Abstract
The exact profiles of the clinical symptoms related to the SARS-CoV-2 Omicron variant (B.1.1.529) remain largely uncertain. Therefore, this study aimed to clarify the clinical manifestations of infection with this variant. We enrolled individuals who were tested by quantitative nasopharyngeal swab reverse transcription-polymerase chain reaction (RT-PCR) test at a large screening center in a city of Japan during the B.1.1.529 Omicron variant wave between January and May 2022, after contact with COVID-19 patients. Swab tests were planned to be performed approximately 4-5 days after contact. The presence of COVID-19-related symptoms was assessed at the swab test site. Among the 2,507 enrolled individuals, 943 (37.6%) were RT-PCR test-positive and 1,564 (62.4%) were test-negative. Among the 943 PCR test-positive participants, the prevalence of the symptoms was as follows: 47.3% with cough, 32.9% with sore throat, 18.4% with fatigability, 12.7% with fever of ≥ 37.5℃, 9.9% with dyspnea, 2.1% with dysosmia, and 1.4% with dysgeusia. The prevalence of cough, sore throat, dyspnea, and fatigability was higher among adults aged ≥ 18 years than among children and adolescents. The prevalence of dysosmia and dysgeusia remarkably decreased during the Omicron wave (1-3%) compared to during the pre-Omicron variant waves (15-25%). In summary, common COVID-19-related symptoms during the Omicron variant wave included cough and sore throat, followed by fatigability, fever, and dyspnea. The prevalence of most of these symptoms was higher in adults than in non-adults. The prevalence of dysosmia and dysgeusia remarkably decreased with the Omicron variant than with pre-Omicron variants.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a major global public health concern in 2022. As of June 2022, the B.1.1.529 (Omicron) variant remains prevalent (Karim and Karim 2021; Petersen et al. 2022). Although this variant is considered to result in milder symptoms with better outcomes compared to prior variants, disease is still not mild (Nealon and Cowling 2022; Zhang et al. 2022). In addition to the change in severity of Omicron, the variant has also been suggested to show a lower prevalence of smell and taste disturbances, and a higher prevalence of sore throat and odynophagia (Piersiala et al. 2022). However, the exact rate and predictive significance of these symptoms remains largely undetermined. Therefore, this study aimed to elucidate the prevalence of key COVID-19-related symptoms in the early phase of SARS-CoV-2 infection during the nationwide Omicron variant wave. This study further compared the clinical features of the infection during the Omicron wave and those during the pre-Omicron waves to delineate the clinical features of Omicron.
Materials and Methods
Study design
We initially recruited individuals living in Sendai City, Miyagi, Japan, who were tested using a nasopharyngeal swab reverse transcription-polymerase chain reaction (RT-PCR) test at a large screening center in the city, after contact with COVID-19 patients between January and May 2022 (n = 2,575). During this period, more than 99% of the SARS-CoV-2 infections in the locality were confirmed to be caused by the B.1.1.529 (Omicron) variant, based on the sampling genome analyses performed by the local governments and public health centers. The local epidemic status before and during the study period, along with the daily number of new COVID-19 patients in Miyagi Prefecture, is shown in Fig. 1. Among the initially recruited individuals, patients who failed to provide information about their completion status and dates of vaccination against COVID-19 (n = 68) were excluded.
To compare the prevalence of key COVID-19-related symptoms among patients between different time periods with different predominant SARS-CoV-2 variants in the locality, we further collected data regarding the prevalence of symptoms from individuals tested in April-June 2021 (B.1.1.7 Alpha wave) and August-October 2021 (B.1.617.2 Delta wave), using the same recruitment criteria. To adjust for potential selection bias for swab test recruitment between the different periods with different predominant variants, demographic data and clinical symptoms were evaluated in both populations with RT-PCR test-positive and test-negative results.
Evaluated variables
Vaccine completion status (0, 1, 2, or 3 doses) was collected before the nasopharyngeal swab test. Data regarding the presence of key COVID-19-related symptoms, including body temperature (measured at home on the day of swab test), cough, dyspnea, sore throat, fatigability, dysosmia, and dysgeusia, were collected from the swab test site. Although not in all participants, the swab tests were scheduled to be performed 4-5 days after contact with patients with COVID-19. Therefore, data collected regarding the key COVID-19-related symptoms in RT-PCR test-positive individuals corresponded to the symptoms at approximately 4-5 days after infection. The presence of sore throat was assessed from February 14, 2022, and the individuals tested before this date were not checked for the presence of sore throat. Dysosmia and dysgeusia were observed in individuals aged ≥ 3 years.
Nasopharyngeal swab quantitative RT-PCR test
The screening test center has been managed by local governments (Sendai City, Miyagi Prefecture) and Tohoku University Hospital since July 2020, which is located remote from Tohoku University Hospital (Ishii et al. 2021). This testing center is a drive-through-type facility, and nasopharyngeal swab samples were collected through a car window with the tested individuals remaining inside the car unless they could not remain seated during the sampling process. For the subsequent probe-based quantitative RT-PCR test, the primer/probe set designed by the National Institute of Infectious Diseases in Japan to detect viral nucleocapsid protein set no. 2 (N2) gene (NIID_2019-nCoV_N_F2, R2, and P2) was used (Shirato et al. 2020). To clarify the clinical features of the B.1.1.529 (Omicron) variant, compared to other previous variants, the prevalence of each key COVID-19-related symptoms among RT-PCR test-positive participants were evaluated by age group.
Statistical analysis
The prevalence of each evaluated COVID-19-related symptom was compared between the RT-PCR test-positive and -negative individuals using the chi-square test or Fisher’s exact test according to the sample size in each cell. The 95% confidence interval (CI) for the prevalence of each symptom was further obtained. Using the number of individuals with RT-PCR test-positive and -negative results for each evaluated symptom, the sensitivity, specificity, odds ratio (OR), positive predictive value (PPV), and negative predictive value (NPV), and the 95% CI were calculated for each symptom. ORs were obtained as crude OR and adjusted OR, which were calculated by performing a logistic regression analysis adjusting for age (years) and vaccine completion status (0, 1, 2, or 3 doses) at the time of the nasopharyngeal swab test. Comparisons of the prevalence of symptoms between participant groups were performed using the chi-square test or Fisher’s exact test, according to the number of participants in each subgroup. The predictive impacts of the clinical symptoms and vaccination status on the subsequent RT-PCR test positivity were evaluated by performing binary logistic regression analysis. Comparisons of the prevalence of symptoms between the three periods with different predominant SARS-CoV-2 variants were performed by the chi-square test. Statistical significance was set at p < 0.05. Adjustment for multiple comparisons was not performed because of the nature of the study. Statistical analyses were performed using R Statistical Software (version 4.0.5; R Foundation, Vienna, Austria).
Ethics
The Institutional Review Board of Tohoku University Graduate School of Medicine approved the present study (approval number: 2020-1-535). The review board waived the need for written informed consent, and informed consent was secured in an opt-out manner.
Results
Participants
This study enrolled a total of 2,507 participants who underwent nasopharyngeal swab RT-PCR test after contact with COVID-19 patients during the B.1.1.529 (Omicron) wave, and for whom detailed information regarding the vaccination status was available. Among the 2,507 enrolled participants, 1,091 (43.5%) were adults aged ≥ 18 years and 1,416 (56.5%) were non-adults aged < 18 years. The RT-PCR test results were positive in 943 participants (37.6%) and negative in 1,564 participants (62.4%). When evaluated by age, 450 (41.2%) of the 1,091 tested adults and 493 (34.8%) of the 1,416 tested non-adults were RT-PCR positive.
Symptoms in the whole participants
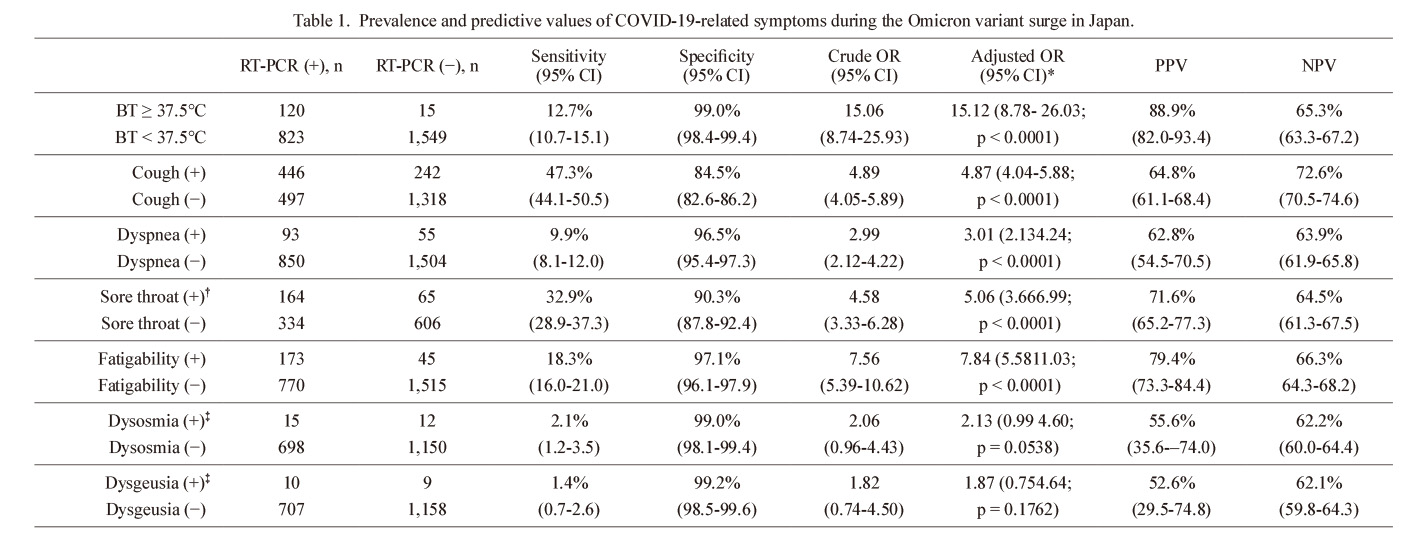
The prevalence of each evaluated key COVID-19-related symptom in the RT-PCR test-positive and negative individuals is summarized in Table 1. The adjusted ORs were significant for body temperature, cough, dyspnea, sore throat, and fatigability (p < 0.0001 for all), but not for dysosmia (p = 0.0538, logistic regression analysis) or dysgeusia (p = 0.1762). The most prevalent key symptoms among the RT-PCR test-positive COVID-19 patients were cough (47.3%) and sore throat (32.9%). Meanwhile, the prevalence of dysosmia (2.1%) and dysgeusia (1.4%) among RT-PCR test-positive participants was much lower. Among the 943 individuals with RT-PCR test-positive results during the B.1.1.529 Omicron wave, only 178 (18.9%) were asymptomatic at the time of nasopharyngeal swab test, without any of the evaluated key COVID-19-related symptoms.
Symptoms between adults and non-adults
The prevalence of each evaluated key symptom in adults aged ≥ 18 years and non-adults aged < 18 years is summarized in Table 2. The prevalence of body temperature ≥ 37.5°C, dysosmia, and dysgeusia was not significantly different between adults and non-adults. Conversely, the prevalence of cough (p = 0.0006), dyspnea (p = 0.0354), sore throat (p < 0.0001), and fatigability (p < 0.0001) was higher in adults than in non-adults.
Furthermore, to exclude the possibility that the prevalence of each symptom was significantly influenced by the completion status of the mRNA COVID-19 vaccines, the prevalence of cough, sore throat, fatigability, and dyspnea among RT-PCR test-positive adults was compared between those who were within 6 months of receiving the second or third dose (n = 262) and others (n = 188). The prevalence of cough (55.3% vs. 50.0%, p = 0.2626, chi-square test), sore throat (41.4% vs. 42.4%, p = 0.8544), fatigability (20.7% vs. 27.7%, p = 0.0862), and dyspnea (11.5% vs. 12.8%, p = 0.6719) did not significantly differ based on the time since vaccination completion.
Binary logistic regression analysis
Finally, to compare the predictive impact of the evaluated demographic data and clinical manifestations for predicting RT-PCR test positivity during the B.1.1.529 (Omicron) wave, a binary logistic regression analysis was performed using the RT-PCR test results as the objective variable. The results of this analysis are shown in Table 3. Age (p < 0.0001), body temperature (p < 0.0001), cough (p < 0.0001), sore throat (p < 0.0001), and fatigability (p = 0.0372) were suggested to have a significant impact on predicting RT-PCR positivity. Sex (p = 0.6036), vaccination status (p = 0.2665), dyspnea (p = 0.7711), dysosmia (p = 0.0952), and dysgeusia (p = 0.2665) were not statistically significant predictors of RT-PCR test positivity.
Symptoms by the periods with different prevalent variants
Finally, to clarify the clinical features of individuals infected with the B.1.1.529 Omicron variant strain, the clinical data collected during the B.1.1.7 (Alpha) wave in April-June 2021 and during the B.1.617.2 (Delta) wave in August-October 2021 were collected and compared. The results are summarized in Table 4. The number of adult patients with COVID-19 significantly decreased in the B.1.1.529 (Omicron) wave. The prevalence of body temperature ≥ 37.5°C was higher in the B.1.617.2 (Delta) and B.1.1.529 (Omicron) waves than the B.1.1.7 (Alpha) wave. The prevalence of cough, dyspnea, and fatigability did not significantly change with the Omicron variant compared with those with the Alpha and Delta variants. Conversely, the prevalence of dysosmia and dysgeusia significantly decreased with the Omicron variant compared to that with the Alpha and Delta variants.
Discussion
This study evaluated the clinical manifestations in the early phase of infection at approximately 4-5 days after infection among RT-PCR test-positive participants with COVID-19 during the B.1.1.529 (Omicron) variant wave in Japan between January and May 2022. The obtained results demonstrated that cough (47.3%) and sore throat (32.9%) were the most common COVID-19-related symptoms in the early phase of SARS-CoV-2 infection during the Omicron variant wave, which agrees with the results of a recent report from China (Xu et al. 2022), and these symptoms were more frequent among adults than non-adults. The prevalence of the evaluated symptoms among RT-PCR test-positive cases was not significantly influenced by the completion status of the second or third vaccine doses. Comparisons of the key COVID-19-related symptoms between the three periods with different predominant variant strains revealed that the prevalence of fever, cough, dyspnea, and fatigability in the early phase of the infection did not differ remarkably between the Omicron variant and the pre-Omicron variants (Alpha and Delta). Meanwhile, the prevalence of dysosmia and dysgeusia with the Omicron variant significantly decreased compared to that with pre-Omicron variants (Akaishi et al. 2021). This finding, along with a remarkable decrease in dysosmia and dysgeusia, suggests that the anatomical site of infection and viral proliferation may have changed between the Omicron and pre-Omicron variants. This is further supported by the fact that the suggested serial interval with Omicron is shorter than with the Delta variant (Backer et al. 2022; Song et al. 2022). Considering that the rate of developing pneumonia with the Omicron variant is lower than that with the pre-Omicron variants, the site of infection and viral proliferation with the Omicron variant might be shifted to the upper airways distal from the lungs. The fact that the incidence of sore throat may increase with the Omicron variant further supports this hypothesis (Menni et al. 2022). Meanwhile, the exact mechanisms of the decreased dysosmia and dysgeusia in spite of the increased tropism toward the upper airway of the Omicron variant remain uncertain. As a possible theory, the levels of tissue and cellular damage caused by SARS-CoV-2 to nasal and tongue epithelial cells, both of which express many angiotensin-converting enzyme 2 receptors on their surfaces, could be alleviated with the Omicron variant from other previous variants (Wang et al. 2020; Xu et al. 2020; Sato et al. 2021). Prior studies have already shown that the clinical spectra in the early phase of SARS-CoV-2 infection may differ with different variants (Akaishi and Ishii 2022), with different rates of severity or fatality in the later stages (Davies et al. 2021; Twohig et al. 2022). Further studies are needed to determine the mechanisms by which altered cell tropism with the Omicron variant could have contributed to its milder clinical manifestations (Abdullah et al. 2022; Nealon and Cowling 2022).
This study has several limitations which should be considered. Firstly, our analyses were limited by the absence of data regarding the prevalence of sore throat during the early phase of the Omicron and pre-Omicron variant waves. This limitation made the eligible sample size used in the binary logistic regression analysis smaller, making it difficult to compare the prevalence of sore throat between the Omicron and pre-Omicron period. Another limitation was that not all enrolled participants were genetically confirmed to be infected with the Omicron variant. To genetically determine that all participants were truly infected by the B.1.1.529 (Omicron) variant, additional laboratory testing to investigate the sequence of the S gene is required. However, as shown in Fig. 1, the nationwide outbreaks with three predominant SARS-CoV-2 variants (Alpha, Delta, and Omicron) created three distinct waves with an obvious non-pandemic period between them. Furthermore, variant-specific RT-PCR tests from random sampling in the locality by the local governments suggested that more than 95% of patients with COVID-19 in each of the three periods (April-June 2021, August-October 2021, and January-May 2022) were infected with the Alpha, Delta, or Omicron variants, respectively. Therefore, we believe that the findings of the present study during the B.1.1.529 Omicron wave can be generalized to the entire population of patients infected during this period. Finally, the present study only evaluated the clinical symptoms approximately 4-5 days after the infection and did not follow-up the symptoms or clinical course thereafter, and the symptoms may have added after the swab test in some of the participants. To gain an overview of the clinical features of the Omicron variant throughout the Omicron course, more detailed data regarding clinical symptoms in the earlier and later infection phases are needed.
In conclusion, the most prevalent key COVID-19-related symptoms in the early phase of SARS-CoV-2 infection during the pandemic with the B.1.1.529 Omicron variant included cough and sore throat, followed by fever, fatigue, and dyspnea. The prevalence of fever, cough, dyspnea, and fatigability was suggested to be largely the same between the periods with Omicron and pre-Omicron waves. Meanwhile, the prevalence of dysosmia and dysgeusia remarkably decreased with the B.1.1.529 Omicron variant (1-3 %) compared to those with the pre-Omicron variants (15-25%). The prevalence of cough, sore throat, dyspnea, and fatigability with the Omicron variant was higher in adults than in non-adults.
Acknowledgments
The authors appreciate all the medical staff and local government staff (Sendai City, Miyagi Prefecture) who joined and cooperated with the drive-through RT-PCR testing project.
Author Contributions
T.A., S. Kushimoto, H.E. and T.I. drafted the manuscript. T.A. and S. Kushimoto performed statistical analyses. N.S. and Y.A. contributed to logistics management of the testing project. S. Kushimoto, Y. Katori, H.E., K. Igarashi, S. Kure, and T.I. supervised the study process. All authors contributed to the collection of samples, and critically reviewed and revised the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Abdullah,
F.,
Myers,
J.,
Basu,
D.,
Tintinger,
G.,
Ueckermann,
V.,
Mathebula,
M.,
Ramlall,
R.,
Spoor,
S.,
de Villiers,
T.,
Van der Walt,
Z.,
Cloete,
J.,
Soma-Pillay,
P.,
Rheeder,
P.,
Paruk,
F.,
Engelbrecht,
A.,
et al.
(2022) Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis., 116, 38-42.
-
Akaishi,
T. &
Ishii,
T.
(2022) Variation in the prevalence of cough symptoms 4-5 days after infection with SARS-CoV-2 between seasons with different prevalent strains. J. Gen. Fam. Med., 23, 248-254.
-
Akaishi,
T.,
Kushimoto,
S.,
Katori,
Y.,
Kure,
S.,
Igarashi,
K.,
Fujita,
M.,
Takayama,
S.,
Abe,
M.,
Kikuchi,
A.,
Tanaka,
J.,
Abe,
Y.,
Imai,
H.,
Inaba,
Y.,
Iwamatsu-Kobayashi,
Y.,
Nishioka,
T.,
et al.(2021) Discriminatory value of self-reported olfactory dysfunction in the prediction of coronavirus disease 2019. Intern. Med., 60, 2905-2910.
-
Backer,
J.A.,
Eggink,
D.,
Andeweg,
S.P.,
Veldhuijzen,
I.K.,
van Maarseveen,
N.,
Vermaas,
K.,
Vlaemynck,
B.,
Schepers,
R.,
van den Hof,
S.,
Reusken,
C.B. &
Wallinga,
J.
(2022) Shorter serial intervals in SARS-CoV-2 cases with Omicron BA.1 variant compared with Delta variant, the Netherlands, 13 to 26 December 2021. Euro Surveill., 27, 2200042.
-
Davies,
N.G.,
Jarvis,
C.I.,
Edmunds,
W.J.,
Jewell,
N.P.,
Diaz-Ordaz, K. & Keogh, R.H.; CMMID COVID-19 Working Group
(2021) Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature, 593, 270-274.
-
Ishii,
T.,
Kushimoto,
S.,
Katori,
Y.,
Kure,
S.,
Igarashi,
K.,
Fujita,
M.,
Takayama,
S.,
Abe,
M.,
Tanaka,
J.,
Kikuchi,
A.,
Abe,
Y.,
Imai,
H.,
Inaba,
Y.,
Iwamatsu-Kobayashi,
Y.,
Nishioka,
T.,
et al.(2021) Predictors of SARS-CoV-2 positivity based on RT-PCR swab tests at a drive-through outpatient clinic for COVID-19 screening in Japan. Tohoku J. Exp. Med., 253, 101-108.
-
Karim,
S.S.A. &
Karim,
Q.A.
(2021) Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet, 398, 2126-2128.
-
Menni,
C.,
Valdes,
A.M.,
Polidori,
L.,
Antonelli,
M.,
Penamakuri,
S.,
Nogal,
A.,
Louca,
P.,
May,
A.,
Figueiredo,
J.C.,
Hu,
C.,
Molteni,
E.,
Canas,
L.,
Österdahl,
M.F.,
Modat,
M.,
Sudre,
C.H.,
et al.(2022) Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet, 399, 1618-1624.
-
Nealon,
J. &
Cowling,
B.J.
(2022) Omicron severity: milder but not mild. Lancet, 399, 412-413.
-
Petersen,
E.,
Ntoumi,
F.,
Hui,
D.S.,
Abubakar,
A.,
Kramer,
L.D.,
Obiero,
C.,
Tambyah,
P.A.,
Blumberg,
L.,
Yapi,
R.,
Al-Abri,
S.,
Pinto,
T.C.A.,
Yeboah-Manu,
D.,
Haider,
N.,
Asogun,
D.,
Velavan,
T.P.,
et al.(2022) Emergence of new SARS-CoV-2 Variant of Concern Omicron (B.1.1.529) - highlights Africa’s research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int. J. Infect. Dis., 114, 268-272.
-
Piersiala,
K.,
Kakabas,
L.,
Bruckova,
A.,
Starkhammar,
M. &
Cardell,
L.O.
(2022) Acute odynophagia: a new symptom of COVID-19 during the SARS-CoV-2 Omicron variant wave in Sweden. J. Intern. Med., 292, 154-161.
-
Sato,
T.,
Ueha,
R.,
Goto,
T.,
Yamauchi,
A.,
Kondo,
K. &
Yamasoba,
T.
(2021) Expression of ACE2 and TMPRSS2 proteins in the upper and lower aerodigestive tracts of rats: implications on COVID 19 infections. Laryngoscope, 131, E932-E939.
-
Shirato,
K.,
Nao,
N.,
Katano,
H.,
Takayama,
I.,
Saito,
S.,
Kato,
F.,
Katoh,
H.,
Sakata,
M.,
Nakatsu,
Y.,
Mori,
Y.,
Kageyama,
T.,
Matsuyama,
S. &
Takeda,
M.
(2020) Development of genetic diagnostic methods for detection for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis., 73, 304-307.
-
Song,
J.S.,
Lee,
J.,
Kim,
M.,
Jeong,
H.S.,
Kim,
M.S.,
Kim,
S.G.,
Yoo,
H.N.,
Lee,
J.J.,
Lee,
H.Y.,
Lee,
S.E.,
Kim,
E.J.,
Rhee,
J.E.,
Kim,
I.H. &
Park,
Y.J.
(2022) Serial intervals and household transmission of SARS-CoV-2 Omicron variant, South Korea, 2021. Emerg. Infect. Dis., 28, 756-759.
-
Twohig,
K.A.,
Nyberg,
T.,
Zaidi,
A.,
Thelwall,
S.,
Sinnathamby,
M.A.,
Aliabadi,
S.,
Seaman,
S.R.,
Harris,
R.J.,
Hope,
R.,
Lopez-Bernal,
J.,
Gallagher,
E.,
Charlett,
A.,
De Angelis,
D.,
Presanis, A.M. & Dabrera, G.; COVID-19 Genomics UK (COG-UK) consortium
(2022) Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect. Dis., 22, 35-42.
-
Wang,
Z.,
Zhou,
J.,
Marshall,
B.,
Rekaya,
R.,
Ye,
K. &
Liu,
H.X.
(2020) SARS-CoV-2 receptor ACE2 is enriched in a subpopulation of mouse tongue epithelial cells in nongustatory papillae but not in taste buds or embryonic oral epithelium. ACS Pharmacol. Transl. Sci., 3, 749-758.
-
Xu,
H.,
Zhong,
L.,
Deng,
J.,
Peng,
J.,
Dan,
H.,
Zeng,
X.,
Li,
T. &
Chen,
Q.
(2020) High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci., 12, 8.
-
Xu,
X.,
Sun,
D.,
Cao,
M.,
Zhang,
W.,
Pu,
Y.,
Chen,
C.,
Sun,
Y.,
Zhou,
S. &
Fang,
B.
(2022) Analysis of clinical characteristics and prognosis of 4 264 patients with asymptomatic and mild novel coronavirus infections in Shanghai. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 34, 449-453.
-
Zhang,
J.,
Chen,
N.,
Zhao,
D.,
Zhang,
J.,
Hu,
Z. &
Tao,
Z.
(2022) Clinical characteristics of COVID-19 patients infected by the omicron variant of SARS-CoV-2. Front. Med. (Lausanne), 9, 912367.