2022 Volume 258 Issue 3 Pages 167-175
2022 Volume 258 Issue 3 Pages 167-175
The prevalence of Alzheimer’s disease (AD) has been rapidly increasing worldwide. We have developed a novel angiogenic therapy with low-intensity pulsed ultrasound (LIPUS), which is effective and safe in animal models of AD and vascular dementia. We performed two trials of LIPUS therapy for AD (mild cognitive impairment due to AD and mild AD); a roll-in open trial for safety, and a randomized, double-blind, placebo-controlled (RCT) trial for efficacy and safety. The LIPUS therapy was performed for whole brain through the bilateral temporal bones for one hour 3 times a week as one session under the special conditions (1.3 MPa, 32 cycles, 5% duty cycle) we identified. The LIPUS therapy was performed for one session in the roll-in trial, and 6 sessions in the RCT trial with 3-month intervals for 1.5 years. The primary endpoint was ADAS-J cog scores. The RCT trial was terminated prematurely due to the COVID-19 pandemic. In the roll-in trial (N = 5), no adverse effects were noted. In the RCT trial (N = 22), the worsening of ADAS-J cog scores tended to be suppressed in the LIPUS group compared with the placebo group at week 72 (P = 0.257). When responders were defined as those with no worsening of ADAS-J cog scores at week 72, the prevalence was 50% (5/10) and 0% (0/5) in the LIPUS and placebo groups, respectively (P = 0.053). No adverse effects were noted. These results suggest that the LIPUS therapy is safe and tends to suppress cognitive impairment although a next pivotal trial with a large number of subjects is warranted.
Along with society aging, the prevalence of Alzheimer’s disease (AD) has been rapidly increasing worldwide. However, effective and safe treatment of AD remains to be developed. For the last decades, amyloid β (Aβ) cascade hypothesis has been in the center of the pathogenesis of the disorder (Hardy and Selkoe 2002). Based on the hypothesis, a number of pharmacological agents that inhibit Aβ synthesis or promote its degradation have been developed without convincing success (Cummings et al. 2020). The effects and safety of aducanumab, a monoclonal antibody against Aβ, for AD has been controversial (Knopman et al. 2021).
It is widely known that AD and vascular dementia (VaD) share common risk factors, such as hypertension, hypercholesterolemia, and diabetes mellitus (O’Brien and Thomas 2015). Long-term exposure to these risk factors result in common outcome, i.e., impairment of vascular endothelial functions (Vanhoutte et al. 2017). Indeed, endothelial dysfunction with reduced nitric oxide (NO) availability has been suggested to play an important role in the pathogenesis of AD (Katusic and Austin 2014). Furthermore, the combination of amyloid pathology (e.g., Aβ deposition and neurofibrillary change) and cerebral ischemic pathology has been found as major triggering mechanisms of dementia (Launer et al. 2008). Thus, vascular dysfunction, especially cerebral microcirculatory dysfunction, should also be regarded as an important pathology of AD (Sweeney et al. 2019).
We have previously developed a low-intensity pulsed ultrasound (LIPUS) therapy that upregulates endothelial NO synthase (eNOS) with resultant therapeutic angiogenesis and suppression of chronic inflammation (Shindo and Shimokawa 2020). We demonstrated that the LIPUS therapy is effective and safe in animal models of chronic myocardial ischemia (Hanawa et al. 2014), myocardial infarction (Shindo et al. 2016), and left ventricular diastolic dysfunction (Monma et al. 2021). We also demonstrated that the LIPUS therapy ameliorates cognitive dysfunctions in mouse models of AD and VaD (Eguchi et al. 2018). The effects of the LIPUS therapy is mainly mediated by its upregulation of eNOS as its beneficial effects are absent in eNOS-deficient mice (Shindo et al. 2016; Eguchi et al. 2018; Shindo and Shimokawa 2020; Monma et al. 2021). Thus, we performed a pilot study to address the effectiveness and safety of our LIPUS therapy in patients with early stage of AD.
Since the accumulation of Aβ in AD extends to the whole brain, we considered that whole-brain irradiation is necessary for the LIPUS therapy as in our animal experiments (Eguchi et al. 2018). Thus, we performed a series of preliminary experiments with human skulls and developed a specialized convex probe that technically enables this concept in accordance with the Japanese Industrial Standards (Supplementary Figs. S1 and S2). Briefly, this probe was set to have an Ispta of 0.15-0.19 MPa at the deepest part (hippocampus; depth of about 8 cm). For safety, Ispta directly below the skull was set to be 1.0-1.5 MPa or less (Supplementary Fig. S1). This probe is a diffusion type probe, so that the cone angle of the probe was set to match the sizes of head width of Japanese elderly adults (Supplementary Fig. S2). Furthermore, based on the preliminary experiments, we changed LIPUS frequency from 1.875 MHz in mice (Eguchi et al. 2018) to 0.5 MHz in humans in the present pilot trial in order to reduce the attenuation of ultrasound transmission due to the difference in temporal bone thickness (Fig. 1).
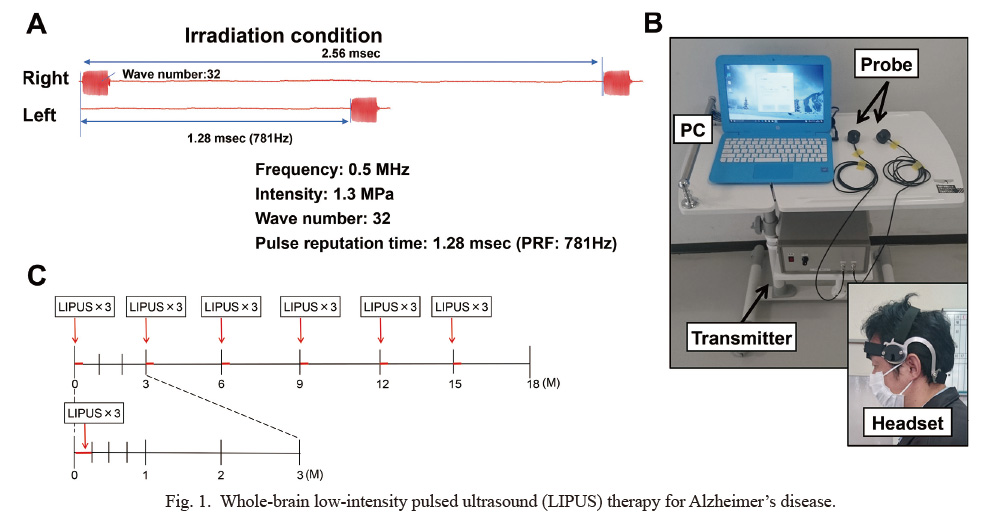
Whole-brain low-intensity pulsed ultrasound (LIPUS) therapy for Alzheimer’s disease.
(A) Irradiation conditions of the LIPUS therapy. (B) LIPUS therapy equipments. (C) Protocol of the randomized, double-blind, placebo-controlled (RCT) trial.
The protocol of the present study was approved by the institutional review board of the Tohoku University Hospital (No. 173008) and was registered as UMIN Trial ID: UMIN000033071. The study consisted of two trials; the roll-in and the randomized, controlled (RCT) trials. In the roll-in trial, the safety of the LIPUS therapy was addressed in an open manner, and in the RCT trial, the efficacy and safety of the therapy were addressed in a randomized, double-blind, placebo-controlled manner. The diagnosis of early stage of AD [mild cognitive impairment (MCI) due to AD and mild AD] was based on the DSM-5 criteria (Fifth edition of the Diagnostic and Statistical Manual of Mental Disorders) by the American Psychiatric Association (2013) and the NIA/AA criteria by the National Institute on Aging and Alzheimer’s Association (Albert et al. 2011). In addition, patients with the Clinical Dementia Rating (CDR) global score 0.5~1.0 and the Japanese version of Mini Mental State Examination (MMSE-J) score greater than 20 were included (Courtney et al. 2004).
The diagnosis of early stage of AD (MCI due to AD and mild AD) was made by board-certified dementia experts (A. I., N. T., and H. A.) with imaging biomarkers, including brain MRI and N-isopropyl-p-(iodine-123)-iodoamphetamine single photon emission computed tomography (123I-IMP-SPECT). Exclusion criteria included unability to receive the LIPUS therapy or brain MRI examination, GCS (Glasgow Coma Scale) score less than 12, symptomatic cerebral infarction or hemorrhage within 12 weeks, cerebral micro-hemorrhage at more than 4 sites, and uncontrolled systemic disease (e.g., heart failure, hepatic failure, renal failure). Stable use of cholinesterase inhibitors and/or memantine was permitted, and a stable dose for a minimum of 4 weeks before screening with no adjustment of dosing during the study was required. Patients were excluded if they had medications that might affect cognitive condition. Patients of both sexes who met the inclusion criteria without the exclusion criteria aged 50~90 years were enrolled in the present study.
Randomization and maskingIn the RCT trial, the patients were randomly divided into 2 groups in a 1:1 fashion to receive either LIPUS or placebo therapy at outpatient clinic for 6 sessions during 18 months at an interval of 3 months (Fig. 1). The present protocol with 18-month of therapy and follow-up was based on the previous pharmacological study (Gauthier et al. 2016). Throughout the trial, patients and physicians in charge were blinded for the therapy and only treating physicians knew the therapy key.
ProceduresThe LIPUS therapy was performed at a single center, Tohoku University Hospital, by physicians who were familiar with the equipment, which consisted of a headset attached to bilateral temporal bones (Fig. 1). LIPUS was irradiated for the whole brain using a convex transducer (Eguchi et al. 2018). The LIPUS therapy was performed for 20 min 3 times with an interval of 5 min each time. One session consisted of the therapy every other day for 3 days (Fig. 1).
In the roll-in trial, the LIPUS therapy was performed for one session during admission under increasing condition of intensity from 0.5 MPa in one subject, 1.0 MPa in 2 subjects, and 1.3 MPa in 2 subjects, while frequency (0.5 MHz), wave number (32), duty cycle (5%), and pulse repetitive frequency (PRF) (781 Hz) were fixed (Eguchi et al. 2018). The patients were followed up for 12 weeks. In the RCT trial, the conditions of the LIPUS therapy were as follows; frequency 0.5MHz, intensity 1.3MPa, wave number 32, duty cycle 5%, and pulse repetitive frequency (PRF) 781Hz with an interval of 1.28 msec (Fig. 1) (Eguchi et al. 2018). The placebo group was treated in the same manner as in the LIPUS group with a convex transducer but without LIPUS irradiation throughout the trial.
OutcomesRegarding the efficacy endpoints, in the roll-in trial, endpoints were usability of the LIPUS equipment. In the RCT trial, primary efficacy endpoint was the changes in summed the Alzheimer’s Disease Assessment Scale-Cognitive Subscale Japanese version (ADAS-J cog) (Connor and Sabbagh 2008; Iwatsubo et al. 2018) at 72 weeks from baseline. Secondary efficacy endpoints included the changes in ADAS-J cog from baseline at 24, 48 and 72 weeks, prevalence of responders for ADAS-J cog, and the changes in the summed values of CDR sum of boxes, Japanese version of Neuropsychiatric Inventory-Questionnaire (NPIQ-J), Japanese version of the Zarit Burden Interview (J-ZBI), Wechsler Memory Scale-revised (WMS-R), MMSE-J, and Functional Activities Questionnaire (FAQ). A responder for the LIPUS therapy was defined as a patient in whom ADAS-J cog did not increase (no deterioration or even improvement from baseline).
Regarding the safety endpoints, in the roll-in trial, the endpoints were MRI findings at 12 weeks and symptoms at 4, 8, and 12 weeks after the LIPUS therapy. In the RCT trial, the safety endpoints included symptoms and adverse events during the trial and MRI findings at 72 weeks.
Statistical analysisContinuous variables are presented as means ± standard error (SE) or medians and interquartile range. Categorical variables are presented as counts and percentages. Changes from baseline in ADAS-J cog and other cognitive function scores were analyzed using the mixed models repeated measures as response values, including group, visit, and interaction term of the group and visit as fixed effects, and each patient as a random effect. Comparison of the prevalence between groups was made by qui square analysis. Sample size calculations were performed using the difference in changes from baseline in ADAS-J cog scores at 72 weeks between the 2 groups with standard deviation at a two-sided alpha level of 5.0 and 70%~80% power. Responder was defined as follows; 0-point or more improvement, 4-point or more improvement, 7-point or more improvement, and 10-point or more improvement on the ADAS-J cog score from baseline were presented as counts and percentages. A P-value of < 0.05 was considered to be statistically significant.
The roll-in protocol was completed with 5 patients from May 2018 to March 2019 as scheduled (Fig. 2). However, due to the COVID-19 pandemic in Japan, the RCT trial was terminated prematurely from April 2019 to August 2020, upon approval by the Pharmaceuticals and Medical Device Agency of Japan (PMDA). As a result, although the planned number of patients was 40, the final number of patients was 22 (Fig. 2).
In the roll-in trial, the 5 patients (4 males and one female, 70.8 ± 9.5 years old), comprising 4 with MCI due to AD and one with mild AD, had MMSE-J score of 24.8 ± 3.4. Twelve weeks after the therapy, no adverse events or abnormal MRI findings were noted. Thus, the safety monitoring committee approved the start of the RCT trial.
In the RCT trial, where 22 patients were initially enrolled (11 in each group), 3 in the placebo group withdrew the consent, and thus 11 in the LIPUS group and 8 in the placebo group received the blinded procedure (Fig. 2, Table 1). After that, one in the LIPUS group and 3 in the placebo group were dropped for various reasons during the trial (Fig. 2). Since the trial was terminated prematurely due to the COVID-19 pandemic, 2 in the placebo group completed 4 sessions. Thus, a total of 19 patients were analyzed (Fig. 2). Among them, 9 had MCI due to AD and 10 had mild AD.
Clinical characteristics of the 19 patients in the RCT trial are shown in Table 1. There were no significant differences in clinical characteristics or cognitive functions between the LIPUS and the placebo groups. For the safety issue, there were no adverse events of the LIPUS therapy including brain MRI findings (data not shown). For the efficacy issue, the changes in ADAS-J cog scores from baseline progressively worsened at 24, 48, and 72 weeks in the placebo group, whereas they remained unchanged in the LIPUS group (Fig. 3). The difference in ADAS-J cog scores at 72 weeks between the 2 groups, which is the primary efficacy endpoint, did not reach a statistically significant level (P = 0.257) due to a small number of patients. A number of 40~50 subjects in each group would reach a statistically significant level. Furthermore, the prevalence of responders with no worsening or even improvement from baseline to 72 weeks was 50% (5/10) in the LIPUS group but 0% (0/5) in the placebo group (P = 0.053) and the prevalence of responders with improvement in cognitive functions progressively increased only in the LIPUS group (Fig. 4). There were no significant differences in other parameters (CDR sum of boxes, NPIQ-J, J-ZBI, WMS-R, MMSE-J, or FAQ) between the 2 groups (data not shown).
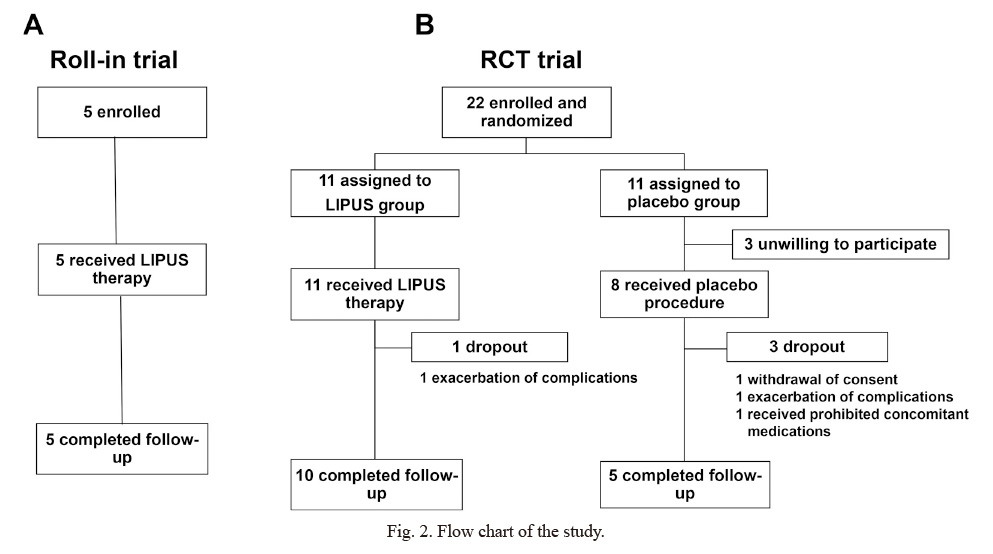
Flow chart of the study.
(A) Roll-in trial. (B) RCT trial.
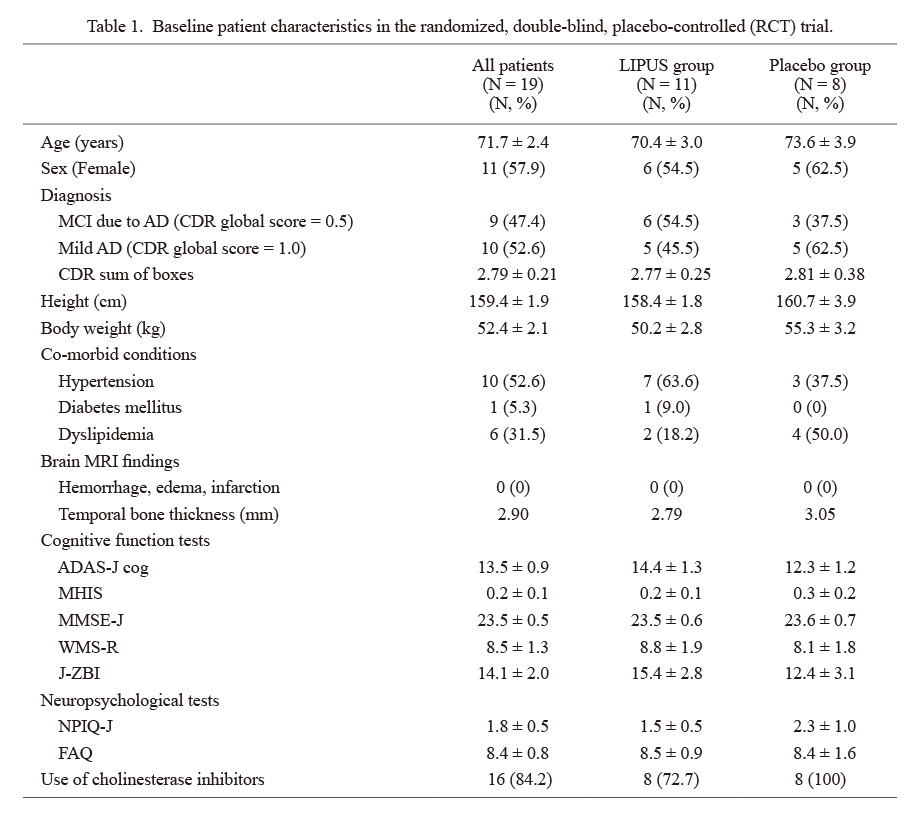
Baseline patient characteristics in the randomized, double-blind, placebo-controlled (RCT) trial.
Results are presented as means ± SE. Categorical variables are presented as counts and percentages.
AD, Alzheimer’s disease; ADAS-J cog, The Alzheimer’s Disease Assessment Scale-Cognitive Subscale Japanese version; CDR, Clinical Dementia Rating; FAQ, Functional Activities Questionnaire; J-ZBI, Japanese version of the Zarit Burden Interview; LIPUS, low-intensity pulsed ultrasound; MCI, mild cognitive impairment; MHIS, Modified Hachinski Ischemic Score; MMSE-J, Japanese version of Mini Mental State Examination; NPIQ-J, Japanese version of Neuropsychiatric Inventory-Questionnaire; WMS-R, Wechsler Memory Scale-revised.
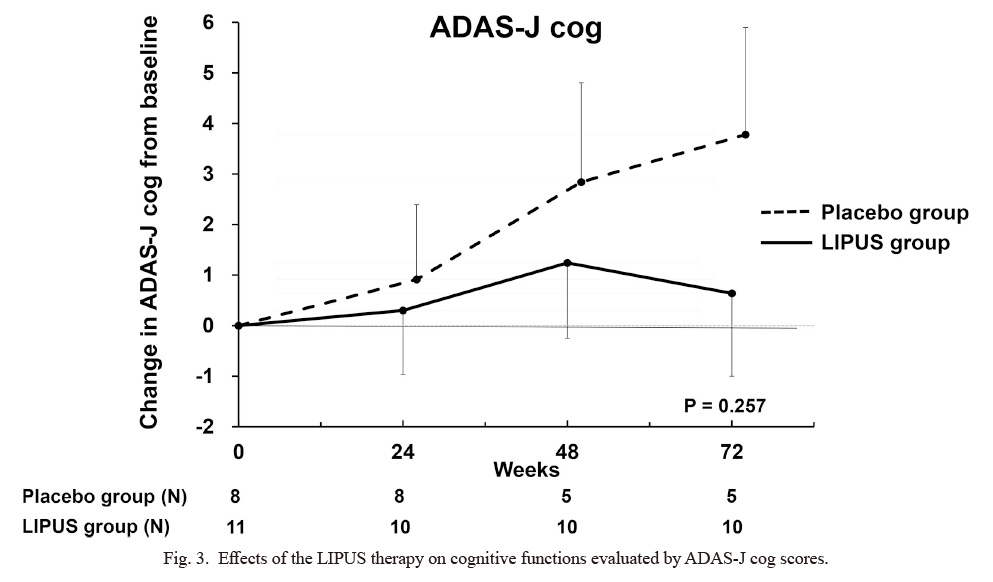
Effects of the LIPUS therapy on cognitive functions evaluated by ADAS-J cog scores.
While cognitive functions tended to progressively worsened in the placebo group (P = 0.09), they remained unchanged in the LIPUS group (P = 0.78). The difference between the 2 groups progressively widened, but did not reach a statistically significant level at 72 weeks (P = 0.257). Results are shown as mean ± SE.
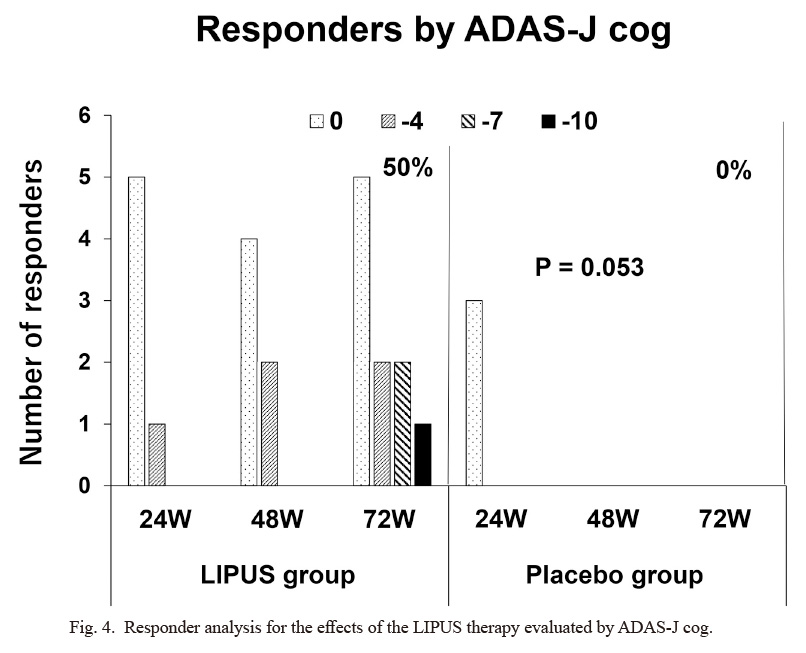
Responder analysis for the effects of the LIPUS therapy evaluated by ADAS-J cog.
While there was no responder at 48 and 72 weeks in the placebo group, the number of responders progressively increased at 24, 48, and 72 weeks in the LIPUS group. The prevalence of responders at 72 weeks was 50% (5/10) in the LIPUS group and 0% (0/5) in the placebo group (P = 0.053).
In the present study, based on our previous experimental findings (Eguchi et al. 2018), we performed a pilot trial to examine the efficacy and safety of our LIPUS therapy for patients with AD. Although the trial was terminated prematurely due to the COVID-19 pandemic and was thus underpowered, the results suggest that the LIPUS therapy is safe and tends to suppress the progression of cognitive dysfunctions in patients with early stage of AD. To the best of our knowledge, this is the first report on the non-pharmacological therapy with LIPUS for the treatment of AD.
Recently, increasing evidence demonstrates that AD is a multifactorial and heterogeneous disease with multiple contributors to its pathophysiology, including vascular dysfunction (Sweeney et al. 2019; Zlokovic et al. 2020). Indeed, regarding the pathogenesis of AD, the roles of vascular dysfunction have attracted increasing attention for the following reasons. First, AD and VaD share common risk factors, such as hypertension, dyslipidemia, and diabetes mellitus, with subsequent vascular dysfunction including endothelial dysfunction (O’Brien and Thomas 2015). Second, experimental evidence demonstrates that endothelial dysfunction with resultant reduced NO availability plays an important role in the pathogenesis of AD (Katusic and Austin 2014). Third, most clinical cases, especially those in persons aged 80 or older, have both classic AD pathology as well as vascular pathology (Dodge et al. 2017). However, despite such situational evidence, it remains to be examined whether vascular dysfunction, especially endothelial dysfunction with reduced NO availability, is involved in the pathogenesis of AD.
In this situation, we have developed a novel therapy with LIPUS that ameliorates microvascular dysfunction through angiogenesis and suppression of chronic inflammation mediated by upregulated eNOS (Hanawa et al. 2014; Shindo et al. 2016; Shindo and Shimokawa 2020; Monma et al. 2021). We first demonstrated that the LIPUS therapy is effective and safe in animal models of heart diseases, including chronic myocardial ischemia (Hanawa et al. 2014), myocardial infarction (Shindo et al. 2016), and left ventricular diastolic dysfunction (Monma et al. 2021). We demonstrated that the therapeutic effects of LIPUS is mediated by mechano-transduction mechanism, by which endothelial β1-integrin/caveolin-1 complex in the caveolae translates the mechanical stimulation of LIPUS into intracellular chemical signaling with resultant upregulations of eNOS and vascular endothelial growth factor (VEGF) in the heart and brain (Shindo et al. 2016; Eguchi et al. 2018; Shindo and Shimokawa 2020), and nerve growth factor (NGF) and brain-derived neurotropic factor (BDNF) in the brain (Eguchi et al. 2018). We then examined the effect and safety of the LIPUS therapy in animal models of dementia, demonstrating that it ameliorates cognitive dysfunctions in both mouse models of AD and VaD, where eNOS also plays an important role (Eguchi et al. 2018). Interestingly, the LIPUS therapy selectively upregulated eNOS, but not neuronal NOS (nNOS) or inducible NOS (iNOS) in the brain (Eguchi et al. 2018). Since NO exerts a variety of physiological effects, including regulation of vascular tone and angiogenesis, eNOS activation is an effective therapeutic strategy for a variety of cardiovascular diseases (Shimokawa 2014; Vanhoutte et al. 2016). Indeed, in the hippocampus, a center of memory function, eNOS plays a crucial role in long-term potentiation (Kantor et al. 1996). We also demonstrated that the LIPUS therapy exerts multiple beneficial effects on cognitive dysfunctions in mice in vivo (Eguchi et al. 2018). In a mouse model of AD, it suppresses amyloid precursor protein (APP) and β-amyloid chelating enzyme (BACE) with resultant decrease in Aβ deposition and suppression of microglial response in the whole brain, and in a model of VaD, it enhances angio-neurogenesis and oligodendrocytes with resultant enhanced re-myelination (Eguchi et al. 2018). These beneficial effects of LIPUS were mediated by eNOS upregulation as they were absent in eNOS-deficient mice (Eguchi et al. 2018). It is important to note that LIPUS is effective to suppress the decline in cognitive functions in both AD and VaD models, suggesting that vascular dysfunction with reduced NO bioavailability locates upstream of Aβ deposition in AD and neuronal damage in VaD (Eguchi et al. 2018).
As compared with drug therapies targeting Aβ cascade (Cummings et al. 2020; Knopman et al. 2021), our LIPUS therapy may have several advantages. First, since LIPUS is directly irradiated for the whole brain, the blood brain barrier, which is a major obstacle for drug development (Pardridge 2002), does not matter. Second, since elderly patients have both classic AD pathology and vascular pathology (Launer et al. 2008; Dodge et al. 2017), the LIPUS therapy, which is effective for both types of dementia at least in animal models (Eguchi et al. 2018), could be an ideal approach for the disorder. Third, since the intensity of LIPUS is within the therapeutic range such as echocardiography (Shindo and Shimokawa 2020), there is no safety issue for the LIPUS therapy. Indeed, there were no adverse events in the present study, including amyloid-related imaging abnormalities (ARIA) (Cummings et al. 2020; Knopman et al. 2021). Fourth, since the LIPUS therapy is less invasive and is specifically for the brain, there are no systemic adverse effects as in the case of drug therapy (Avgerinos et al. 2021; Saeedi and Mehranfar 2022).
To the best of our knowledge, two studies have recently examined the therapeutic potential of focused ultrasound for the treatment of dementia. Jeong et al. (2021) examined the acute effects of low-intensity transcranial focused ultrasound to the right hippocampus combined with intravenous injection of microbubble in 4 cases with AD and found some improvement of cognitive function within a day. Popescu et al. (2021) examined the effects of 2-4 weeks of transcranial ultrasound pulse stimulation (shock wave) to the cortical atrophy lesions as determined by MRI and found the reduction in the atrophy associated with some cognitive improvement. In these studies, there was no placebo group. Our LIPUS therapy is totally different from these therapies in terms of whole-brain LIPUS irradiation (versus focused ultrasound) with multiple treatments for a long-term period.
Several limitations should be mentioned for the present study. First, since the number of patients was too small to achieve a statistical significance, the present findings need to be confirmed in a next pivotal trial with a large number of patients. Second, in the present study, imaging evidence, such as Aβ PET imaging, for the diagnosis of AD was not obtained. However, the diagnosis of AD was made by the board-certified dementia experts with imaging biomarkers based on the international criteria (Albert et al. 2011; American Psychiatric Association 2013). Furthermore, it was reported that among patients with AD diagnosed by NIA-AA, 92% of them were positive for Aβ by PET and/or cerebral spinal fluid (pathological AD) (Lowe et al. 2013). Third, for the efficacy of the LIPUS therapy, no detailed mechanistic information, such as Aβ PET imaging or metabolites of NO in cerebrospinal fluid, was obtained. Fourth, although more LIPUS therapy could exert more beneficial effects, this point remains to be examined in future studies. Fifth, it remains to be examined how long the effects of the LIPUS therapy last in patients with AD. Sixth, for obvious ethical reasons, we were unable to obtain direct evidence for eNOS upregulation in the brain of the patients in the LIPUS group.
In summary, the present study suggests that the whole-brain LIPUS therapy is safe and tends to suppress the cognitive impairment in patients with AD although underpowered due to a small number of subjects. The present findings warrant a next pivotal trial with a large number of patients.
The present study was conducted in part by the grant from the Japan Agency for Medical Research and Development (No. 19lk1403011h0003). We thank Prof. Yasuyuki Taki and Dr. Tatsushi Mutoh, Institute of Development, Aging and Cancer, Tohoku University, Japan, and Koji Ikeda and Yoshimasa Yamazaki, Clinical Research Innovation and Education Center Tohoku University Hospital, Sendai, Japan for cooperation in this study. We also thank Yuko Yamamoto and Hironori Shimosato, EPS Corporation, Tokyo, for statistical analysis. Appreciation is also expressed to all patients and their families who made our work possible.
The authors declare no conflict of interest.