2022 年 258 巻 1 号 p. 43-48
2022 年 258 巻 1 号 p. 43-48
Amyloidosis is characterized by systemic or local deposition of amyloid fibrils outside organs and tissues. Amyloidosis is rarely seen on cornea. A 30-year-old woman patient had had trichiasis in both eyes for 8 years. Trichiasis was observed, which touched the cornea. Slit lamp microscopy showed white gelatinous droplet-like eminences and trichiasis in the lower cornea of the right eye. Optical coherence tomography showed that the lesion involved most of the cornea. Hematoxylin and eosin staining showed that most of the stroma stained red, with scattered inflammatory cells. High expression of lactoferrin was detected by mass spectrometry, and the case was diagnosed as secondary corneal lactoferrin amyloidosis in the right eye.
Amyloidosis is the name given to a heterogeneous group of disorders characterized by systemic or local deposition of amyloid fibrils outside organs and tissues. In amyloidosis, abnormally folded precursor proteins form unstable polymers, resulting in the formation of β-folding structures of amyloid fibrils. The most commonly involved organs are the heart, kidney, liver, gastrointestinal tract and nervous system, causing a series of clinical symptoms. Clinical manifestations vary significantly, making amyloidosis difficult to diagnose and treat (Merlini and Bellotti 2003; Wechalekar et al. 2016).
The patient was a 30-year-old woman. She had had trichiasis in both eyes for 8 years.
In the eye examination, a small amount of trichiasis was observed, which touched the cornea. The trichiasis was more obvious in the right eye than the left. Slit lamp microscopy showed white gelatinous droplet-like eminence and single trichiasis (Fig. 1), with corneal neovascularization in the right eye; corneal fluorescein staining was positive. Optical coherence tomography of the right eye showed that the lesion involved most of the cornea and invaded deeply (Fig. 2). Binocular visual acuity was 0.6, right intraocular pressure was 15.7 mmHg, and left intraocular pressure was 14.0 mmHg.
Pathological examination was performed on the biopsy specimens from the right corneal abnormality, which was a gray-white tissue of about 0.1 cm. Hematoxylin and eosin (HE) staining showed that the surface was covered with a small amount of scaly, lamellar epithelium, and that most of the stroma had stained red with a scattering of a small number of inflammatory cells (Fig. 3). Congo red staining of an 8-μm-thick paraffin section showed no typical brick-red coloration (Fig. 4), and observation under polarized light showed no green birefringence (Fig. 5).
The remaining biopsy specimens in paraffin section were subjected to mass spectrometry using a ThermoFisher Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with a label-free method. After protein extraction, trypsin digestion and purification, the samples were analyzed using liquid chromatography-mass spectrometry. Then the mass spectrometry data were searched against the protein spectrum database; the 10 most-abundant proteins we detected are listed in Table 1 and Fig. 6. Of those 10, lactoferrin (LF), a protein associated with corneal amyloidosis, was highly abundant. The final diagnosis was secondary LF amyloidosis in the right eye. Treatment of this patient involved excision of corneal lesion and therapeutic excimer laser keratectomy of right eye. At present, the patient has recovered well.
All experiments were performed in compliance with the relevant laws and institutional guidelines, and the study was approved by the institutional review board. Informed consent was obtained from the patient.
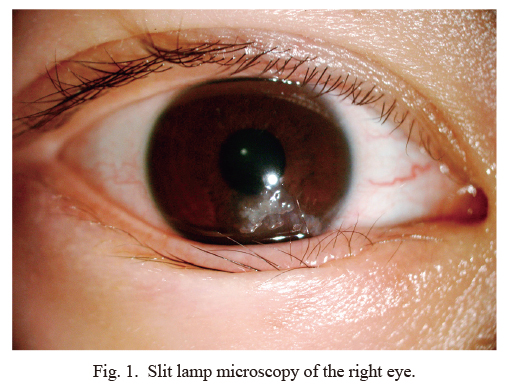
Slit lamp microscopy of the right eye.
Slit lamp microscopy showed corneal protrusions below the right eye and single trichiasis, with a grayish, neoplastic appearance.
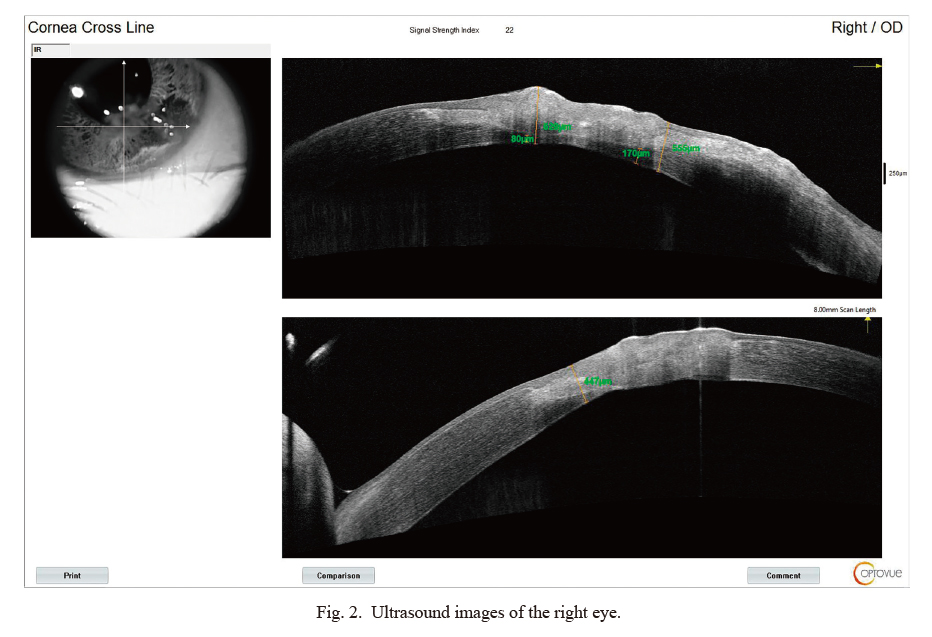
Ultrasound images of the right eye.
Ultrasound images showed that the lesion involved almost the entire thickness of the cornea. The thickness of the unaffected cornea under the lesion was about 80 µm.

Hematoxylin and eosin (HE) staining of biopsy specimens.
There were a large number of amorphous, eosinophilic materials deposited under the skin, accompanied by brittleness and fragmentation.

Congo red staining which showed a red color rather than the typical brick-red color.

Polarized-light microscopy of Congo red-stained specimen.
No typical apple-green birefringence was observed using a polarized-light microscope.

The 10 most-abundant proteins detected.
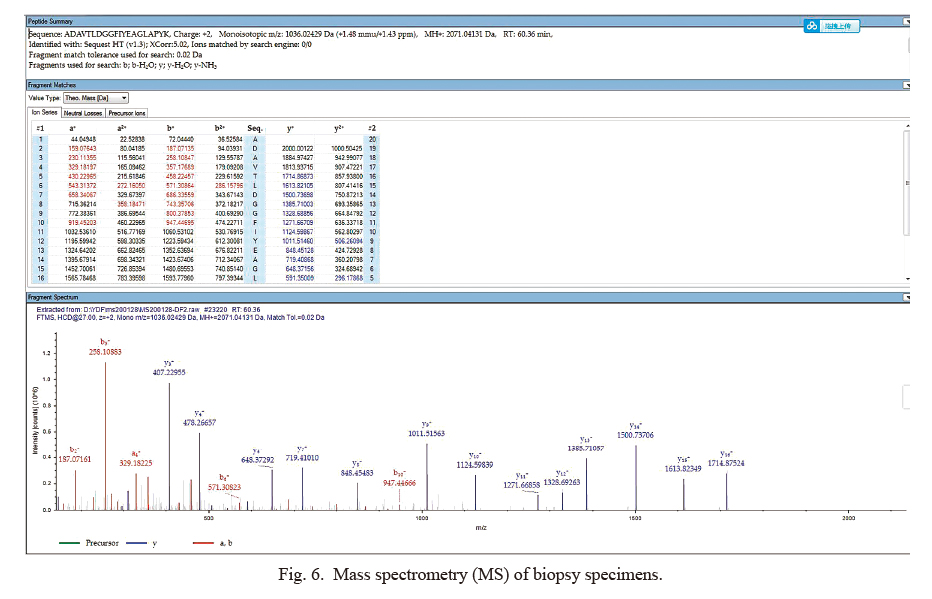
Mass spectrometry (MS) of biopsy specimens.
A specific peptide of lactoferrin (LF), ADAVTLDGGFIYEAGLAPYK, charge +2, was identified by mass spectrometry (MS). In the middle panel, there listed all possible ion fragments from the peptide mentioned above in secondary mass spectrum. The red and blue colored data is the m/e ratios that are all detected by MS. Red and blue represented b-type and y-type ions, respectively. The lower panel showed secondary mass spectrum of the peptide from LF. Ions are marked by their m/e ratios, with red and blue representing different ion types.
Eyelids and the conjunctiva are common sites for ocular amyloidosis. However, Garner (1969) first reported corneal amyloidosis complicated by trichiasis, i.e., localized amyloidosis of the cornea secondary to trichiasis. Since then, a few cases have been reported from Japan, America and France (Shimazaki et al. 1995; Aldave et al. 2005; Mora et al. 2011).
Congo red staining is a traditional technique for demonstrating amyloid protein aggregates in biopsy specimens. Congo red staining is due to the strong affinity between the hydrogen bonds of Congo red amino groups and the main hydroxyl groups in the β-folded amyloid proteins. This results in the change of the absorbance peak from 490 nm to 512 nm, and produces a unique shoulder peak at about 540 nm, which is brick red under the microscope and shows characteristic yellowish-green or bluish-green birefringence under a polarized-light microscope. In previous studies, the characteristic brick-red staining and apple-green birefringence under polarized light were considered the gold standard for the diagnosis of amyloidosis (Lin et al. 2003). However, in recent studies of amyloid protein identification, non-specific binding of Congo red has been shown, which may cause many false-positive results (Yakupova et al. 2019). When the deposition of amyloid protein is low, Congo red staining may show false-negative results in a polarized-light microscope (Yakupova et al. 2019). Therefore it is not necessary to confirm amyloidosis only by HE or Congo red staining. There needs further solid evidence from immunohistochemical, immunofluorescence, electron microscopy, gene detection and mass spectrometry techniques. Through these results, we can obtain more adequate and multi-faceted proof of corneal amyloidosis, and then get the molecular type of amyloid deposits.
Immunofluorescence and immunohistochemistry are common techniques based on antigen-antibody reactions. Some studies have shown that the success rate of immunohistochemical methods for amyloidosis is 38%-87%, and the success rate of immunofluorescence methods is 65%-87% (Sun and Li 2014). Gene detection should be used as a necessary supplement to other typing methods, but the interpretation of the results should be combined with the specific situation (Shen et al. 2015). Mass spectrometry offers the following advantages for improving the accuracy of amyloidosis protein typing: the detection information is more comprehensive, with no need to determine subgroups one by one, and the diagnosis does not rely on clinical data. Moreover, the possibility of finding new pathogenic proteins and chaperones is increased. At present, the newly developed laser microdissection mass spectrometry technique has obvious advantages compared with the traditional mass spectrometry method, which is considered the gold standard for judging the subtypes of amyloidosis (Vrana et al. 2009).
The TGFBIp protein is an RGD-containing protein that binds to type I, II and IV collagens. The RGD motif is found in many extracellular matrix proteins modulating cell adhesion and serves as a ligand recognition sequence for several integrins. This protein plays a role in cell-collagen interactions and may be involved in endochondrial bone formation in cartilage. Mutations in this gene are associated with multiple types of corneal dystrophy. If the TGFBI cannot be a protein in a high level, it cannot be the main reason that cause secondary corneal amyloidosis in this case.
In the present case, the relative abundance of LF was the highest on the mass spectrometry analysis, and therefore, LF was considered to be the main amyloid deposition protein. LF is an iron-binding glycoprotein. LF is one of the precursor proteins in secondary corneal amyloidosis. LF is present in exocrine secretions such as milk, tears, nasal exudates, saliva, bronchial and cervico-vaginal mucus, seminal plasma and gastrointestinal fluid. It has antimicrobial activity, which depends on the extracellular cation concentration. It is reported that LF isoform 1 is expressed in brain, testis and peripheral blood leukocytes. So, it may be synthesized and secreted from blood leukocytes.
The concept of corneal amyloidosis was first proposed by Lewkojewa (1930). Because the clinical appearances of different types of corneal amyloidosis are similar, many techniques were invited to make an accurate differential diagnosis of corneal amyloidosis. Corneal amyloidosis was first divided into primary and secondary amyloidosis. Then some techniques, like slit-lamp biomicroscopy, Congo Red dyeing, immunohistochemistry and electron microscopy, are applied to observe amyloid samples (Takahashi et al. 1983). The corneal amyloidosis was then classified by gelatinous drop-like dystrophy (GDLD), lattice corneal dystrophy (LCD)-like and the combined type (Araki-Sasaki et al. 2005, 2013; Tsujikawa 2012). After that, as gene sequencing and protein mass spectrometry techniques matured, people found many genes or proteins are as direct drivers or main compositions of corneal amyloidosis, such as TACSTD2, TGFBI and LF (Suesskind et al. 2006; Kinoshita et al. 2012; Lai et al. 2014). Then, experts from the association of amyloidosis tend to classify amyloidosis by the proteins or genes mainly involved, such as amyloidosis with lactoferrin (ALac) (Picken 2020). Amyloidosis is a big class of highly heterogeneous diseases, of which 36 species have been identified so far (Sipe et al. 2016). Amyloid light–chain amyloidosis (AL amyloidosis) can affect the kidneys, spleen, heart, and other organs. AA amyloidosis is caused by fragments of amyloid A protein, and affects the kidneys in about 80 percent of cases. ALac amyloidosis can affect cornea (Benson et al. 2020).
In summary, the key to diagnosis and treatment of amyloidosis includes characteristic clinical manifestations and pathological histology to identify the existence of amyloid sediments and deposited proteins for accurate classification. In situ pathological examination plays an important role in the characterization of secondary amyloidosis and the classification of deposited proteins.
The authors declare no conflict of interest.