severe COVID-19 (Kos et al. 2021). In the present study, patients infected with B.1.1.7 had higher levels of circulating monocytes which were associated with a decreased risk of mortality (OR 0.87). Eosinophils are cells that have various functions such as immunoregulation and antiviral activity, but their role in COVID-19 is not well studied. Previous studies reported eosinopenia in patients with acute respiratory deterioration during COVID-19 (Zhang et al. 2020). In the present study, eosinophil levels remained significantly lower in the B.1.617.2 infected group. In the light of the present study and previous reports, platelets, eosinophils and monocytes seem to be playing essential roles in COVID-19. Investigating the causes of reduced levels of monocytes, eosinophils, and platelets might be helpful in the fight against COVID-19.
Recently, ratios that are more accessible to researchers are used in diagnosis and prognosis assessment. A high NLR was reported in patients who tested positive for SARS-CoV-2 compared to controls (Seyit et al. 2021). In a study from Wuhan/China, Yang et al. (2020) reported elevated NLR, which was significantly associated with illness severity. In the same study, PLR of severe COVID-19 patients was higher than those of non-severe COVID-19 (Yang et al. 2020). This study hypothesized that the ratios of essential parameters in terms of disease severity and prognosis in COVID-19 might help differentiate B.1.1.7 infected patients from B.1.617.2 infected group. In the present study, the B.1.617.2 infected group had higher NLR, PLR, FLR, and CLR. A cut-off value above 134.5 for FLR and above 19.3 for CLR might help differentiate B.1.617.2 infected patients from B.1.1.17 infected group. The B.1.1.7 group had a lower FDR, which was associated with a decreased risk of mortality (OR 0.99).
Constant efforts to prevent or reduce the harmful effects of COVID-19 are actively carried out around the world. Previously, the messenger RNA vaccine BNT162b2 (Pfizer-BioNTech) had 95% efficacy against COVID-19 (Polack et al. 2020). In a report from Qatar, vaccine effectiveness against severe, critical, or fatal disease due to infection with any SARS-CoV-2 (The B.1.1.7 and B.1.351 variants were predominant at the time the study was conducted in Qatar) was found to be 97.4% (Abu-Raddad et al. 2021). Inactivated vaccines (CoronaVac) were previously reported to be safe for use (Tanriover et al. 2021; Wu et al. 2021). But the effectiveness of inactivated vaccines against the B.1.617.2 variant remains unclear. In the present study, 53.9% of survivors were vaccinated with at least one dose of Pfizer-BioNTech or CoronaVac.
This study has some limitations, in addition to being a retrospective study, even though all laboratory parameters belong to the first admission day before an in-hospital treatment, they might have been affected by comorbid diseases or medications used for those diseases. Early diagnosis and treatment undoubtedly affect the course of the disease. In the present study, the time between the onset of complaints and hospitalization was not appropriately obtained. Delayed initiation of treatment in some patients may have resulted in more extended clinical and intensive care unit stay, which might affect the results. Since there is no detailed information about vaccination, the study provides limited information about vaccine affectiveness against Delta and UK variants.
In conclusion, the B.1.617.2 variant more frequently affects those with prior history of coronary artery disease and hypertension, leading to more frequent acute coronary syndrome and mortality. In the B.1.1.7 infected group, cough and fever were more common whereas in the B.1.617.2 group tiredness was a more frequent complaint. Vaccination with at least one dose of Pfizer-BioNTech or CoronaVac is independently associated with a decreased mortality risk caused by two variants (OR 0.4).
The authors declare no conflict of interest.