2022 Volume 258 Issue 4 Pages 309-317
2022 Volume 258 Issue 4 Pages 309-317
A previous study confirmed that miRNAs play an important role in the chemosensitivity of seminoma. Increasing evidence reveals that exosomes participate in the regulation of cisplatin resistance by carrying miRNAs. In this study, we further explored whether exosomes regulated the chemosensitivity of seminoma TCam-2 cells to cisplatin. Initially, cisplatin-resistant TCam-2 cells were induced. Our results revealed that exosomes from cisplatin-resistant TCam-2 cells (rExos) could affect the viability of TCam-2 cells in the context of cisplatin treatment through regulation of both cell apoptosis and the cell cycle. Meanwhile, the levels of γ-H2AX were negatively modulated by rExos, which indicated that rExos could decrease the DNA damage from cisplatin. Furthermore, miR-193b-3p was enriched in rExos, and exosomal miR-193b-3p enhanced the proliferative ability of TCam-2 cells under cisplatin treatment. Mechanistically, exosomal miR-193b-3p targets ZBTB7A, which further decreases apoptosis and promotes cell cycle progression. Taken together, these findings indicate that exosomal miR-193b-3p regulates the chemosensitivity of TCam-2 cells to cisplatin through ZBTB7A signaling and could be a promising drug target for patients with chemoresistant seminoma.
Seminoma accounts for approximately 50% of testicular germ cell tumors (TGCTs), and its incidence is increasing worldwide. Fortunately, seminoma is a neoplasm with a high cure rate, especially after the introduction of cisplatin (cis‐diamminedichloroplatinum II, CDDP), a key drug of chemotherapy. Although most patients are highly sensitive to CDDP-based chemotherapy, either refractory disease or relapse after therapy has been reported. Patients in an advanced stage commonly cannot achieve an ideal prognosis and eventually die (Mortensen et al. 2014; Cheng et al. 2018). Resistance to CDDP remains a pivotal cause of death in these patients. Moreover, early aging after cytotoxic treatment for testicular cancer merits our attention (Lubberts et al. 2020). Therefore, approaches to reduce the CDDP dose and increase sensitivity so as to avoid short- and long-term complications are also issues to consider. However, given the largely favorable responses of testicular tumors to chemotherapy, there are very few studies on the mechanisms of chemoresistance, which poses a huge challenge for doctors.
Exosomes, which were discovered in 1983, arise from the membranes of multivesicular bodies (Harding et al. 1983; Pan et al. 1985), with diameters ranging from 50 nm to 150 nm (Zhang and Yu 2019). Exosomes can transport a diversity of molecules, such as proteins, messenger RNAs, and small non-coding RNA to other cells, so they serve a key role in intercellular communications (Wang et al. 2016). According to data from the ExoCarta database, identified exosome contents include 9,769 proteins, 3,408 mRNAs, 2,838 miRNAs and 1,116 lipids (Tang et al. 2021).
Compared to normal cells, cancer cells usually release more exosomes, and cancer-derived exosomes (CDEs) affect the development, metastasis and immunity by altering local and distant microenvironments (Zhang and Yu 2019). Recently, more studies have identified that CDEs were involved in cellular resistance to certain drugs by inducing of epithelial mesenchymal transition (EMT) (Zeng et al. 2017), promoting anti-apoptotic pathways (Fornari et al. 2017), drug efflux or sequestration (Paolillo and Schinelli 2017) and alerting signal transduction (Hu et al. 2019). Among these mechanisms, miRNAs have attracted the most attention, due to their regulatory roles in gene expression (Zhang et al. 2015). miRNAs are short, noncoding RNA molecules that regulate target genes at an epigenetic level. Exosomes are important carriers of miRNAs, which are transported to gain specific functions in different cells. Exosomal miR-105 released from MCF-10A and MDA-MB-231 cells promoted metastases to the lung and brain by reducing ZO-1 gene expression in endothelial cells (Zhou et al. 2014). Exosomal miR-214 from HMEC-1 cells stimulated migration and angiogenesis in neighboring HMEC-1 cells (van Balkom et al. 2013).
In our previous study, we compared the transcriptomes of newly established CDDP-resistant seminoma TCam‐2 cells and their parental origins as to define differentially expressed miRNAs (Wei et al. 2018), and found numerous differentially expressed miRNAs. However, whether exosomes participate in the regulation of CDDP chemosensitivity by carrying miRNAs in seminoma is still largely unknown, and further molecular mechanisms must be unveiled. In this study, we determined the role of exosomal miR-193b-3p in conferring chemosensitivity in seminoma.
The tumor cell line TCam-2 was kindly gifted from Dr. Riko Kitazawa (Department of Diagnostic Pathology, Ehime University Hospital, Toon, Ehime, Japan). Cells were cultured at 37°C with 5% CO2 in complete RPMI 1640 medium (Gibco, Grand Island, CA, USA) with 10% fetal calf serum (Gibco), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Gibco). In our previous study, we established seminoma TCam-2/CDDP cell lines. Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibodies used in this study included antibodies against γ-H2AX (Abcam, Cambridge, UK), CD63 (Proteintech, Wuhan, China), CD9 (Proteintech), FZD8 (Proteintech), ZBTB7A (Abcam), FZD8 (Proteintech), caspase-3 (Abcam), and β-actin (Proteintech).
Isolation and identification of exosomesCells were cultured in exosome-free RPMI 1640 medium. When the cells were at 90% confluence, the culture media was collected and centrifuged at 3,000 × g at room temperature for 15 min followed by straining through a 100-kDa ultrafiltration centrifuge tube. A total of 500 mL of supernatant was centrifuged at 4°C and 17,500 × g for 30 min, and then collected and centrifuged at 4°C and 110,000 × g for 70 min. The precipitate was resuspended in phosphate-buffered saline (PBS) and again centrifuged for 70 min at 4°C and 110,000 × g. Finally, the precipitate was resuspended in PBS followed by filtering with 0.22-mm filters (Millipore, Burlington, MA, USA). The BCA protein quantification kit (Solarbio, Beijing, China) was used to detect the concentration of exosomes. The samples were dropped onto the copper mesh and negatively stained with 2% phosphotungstic acid for 2 min. After natural air drying for 15 min, the samples were observed under transmission electron microscopy (Hitachi, Tokyo, Japan). Exosome particle size was detected using a NanoSight NS300 (Malvern Panalytical, Malvern, UK). The exosome-specific proteins CD63 and CD9 were detected by Western blotting.
Exosomal labeling and uptakeStaining of exosomes was performed using DiD dye (AAT Bioquest, Sunnyvale, CA, USA) according to the manufacturer’s instructions. Then, the labeled exosomes were incubated with the cells for 1 h or 4 h. Next, the cells were fixed and stained with DAPI. The stained cells were examined under confocal microscopy (Leica, Germany) for quantification of exosomal uptake.
Cell viability assayThe cytotoxic effect of CDDP on TCam-2/CDDP cells was measured by a cell counting kit 8 (CCK8) assay (Vazyme, Nanjing, China). TCam-2 cells were seeded into 96-well plates at a density of 5 × 103 cells/well and cultured for 24 h. Then, the cells were treated with cisplatin or Exos + cisplatin for different durations. Thereafter, the culture media were replaced with fresh media containing CCK-8 and mixtures were incubated at 37°C for an additional 1 h. The optical density (OD) at 450 nm was determined with a microplate reader (Bio-Tek, Winooski, VT, USA).
Cell cycle analysisCell cycle analysis was performed using cell cycle assay kit (Solarbio) according to the kit instructions. Briefly, the cells were collected, washed and centrifuged at 1,000 rpm and then fixed with 75% cold ethanol at 4℃ overnight. The cells were washed and centrifuged at 1,000 rpm, treated with RNase A at 37°C for 30 min and then stained with propidium iodide (PI) (Sigma-Aldrich) in the dark for 30 min. The cell cycle was analyzed by a flow cytometer (Beckman Coulter, Brea, CA, USA).
Cell apoptosis analysisApoptosis was measured by an Annexin V-FITC/PI apoptosis detection staining kit (Beyotime, Shanghai, China). Briefly, after treatment with cisplatin for 72 h, cells were collected, washed with PBS, and labeled with Annexin V and PI in the dark. Cell apoptosis was subsequently analyzed by flow cytometry (Beckman Coulter).
Immunofluorescence detection of γ-H2AXThe level of γ-H2AX in TCam-2 cells was detected by immunofluorescence to reflect the degree of DNA damage. TCam-2 cells with different treatments were cultured on coverslips and fixed in 4% paraformaldehyde for 30 min at room temperature. After washing with PBS, the cells were permeabilized with 0.1% Triton X-100 (Solarbio) in PBS at room temperature for 20 min and then blocked with 5% BSA (Beyotime) for 1 h followed by incubation with primary antibodies against γ-H2AX at 4°C overnight. On the next day, the cells were incubated with fluorescently labeled secondary Abs. Nuclei were stained with DAPI (Beyotime). Following staining, the stained cells were examined under a fluorescence microscope (OLYMPUS, Tokyo, Japan).
RNA extraction and quantitative reverse transcription-PCR (RT-qPCR)For quantitative real‐time PCR (qPCR), total RNA was extracted using an RNA kit (Omega Bio-Tek, Norcross, GE, USA) according to the manufacturer’s instructions. The procedures for these assays can be found as previously reported (Wei et al. 2018). Reverse transcription was performed using a HiScript II 1st strand cDNA synthesis kit (Vazyme). qRT-PCR was performed on a step-one plus real-time PCR system (Applied Biosystems, Waltham, MA, USA) using ChamQ Universal SYBR qPCR Master Mix (Vazyme). All primers were synthesized by RiboBio Co., Ltd. (Guangzhou, China). Primer sequences are listed in the Table 1. U6 small nuclear RNA and β-actin were used as internal controls. The relative levels of RNAs were calculated using the 2-ΔΔCt method.
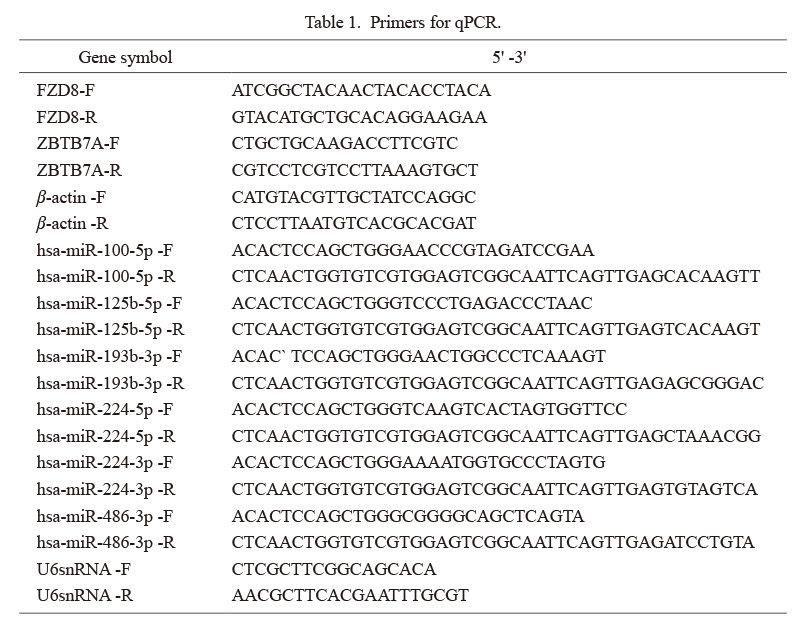
Primers for qPCR.
F, forward; R, reverse.
Cell transfection was performed as previously described (Wei et al. 2018). miR-193b-3p mimics, miR-193b-3p inhibitor and the negative control were purchased from RiboBio Co., Ltd. to investigate the effects of miR-193b-3p on seminoma. The mimics and inhibitor were transfected with Lipofectamine 3000 reagent (ThermoFisher, Waltham, MA, USA) when the cells reached 70% confluence. The lentivirus for ZBTB7A overexpression was obtained from the Genechem (Shanghai, China).
Western blottingCells were lysed in a lysis buffer containing protease inhibitors (Solarbio) for 30 min. The protein concentration of lysates was determined by the BCA kit. An equivalent amount of protein (30 μg) from each sample was separated by 10% SDS‐PAGE and then transferred to PVDF membranes (Millipore, Boston, MA, USA). The membranes were blocked with 5% nonfat dry milk in 0.2% Tween-20 in Tris-buffered saline for 1 h at room temperature and then probed with primary antibodies. After incubated with the secondary antibody, immunoreactivity was detected by the enhanced chemiluminescence method (Affinity Biosciences, Changzhou, China).
Statistical analysisStatistical analysis of the results was performed using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). The quantitative data are expressed as the mean ± standard deviation (mean ± SD) of 3 or more independent experiments. The unpaired, two-tailed Student’s t test was used to compare group means. A value of P < 0.05 was considered to indicate a statistically significant difference.
Exosomes isolated from TCam-2 and TCam-2/CDDP cells, referred to sExos and rExos respectively, were positive for CD63 and CD9 by Western blotting (Fig. 1A). The results showed that the levels of both CD63 and CD9 were not significantly different between the two groups. Furthermore, we extracted exosomes from TCam-2 and TCam-2/CDDP cells and assessed their morphological characteristics using transmission electron microscopy (TEM) (Fig. 1B). The size of exosomes measured between 30 and 200 nm (Fig. 1C). To confirm the internalization of exosomes, we cultured the TCam-2 with media containing the rExos (labeled with DiD) and used confocal microscopy to detect these rExos into the TCam-2 cells. As shown in Fig. 1D, the fluorescence intensity increased with time. However, GW4869, an exosome secretion inhibitor, significantly weakened the fluorescence. The aforementioned results indicate that both TCam-2 and TCam-2/CDDP cells can secrete exosomes, and that rExos can be internalized by TCam-2 cells.
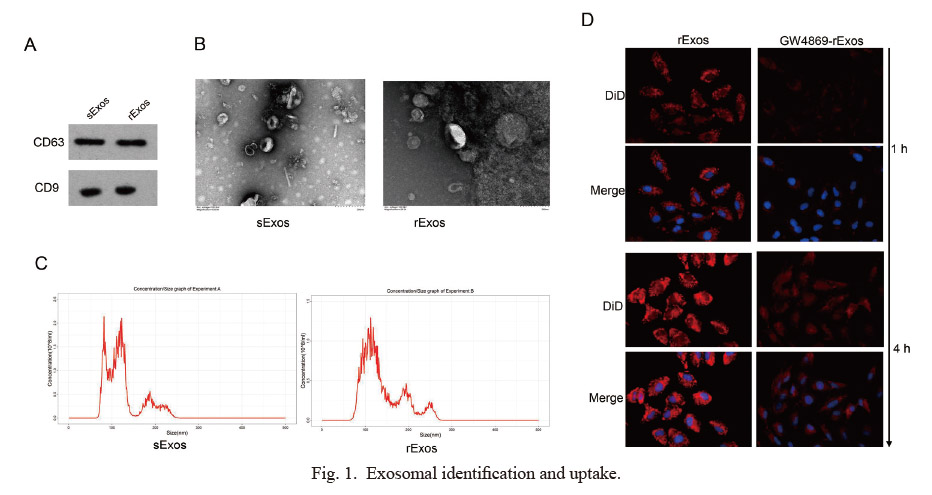
Exosomal identification and uptake.
(A) Exosomal-positive markers CD63 and CD9 were detected in TCam-2-derived and TCam-2/CDDP-derived exosomes. (B) TEM images of exosomes derived from TCam-2 and TCam-2/CDDP cells. (C) The size distribution of the isolated exosomes was measured. (D) Confocal microscopy revealed exosomal internalization by TCam-2 cells after incubation with DiD-labeled (red fluorescence) rExos treated with or without GW4869. DAPI was used to stain the nuclei of TCam-2 cells with blue fluorescence.
In the presence of cisplatin, we tested the cell viability of TCam-2-sensitive cells cultured with rExos and rExos with GW4869. Initially, it was clear that TCam-2 cells gained resistance to cisplatin when cultured with rExos. However, TCam-2 cells were also sensitive to cisplatin when TCam-2/CDDP was incubated with GW4869, indicating a potential transfer of resistance from resistant TCam-2 to susceptible TCam-2 through the exosomes (Fig. 2A). Additionally, in contrast to the control treatment, rExos attenuated the antitumor effect of CDDP in TCam‐2 cells. The results from cell cycle assays and cell apoptosis assays demonstrated that rExos promoted the cell cycle progression (Fig. 2B) and decreased apoptosis (Fig. 2C) under drug treatment condition. In contrast, rExos derived from TCam‐2/CDDP cells treated with the exosome inhibitor GW4869 inhibited cell cycle progression and enhanced apoptosis. Finally, cell cycle progression and apoptosis are tightly correlated with DNA damage. From this point of view, we further examined the DNA damage status of TCam-2 cells in the CDDP treatment context after rExos incubation and found that DNA damage was attenuated under rExos condition (Fig. 2D). These data together demonstrate that rExos enhances the cisplatin tolerance of TCam-2 cells.

The CDDP tolerance in TCam-2 cells was increased by rExos.
(A) Cell viability was measured by CCK8 assay at 0, 24, 48, and 72 h after treatment. Both the distribution of the cell cycle (G0/G1, S, and G2/M phases) (B) and the quantity of apoptotic cells (C) were analyzed by FCM assays at 48 h after drug treatment. (D) DNA damage was detected by γ-H2AX immunofluorescence staining. **P < 0.01, *** P < 0.001.
NC, control.
To uncover the expression profiles of miRNAs in CDDP‐resistant seminoma, a comparative analysis of the expression profiles of miRNAs between TCam‐2/CDDP cells and their parental origins was performed by microarray (Wei et al. 2018). As shown in Fig. 3A, the miRNA expression profiles in the drug-resistant cells were significantly changed. miR-148a-3p, miR-486-3p, miR-125b-5p, miR-193b-3p, miR-374a-5p, miR-224-3p, miR-99a-5p, miR-100-5p, miR-619-5p, miR-224-5p, and miR-22-5p were obviously upregulated. To confirm whether differentially expressed miRNAs affect cellular chemosensitivity through exosomes, we tested the expression of the miRNAs miR-100-5p, miR-125b-5p, miR-193b-3p, miR-224-3p, miR-224-5p, and miR-486-3p and observed that miR-193b-3p was significantly increased in rExos compared with sExo. Moreover, GW4869 markedly lowered the level of miR-193b-3p in rExos (Fig. 3B). Next, we inhibited miR-193b-3p in TCam-2/CDDP cells and confirmed its efficacy in rExos using qRT-PCR. The results showed that inhibition of miR-193b-3p significantly decreased the level of miR-193b-3p levels relative to the exosomal levels; that is, at a constant microgram levels of exosomes, miR-193b-3p levels were significantly decreased (Fig. 3C). To further clarify the effect of exosomes highly expressing miR93b-3p on chemosensitivity, we tested the cell viability of TCam-2-sensitive cells cultured with exosomes from TCam-2/CDDP (rExos) and TCam-2/CDDP with miR193b-3p inhibitors (193b-3p-down rExos) in the presence of CDDP for 48 h. Initially, it was clear that TCam-2 cells gained resistance to cisplatin when cultured with rExos. However, CDDP could significantly decrease the viability of cells treated with 193b-3p-down rExos compared with rExos. These data indicated that downregulation of miR-193b-3p could significantly decrease the effect of rExos in conferring resistance to cisplatin treatment (Fig. 3D). The results from cell cycle and cell apoptosis assays demonstrated that knockdown of miR-193b-3p in rExos inhibited the cell cycle progression (Fig. 3E) and enhanced apoptosis (Fig. 3F). DNA damage status was also detected, and inhibition of miR-193b-3p led to an increased DNA damage rate (Fig. 3G). These data provide strong evidence that miR-193b-3p carried by exosomes plays a vital role in conferring cisplatin resistance.

miR-193b-3p in rExos was correlated with cisplatin resistance of TCam-2.
(A) Heatmap of the miRNAs in sExos (A1, A2, A3) and rExos (B1, B2, B3). Rows correspond to genes and columns correspond to samples. Red and blue colors indicate different extents of high or low expression. (B) Relative expression levels of miRNA-100-5p, miRNA-125b-5p, miRNA-193b-3p, miRNA-224-3p, miRNA-224-5p and miRNA-486-3p in exosomes derived from TCam-2, TCam-2/CDDP and TCam-2/CDDP+GW4869 cells were measured by qPCR. (C) Relative expressions of miR-193b-3p in the indicated groups. (D) Relative cell viability of TCam-2 cells in different groups with or without cisplatin treatment for 48 h. Both the distribution of cell cycle (E) and the quantity of apoptotic cells (F) were analyzed by FCM assays at 48 h after drug treatment. (G) DNA damage was detected by γ-H2AX immunofluorescence staining. * P < 0.05, **P < 0.01, ***P < 0.001.
ns, not significant; NC, control.
Using prediction mapping, we further identified that miR-193b-3p potentially binds and affects the expression of ZBTB7A and FZD8 (Fig. 4A). We assessed the mRNA and protein levels of the two genes and observed that the expression of ZBTB7A was significantly increased in the presence of GW4869, while FZD8 did not change significantly (Fig. 4B, C). Thereafter, we further detected the expression of ZBTB7A in the presence of miR-193b-3p inhibitors at both the mRNA and protein levels. However, the expression of ZBTB7A was significantly increased in the miR-193b-3p inhibitor group (Fig. 4D, E). These results clearly confirmed that exosome-derived miR-193b-3p downregulated the expression of ZBTB7A in TCam-2 cells.
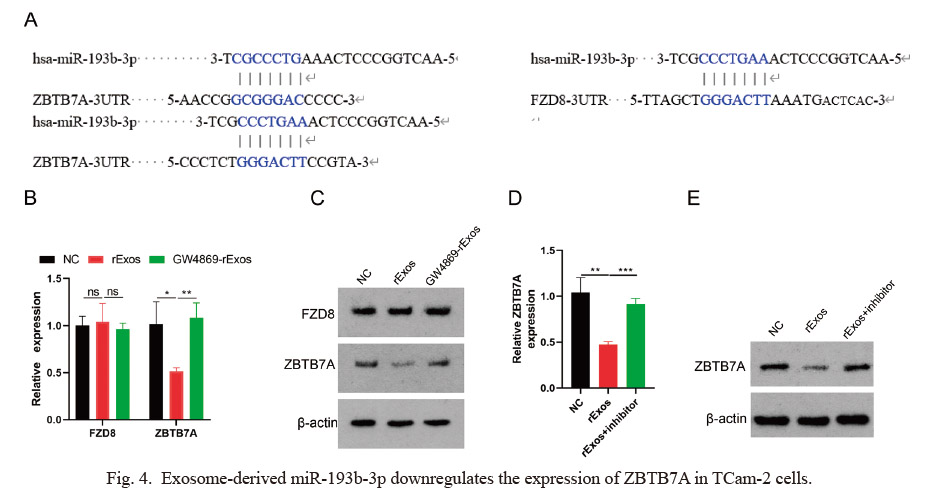
Exosome-derived miR-193b-3p downregulates the expression of ZBTB7A in TCam-2 cells.
(A) A schematic diagram of the predicted binding region of miR-193b-3p and its target genes ZBTB7A and FZD8. Relative expression of FZD8 and ZBTB7A in TCam-2 cells incubated with rExos, or GW4869 + rExos at the mRNA (B) and protein (C) levels. miR‐193b‐3p was inhibited, and the levels of ZBTB7A mRNA (D) and protein (E) were detected. *P < 0.05, **P < 0.01, ***P < 0.001.
ns, not significant; NC, control.
We used TCam-2 cells with or without rExos to assess the mechanistic influence of miR-193b-3p on ZBTB7A; subsequently, these cells were also overexpressed ZBTB7A. Initially, we assessed the viability of these cells and observed that rExos significantly increased the viability of TCam-2 cells. Interestingly, overexpression (OE) of ZBTB7A decreased the viability of these cells after CDDP treatment (Fig. 5A, B). Similarly, the cell cycle and apoptosis were assessed. As shown in Fig. 5C and D, cell cycle progression was inhibited, and the apoptosis rate was significantly higher in the rExos+ZBTB7A-OE group, and lower in the rExos group. Meanwhile, apoptosis-related protein cleaved-caspase 3 was much higher than that in the rExos group after ZBTB7A was upregulated (Fig. 5E). At the same time, DNA damage status was also detected, and overexpression of ZBTB7A led to an increased immunofluorescence of γ-H2AX. However, this phenotype could be partly reversed by miR-193b-3p mimics (Fig. 5F). These findings support the hypothesis that miR-193b-3p increases cisplatin resistance by regulating ZBTB7A.

Exosome-derived miR-193b-3p targeted ZBTB7A to regulate CDDP resistance.
(A) Levels of ZBTB7A in TCam-2 cells after transfection with ZBTB7A overexpression (OE) lentivirus were detected by qPCR. (B) Relative cell viability of TCam-2 cells in different groups with or without cisplatin treatment for 48 h. Both the distribution of cell cycle (C) and the quantity of apoptotic cells (D) were analyzed by FCM assays 48 h after drug treatment. (E) Levels of ZBTB7A and the apoptotic protein cleaved-caspase 3 in TCam-2 cells after treatment with different exosomes with cisplatin for 48 h were detected by Western blotting. (F) DNA damage was detected by γ-H2AX immunofluorescence staining. *P < 0.05, **P < 0.01, ***P < 0.001.
NC, control.
Chemoresistance is a major problem in tumor treatment. Platinum-based chemotherapy is an important treatment method for TGCTs. However, the heterogeneity of tumors contributes to CDDP resistance in some relapsed cases of seminoma, for which outcomes are dismal. Mechanisms of CDDP resistance are poorly investigated in seminoma, which results in a lack of tailored therapy for cisplatin-resistant seminoma.
Exosomes are involved in the transportation of miRNA, mRNA and proteins from one cell to another cells and serve an important role in cell-to-cell communication, including chemoresistance (Kulkarni et al. 2019). It was found that the secretion of exosomes is drastically amplified for all cells in the tumor microenvironment. Exosomes carry miRNA and mRNA, protecting them from enzyme ribonuclease in the tumor microenvironment. Therefore, exosomes represent important mediators of intercellular communication. In recent years, the importance of exosomes for chemoresistance has gradually been elucidated. Mao et al. (2016) reported that in breast cancer, adriamycin resistance was acquired via exosomal miRNA (miR-23a, miR-24 and miR-222) by targeting of p27, and phosphatase and tensin homolog (PTEN) expression. And Kulkarni et al. (2019) discovered that exosomes-encapsulated miRNAs exacerbate tumor chemoresistance by promoting tumor stem cell self-renewal and inducing autophagy. Other researchers found that exosomal transfer of miR-21 from cancer-associated fibroblasts (CAFs) to normal ovarian cells activates the chemoresistant phenotype through apoptotic protease activating factor 1 in ovarian carcinoma (Au Yeung et al. 2016). Our study also confirmed that exosomes are involved in the regulation of chemosensitivity in seminoma. rExos significantly reduced cisplatin-induced apoptosis of parental TCam-2 cells. However, more evidence is still needed to uncover the mechanism.
Epigenetic modifications, such as miRNA regulation, may serve important roles in tumoral resistance to chemotherapy (Dong et al. 2021). A previous comparative analysis of the expression of miRNAs between CDDP-resistant TCam‐2 cells and parental origins indicated significant change in the miRNA profile, and further studies validated that the H19/miRNA‐106b‐5p/TDRG1 axis was involved in homeostatic seminoma as well as in a CDDP‐resistant context (Wei et al. 2018). In this study, we observed that miR-193b-3p, an unexploited miRNA among the differentially expressed miRNA profiles, can also affect cellular chemosensitivity through exosomes. miR‐193b is located on chromosome 16 (chr16: 14303967-14304049), and the pre‐miR‐193b generates two mature miRNAs consisting of miR‐193b‐3p and miR‐193b‐5p (Khordadmehr et al. 2019). The role of miR-193b-3p in tumorigenesis remains controversial. On the one hand, miR‐193 acts as a tumor suppressor by targeting different genes that contribute to cancer cell proliferation, invasion, migration and metastasis (Khordadmehr et al. 2019). On the other hand, researchers recently found that miR-193b-3p also functioned as an oncomiRNA, which inhibits the translation of the oncosuppressive methyltransferase SUV39H1 and promotes tumor cell proliferation (Dinami et al. 2022). The expression of miR-193b-3p was associated with cisplatin sensitization by regulating the DNA binding ability of CEBPD in the CDDP response (Lin et al. 2017). In this study, exosomal miR-193b-3p was found to be highly upregulated in TCam-2/CDDP cells, and a reduction in miR193b-3p in rExos increased the apoptosis rate caused by CDDP. This suggests that rExos induce cisplatin resistance partly through upregulation of miR-193b-3p.
We also found that miR-193b-3p negatively regulates the mRNA level of ZBTB7A. The role of ZBTB7A varies among tumors, and it can act as an oncogene and tumor suppressor gene. Likewise, it also has dual effects of anti-apoptosis and pro-apoptosis effects. ZBTB7A silencing potently induces the p53 pathway and the subsequent intrinsic and extrinsic apoptotic signaling pathways (Constantinou et al. 2019). Negatively, ZBTB7A overexpression promoted cell apoptosis in hepatocellular carcinoma (Liang et al. 2018). ZBTB7A transactivates TRAIL-R2, which sensitizes cells to cisplatin-induced apoptosis (Yeh et al. 2020). Our findings support the hypothesis that low levels of ZBTB7A increase cisplatin resistance in seminoma via the exosomal miR-193b-3p/ZBTB7A axis.
In general, we expanded our knowledge of the exosomal miR-193b-3p/ZBTB7A axis in the CDDP‐resistant communication between seminoma cells. Exosomes were found to act as a vesicular cargo to carry miR-193b-3p into adjacent cells, and inhibited the ZBTB7A to promote CDDP resistance. Interrupting this pathway may provide new targets for CDDP-resistant seminoma.
We thank Dr. Riko Kitazawa (Ehime University Hospital, Toon, Ehime, Japan) for kindly providing TCam-2 cells. This work was supported by Hunan Natural Science Foundation (Grant numbers: 2019JJ80008). Thanks to AJE for providing professional English editing service.
Y.W., Q.Z. and Y.X.D. conceived the experiments. Y.W., and J.J.L. performed the experiments. S.H. contributed to the materials. Y.W. and Q.G. developed the image processing algorithms. Y.W., X.G. and W.G.R. performed analysis and interpretation of the data. Y.X.D. supervised the whole process. Y.W. and Y.X.D. wrote the manuscript. All authors read and approved the final manuscript.
The authors declare no conflict of interest.