2022 Volume 258 Issue 4 Pages 243-255
2022 Volume 258 Issue 4 Pages 243-255
Human Monkeypox (HMPX) outbreak in the year 2022 occurs in many countries outside of the African regions, a common location of such outbreaks, with a considerable rate of human-to-human transmission, which was an uncommon route of infection before. The epidemiological reports also represent a sharping pace of infection spreading between communities rather than in previous outbreaks as the following pace of afflictions is unpredictable. Also, the cautions regarding the sexually transmitted infection of the such virus have been raised in this outbreak. Further, the main reservoirs of the recent outbreaks are yet to be revealed. As a consequence, the World Health Organization (WHO) has declared the 2022 HMPX outbreak as an “Atypical” phenomenon compared to its previous characteristics. To better recognize the properties of this outbreak, herein we systematically described and compared the historical evidence of monkeypox virus outbreaks in the aspects of epidemiological, clinical, and molecular evolutions since its emergence, as well as an explanation of the previous investigations and considerations of WHO and other international health societies over time. The history of human and monkeypox virus interaction during the past 64 years provides viewpoints on preventing strategies and assessing the present and potential future hazards of health implications.
The Monkeypox virus (MPXV), a zoonotic orthopoxvirus DNA-virus associated with the smallpox virus, was firstly identified in humans in 1970 in the Democratic Republic of Congo (DRC) (Chastel 2009). Africa has had sporadic infection outbreaks, which are mainly brought on by contact with animal reservoirs (Yao 2022). Due to the minimal secondary spread of such epidemics and travel-associated cases out of Africa, the between-humans transmission was labeled as ineffective (Quarleri et al. 2022). Monkeypox research has been ignored and underfunded despite the fact that the MPXV has been circulating for years in areas where it has historically been prevalent (Kaler et al. 2022). Meanwhile, due to recent outbreaks of disease, the World Health Organization (WHO), on June 23, 2022, classified Monkeypox as an “emerging threat of moderate public health concern” as a result of more than 3,000 instances of MPXV infection that had been documented in more than 50 countries across five regions since early May 2022. Large respiratory droplets, intimate contact with skin lesions, and potentially infected fomites are all routes by which the MPXV is spread (Vaughan et al. 2020). Additionally, there are also recent data that suggests sexual transmission (Vaughan et al. 2020; Heskin et al. 2022). There have also been reports of vertical transmission and fetal deaths (Heskin et al. 2022). In general, endemic monkeypox resolves on its own, with case fatality rates ranging from 1-10%, depending on the lineage. The majority of the time, the clinical symptoms start with fever and progress to include noticeable lymphadenopathy and many widespread, ulcerative, and vesiculopustular lesions on the face and body (Saied et al. 2022). Keratitis, encephalitis, pneumonitis, and subsequent bacterial infections are among the complications (Saied et al. 2022). According to reports, the danger of serious consequences is higher in young children and immunocompromised adults, such as those who have HIV infection (de Sousa et al. 2022). The current worldwide MPXV infection outbreak in humans raises the possibility that the virus may have changed biologically, that human behavior may have changed or both. These alterations may be brought on by declining smallpox immunity, dwindling COVID-19 preventive strategies, sexual interactions, and the restart of international travels (Zhu et al. 2022). According to phylogenetic analysis, the virus has been silently circulating outside of its endemic regions for some time. In fact, it is possible that the virus has been passing itself off as several sexually transmitted diseases (Adalja and Inglesby 2022). The existing worldwide case definition could not be sufficient to capture the constantly evolving range of clinical manifestations; as a result, it might not enable early detection, explain means of transmission, and guide global public health strategies and clinical studies. The present article sought to lay out a thorough review of the emergence and historical epidemiology of the MPXV to elucidate the atypical occurrence of the 2022 outbreak and to determine plausible preventive and management strategies based on previous evidence.
Since the authors aimed to gather all epidemiologic data regarding MPXV epidemics, this study was based on a systematic review that followed international criteria for carrying out systematic review articles, such as guidelines of the Cochrane Collaboration (Korfitsen et al. 2022). Comprehensive searches were performed with no language restrictions in 4 major databases, including PubMed, EMBASE, the Internet Library sub-Saharan Africa (ilissAfrica), African Journals Online (AJOL), and MEDLINE (accessed using PubMed). All relevant published literature recorded until July 30, 2022, as the last search date, has been designated for eligibility. In Embase explorations consisted of ‘monkey pox’: ti,ab OR monkeypox: ti,ab OR ‘monkeypox virus’/exp OR ‘monkeypox’/exp. On PubMed, explorations were comprised of “Medical Subject Headings (MeSH)” keywords of Monkeypox virus [MeSH] OR Monkeypox [MeSH] as well as “abstract and title” search string using “variole simienne” OR “variole du singe” OR “monkey pox” OR “monkeypox.” We also looked up the illissAfrica and AJOL separately for the mentioned words: variole simienne, variole du singe, and monkeypox. Furthermore, seven other search databases for further relevant information were investigated. These sources included the WHO website, the Centers for Disease Control and Prevention (CDC) in the United States of America, African Field Epidemiology Network, Nigeria CDC and Africa CDC, along with Pro-Med and Epicentre. Furthermore, a Google search on the African nations identified with having monkeypox patients was conducted, as well as a brief search on the webpages of the associated health ministries. Because no formal exploration technique has been used, we could not specify a denominator for the number of studies. One research investigator (L.M.) analysed the grey literature, while another research investigator (E.M.B.) assessed the results and added pertinent data to the information sheets. The goal of this study was to look at how MPXV epidemiology has changed in terms of fatality rate, transmission, patients’ characteristics, and incidence. Additionally, we wanted to look at the risk factors suggested for obtaining human MPXV. After identifying all studies from the four data sources and removing duplicate files, three researchers did a duplicate screening on the abstract and title (S.A., F.E.T., and F.S.R.). For full-text screening, articles with relevant data for the review aims were chosen. Non-human research, modeling research without original information, papers focusing solely on other orthopoxvirus members, and reports containing data extraneous to the themes of interest were also excluded. When in doubt, the articles were chosen for the full-text check. The full-text papers have been then assessed to see if one of the study objectives had at least been accomplished. At this point, other works, including conference abstracts, were removed. Each study was then checked again during the process of extracting data. This step resulted in some extra exclusions. For example, in studies with similarly formed conclusions from data sets that were virtually alike, just one study was included (usually the more recent). In certain circumstances, there has been some data overlap, so several articles covered the same examples as well as some unique cases. In these circumstances, the data extraction sheet only contained unusual examples from each article. The information extraction sheets for the qualifying publications were generated by one researcher (F.E.T.) and it was evaluated by a second researcher (S.A.). Surveillance research, epidemiological articles, epidemic studies, and case reports were among the articles acceptable for use from the search series conducted on the literature. Because no systematic checklists for critical appraisal were available for these sorts of papers, no official quality assessments were done. The process of methodology has been portrayed in Fig. 1.
Moreover, it is worth mentioning that in order to achieve a better grasp of the viral spread, we decided to illustrate the global distribution of the confirmed Monkeypox cases based on the data provided by the CDC, using the Illustrator software (CDC 2022). The results of our attempts are visualized in Fig. 2.
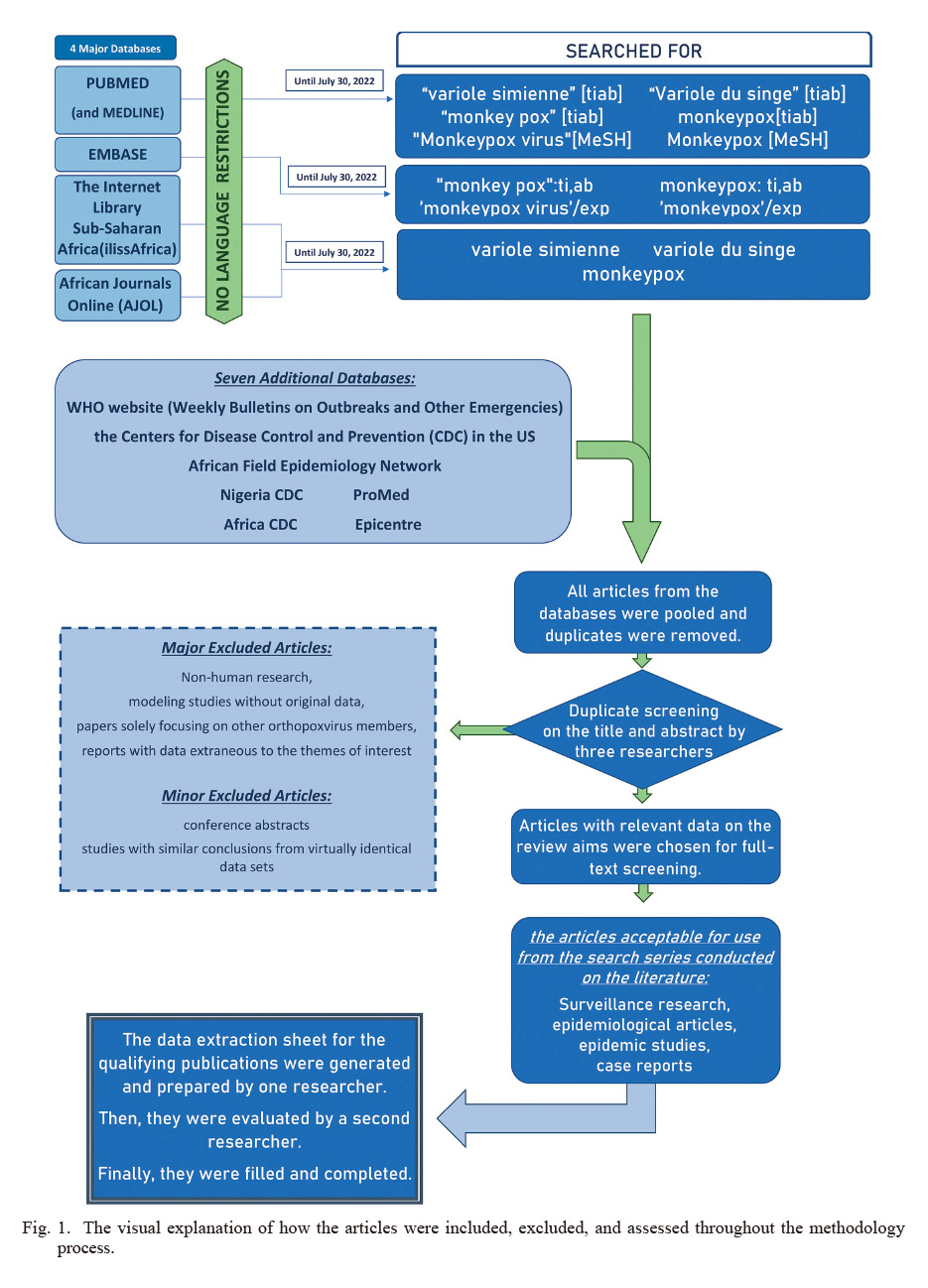
The visual explanation of how the articles were included, excluded, and assessed throughout the methodology process.
ilissAfrica, Internet Library of Sub-Saharan Africa; AJOL, African Journals Online; MeSH, Medical Subject Headings; CDC, Center for Disease Control and Prevention.
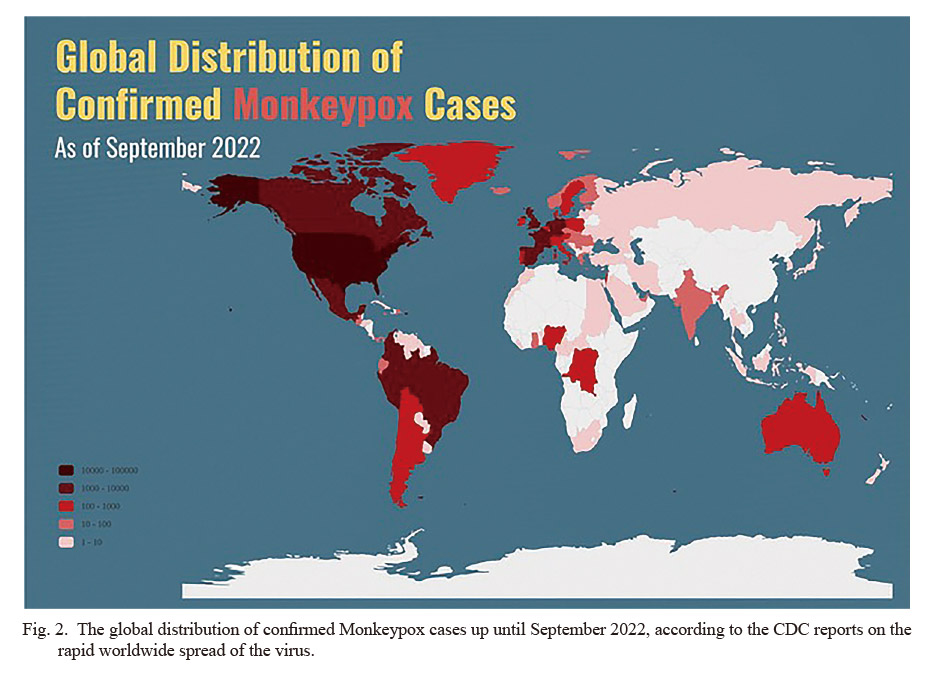
The global distribution of confirmed Monkeypox cases up until September 2022, according to the CDC reports on the rapid worldwide spread of the virus.
Historical studies on the evolution of the orthopoxviruses genome showed that the orthopoxviruses as a Poxviridae family diverged 40,000 years ago. In fact, as depicted in Fig. 3, the orthopoxviruses existing in modern life originated over 4,500 years ago (Babkin et al. 2022; Bunge et al. 2022). Meanwhile, research on the history of molecular evolution suggests that MPXV, a relatively recent member of orthopoxviruses, evolved into the West African branch about 500 to 1,000 years ago, almost 3,500 years after initial orthopoxviruses occurrence (Vora et al. 2015; Babkin et al. 2022). Of note, the orthopoxviruses genus is the most studied poxviruses, and two of its members have played essential roles in the history of human disease and preventive medicine: 1. First, the variola virus (VARV), which caused a unique systemic illness in humans called smallpox and is now eradicated in the nature; and 2. Second, the Vaccinia virus (VACV) is used to prepare vaccines and plays a vital role in eradicating smallpox. It is also utilized to prevent or mitigate smallpox in the early days of contact with the cowpox virus (CPXV).
Regarding the recognition of MPXV, von Manguns et al. (1959) recorded two epidemics of non-fatal pox-like diseases amongst cynomolgus macaque monkeys transported from Singapore to Denmark in 1958. This isolated and cultured virus which had different molecular characteristics from other known Orthopoxviruses was named MPXV (Kaynarcalidan et al. 2021; Diaz-Cánova et al. 2022). Controversially, MPXV and VARV were often considered close relatives, although comparative genomics has shown that MPXV was a unique virulent species derived from another orthopoxvirus ancestor. As a matter of fact, the terminal region of the MPXV genome encodes sequences related to virulence and host range, which are pretty distinct from other orthopoxviruses. Further, MPXV cannot be easily derived into VARV, so there is no direct relationship between MPXV and VARV (Reeves et al. 2011; Kaynarcalidan et al. 2021; Bunge et al. 2022).
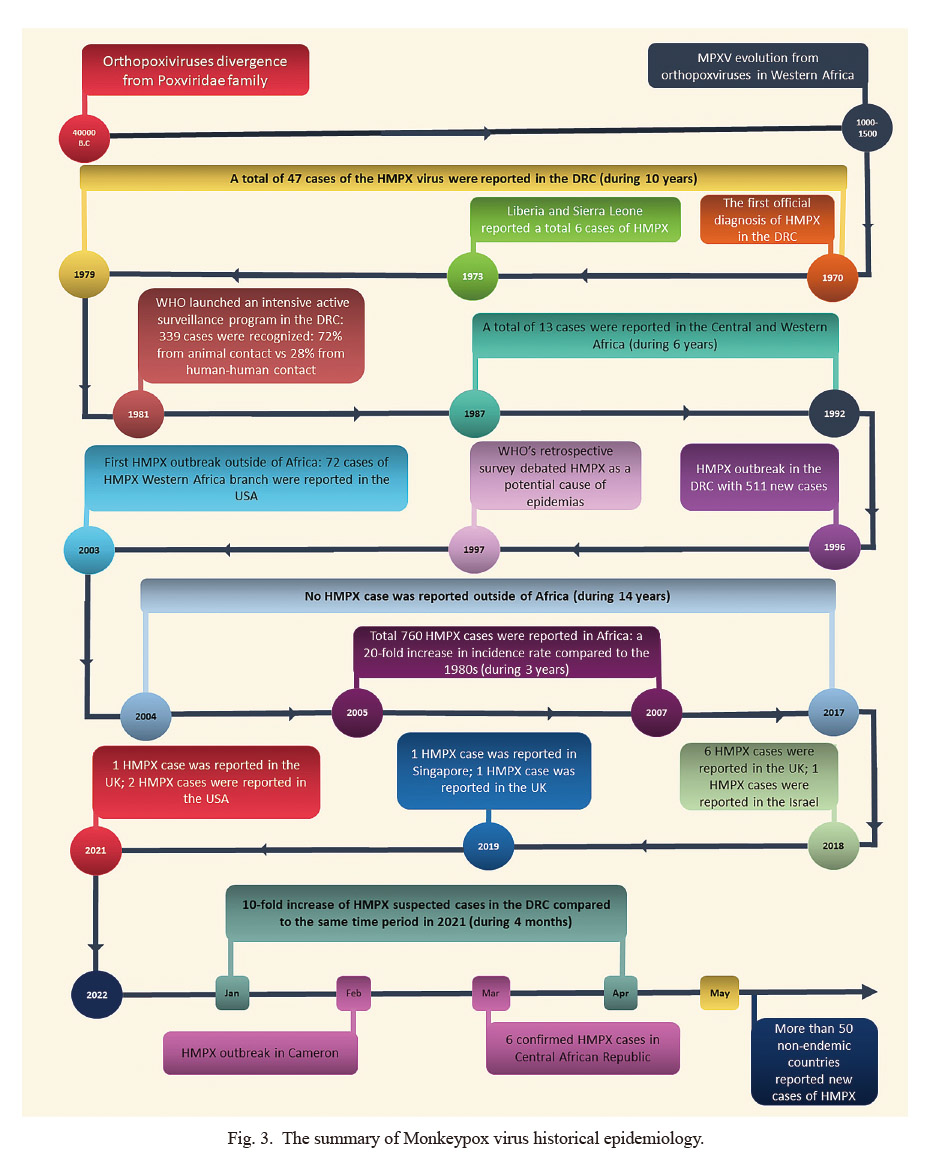
The summary of Monkeypox virus historical epidemiology.
MPXV, Monkeypox Virus; HMPX, Human Monkeypox Virus; DRC, Democratic Republic of Congo.
MPXV was initially isolated from monkeys and was named accordingly. However, the reservoir and accidental hosts in its natural ecology are yet to be fully elucidated. Studies have shown that a variety of animals play parts in maintaining MPXV’s virulence and infectivity. In fact, its cycle of life is a complicated process of interaction between incidental species and reservoir hosts (Takemura 2001). Notably, the broad range of hosts for MPXV has attracted widespread attention because this feature may facilitate unwelcome adaption to new hosts and territories (Nolen et al. 2015). In the 1980s, a serological survey conducted by WHO in the DRC showed that MPXV infection in monkeys was sporadic as in humans, and MPXV had a wide range of animal hosts (Tesh et al. 2004). For instance, 39-50% of West African squirrels and 50% of red-legged sun squirrels were positive for MPXV-specific antibodies in serum, and the virus could also be isolated from sick mice (Kaler et al. 2022). Moreover, in the 2003 Human Monkeypox (HMPX) outbreak in the United States, the investigation found that MPXV originated from several African rodents imported from Ghana (Alakunle et al. 2020). Other studies indicated the infected hosts of MPXV within in-vitro settings included cynomolgus monkeys, prairie dogs, and ground squirrels (Fenner 2012), in which all ground squirrels infected with MPXV showed fulminant disease and died 6 to 9 days later (Jahrling et al. 2007). Furthermore, in the first decade after MPXV discovery, nine outbreaks of animal monkeypox were observed in non-human primates in the United States, the Netherlands, France, and some Asian countries, e.g., Malaysia (Likos et al. 2005; Sadeuh-Mba et al. 2019). The MPXV-infected animals’ clinical severity varied among primate subjects, ranging from asymptomatic cases to a self-limiting mild disease with generalized rashes and even to death during the acute viremia phase before the onset of any clinical symptoms (Bunge et al. 2022). However, at the time, there has been no early incidence of infection among humans while handling monkeypox in such species (Likos et al. 2005).
MPXV strainsShortly after the isolation and identification of MPXV, two distinct strains were identified in animal monkeypox outbreak studies; one with biological characteristics easily distinguishable from VARV and CPXV; and the other with several features arduously distinguished from VARV (Likos et al. 2005). Further, genome-wide analysis on single-gene phylogeny and restriction fragment length polymorphism have demonstrated that MPXV exists in two regional clades, West Africa and Congo Basin (West African and Central African) (Likos et al. 2005). This was subsequently confirmed by whole-genome sequencing (Likos et al. 2005; Sadeuh-Mba et al. 2019). MPXV evokes both innate and adaptive immune responses as MPXV cases elucidate an Orthopoxvirus-specific immunoglobulin M (IgM), IgG, CD8+ and CD4+ T-cells, B-cell responses, and cytokine production. Sufficient IgG levels seem to diminish the severity of MPXV infection, emphasizing the importance of memory humoral immune response against MPXV (Karem et al. 2007). The complement system is a crucial member of the innate immune system as it facilitates neutralization and phagocytosis of pathogens and boosts the adaptive immune response against them (Agrawal et al. 2020). Identifying two distinct branches of MPXV has helped to understand the pathological differences in monkeypox. One of the immunogenicity differences between the two strains was the functionality level defined for regulators of complement activity; the West African complement control protein (CCP) does not function. CCP inhibits both classical and alternative complement activation routes and blocks virus neutralization mediated by complements. Therefore, compared to the Central African clade MPXV, the CCP-deleted West African clade MPXV is less virulent to cynomolgus monkeys. Moreover, it has been demonstrated that Central African MPXV hampers receptor-mediated activation of T-cells, possibly through downregulating the production of histocompatibility complex class I (MHC I) in the afflicted cellular structures, resulting in a weakened immune response (Nolen et al. 2015). Besides, epidemiological analysis of HMPX also supports this difference in toxicity. The case fatality rate of West African HMPX is usually < 1%, while the case fatality rate of Central African HMPX is often > 10% (Adalja and Inglesby 2022). Accordingly, a recent systematic review analysis also showed a pooled HMPX case fatality rate of 3.6% and 10.6% in West and Central Africa, respectively (Likos et al. 2005).
The disease caused by HMPX compared to mild smallpox was difficult to distinguish clinically, so it was not recognized whether HMPX was another disease until the smallpox epidemic effectively ceased (Heskin et al. 2022). In 1970, a 9-month-old boy with a primary diagnosis of smallpox appeared in an area free of smallpox cases for two years in the DRC and was later diagnosed with MPXV infection. This was the very first confirmed case of HMPX in history (Breman et al. 1980). As Cho and Wenner (1973) summarized in 1973, DRC, Liberia, and Sierra Leone reported a total of 6 cases of HMPX (including the first case), aged 9 months to 9 years old; who were all first misdiagnosed as “smallpox.” Four of them were classmates, and two others had close contact with monkeys. All six children survived, and none of the family members became afflicted. Breman et al. (1980) reported that between 1970 and 1979, a total of 47 cases of HMPX were recorded in certain villages of DRC, mostly diagnosed during the warm seasons and hunting locations. Four cases were reported to belong to human-to-human transmission, and the secondary incidence rate of all susceptible contacts was estimated at 3%. Moreover, another piece of the literature showed that by 1980, a total of 59 HMPX cases were reported from 6 African countries (Heymann et al. 1998). In fact, secondary cases of human-to-human transmission raised the question of whether HMPV may replace smallpox in unimmunized populations. Since the emergence of HMPX shortly after the disappearance of smallpox cases, a serological survey in Sierra Leone and DRC was conducted on 10,300 children in November 1981 (Arita et al. 1972; Bunge et al. 2022). This study found that the average positive rate of orthopoxviruses and MPXV-specific antibodies was about 15.4% and 0.7%, respectively. 30% of MPXV-specific antibody-positive cases had no medical history and not even acne scars which were then considered sub-clinical infection cases (Heymann et al. 1998). Meanwhile, in 1981, WHO planned to launch an intensive active surveillance program in the epidemic foci of DRC, the area where 48 cases of HMPX had been recently reported. During the monitoring period, 338 HMPX cases were found, with an estimated incidence of nearly 1/100,000 in an area much lower than the incidence of malaria (32.1/100,000) and helminthiasis (27.6/100,000) in the same area and time. Of these, 245 infections (72%) were revealed to be animal-originated, and 93 cases (28%) were secondary cases of human-to-human transmission (Heymann et al. 1998), with the total mortality at about 10% (Breman et al. 1980). This surveillance initiative confirmed that HMPX could be transmitted from person to person and passed down continuously for several generations. Also, the above-mentioned results suggested that direct physical contact could increase the risk of infection, and the possibility of airborne transmission was slight. Serological studies on HMPX contacts showed that 18% of non-immunized and 28% of immunized patients had a sub-clinical infection, and vaccine-immunized patients generally had mild disease with few rashes (Heymann et al. 1998; Karem et al. 2007). Further, analytical modeling conducted by WHO suggested that 78% of the initial cases had no secondary cases, and all the outbreaks were restricted on their own, even without special health interventions (Heymann et al. 1998; Kaler et al. 2022). Consequently, this model indicated that MPXV could not achieve permanent infection through human-to-human transmission alone in the human population; therefore, persistent HMPX must rely on animal hosts to frequently re-introduce infection into the population. Thus, HMPX was not considered a serious health concern (Arita et al. 1972; Likos et al. 2005), and the research interest was directed to emerging infections caused by HIV at the time. Since then, the reports of HMPX cases in West and Central Africa have decreased significantly, where 13 cases were reported from 1987 to 1992 (Heymann et al. 1998). In 1996, Medecins Sans Frontieres notified WHO of a suspected epidemic of HMPX in the DRC foci; the first patient, a 35-year-old male, was detected in mid-February. Subsequent cases spread to 13 villages by the end of August, with a comprehensive report of 511 new case (Heymann et al. 1998; Hutin et al. 2001). The following year, WHO conducted its second retrospective survey, highlighting that HMPX might have led to a full-on epidemic with secondary cases of human-to-human transmission (Hutin et al. 2001). Compared with previous outbreaks, the proportion of secondary cases in this outbreak was significantly higher than that of the 1980s (78% vs. 28%), and the case fatality rate was much lower (1.5% vs. 10%). It is hypothesized that weakening population immunity due to the cessation of universal vaccination may be responsible for soaring new secondary cases. However, if that was the case, the case fatality rate should have increased, but it has actually decreased significantly. At that time, it could not be ruled out that the less virulent and more transmissible types were spreading in the DRC. Relevantly, Di Giulio and Eckburg (2004) believed that the case definition used adopted the 1996-1997 epidemic was less specific than the 1981-1986 active surveillance period. Most cases were probably varicella, not HMPX.
Before 2003, cases of HMPX were not reported outside of Africa. In 2003, 72 patients with HMPX were reported in the United States, with a median age of 28 years (Di Giulio and Eckburg 2004), and children younger than 18 years accounted for 29% (10/34) (Bunge et al. 2022). Of the 69 patients whose data could be traced back, 18 (26%) were hospitalized, the two severely ill patients were children, and there were no deaths. The outbreaks of HMPX in the United States are all animal-to-human and have no human-to-human transmission. A retrospective investigation confirmed that all HMPX cases have been linked to purchasing prairie canine species from an animal dealer. The prairie canine species became infected by contact with rodents imported from Ghana (west of Africa) to Texas. Later virological studies also proved that the outbreak was caused by the West African branch of MPXV (Reynolds et al. 2010; Bunge et al. 2022; Quarleri et al. 2022). The investigators believed that the failure of endemics due to zoonotic disease was due to higher natural resistance in the US population, the better health status of the patient population, fewer secondary infections, and better medical care. However, this outbreak again demonstrated this zoonotic disease’s ability to adapt to a new ecological environment.
From 2003 to 2017, no cases of HMPX were reported in countries and regions outside Africa. Meanwhile, population-based active surveillance of HMPX in African countries from 2005 to 2007 on 7,600 patients estimated an average annual incidence rate of 5.53 per 10,000 people (ranging from 2.18 to 14.42 per 10,000 based on the region), which increased about 20-fold compared to incidence reports in the 1980s. This surveillance also found that risk factors that increase HMPX include: living in rainforest areas, male sex, age < 15 years, and unvaccinated smallpox; the risk of developing HMPX among unvaccinated persons was estimated to be approximately 5.2 times that of vaccinated persons (4.05/10,000 vs. 0.78/10,000) (Luo and Han 2022). In mid-2018, the first report of people taking the HMPX virus out of Africa appeared in 2003 was occurred (Yinka-Ogunleye et al. 2018; Erez et al. 2019). In September 2018, the United Kingdom reported two other cases of HMPX returning from Nigerian tourism, one of which, during treatment, may have infected a caregiver through contaminated bedding. He became ill, and 4 of the 134 people he came into contact with became ill (Erez et al. 2019; Vaughan et al. 2020). In late 2018, Israel reported a case of a 38-year-old man who became afflicted after returning from Nigeria who had handled rodents 12 days before the appearance of the rash. The patient had five family members and 11 medical staff who had contact with him in Israel. Merely one person agreed to be vaccinated, and no one became afflicted during follow-up (Erez et al. 2019). In 2019, 2 cases of HMPX virus were reported out of Africa: first, a 38-year-old Nigerian male reported in Singapore who had a history of eating suspicious barbecue 2 weeks before the symptom occurrence (Yong et al. 2020), and second, a 39-years-old man was diagnosed in the United Kingdom who had a recent trip to Nigeria (Rao et al. 2022). No HMPX cases were reported outside of Africa in 2020. That year, Nigeria reported 8 cases of HMPX, the year with the fewest reported cases since the outbreak in 2017 (Costello et al. 2022). In May 2021, a British national who worked and lived in Nigeria became ill after returning to China and became ill during home isolation due to the prevention and control of the novel coronavirus pneumonia (Velavan and Meyer 2022). In July 2021, the United States reported a middle-aged male American case from Nigeria to Dallas, believed to be the 7th case of HMPX reported by countries outside Africa since 2017 (Guarner et al. 2022; Velavan and Meyer 2022). Among 223 of these human-human contacts, 189 were classified as low-risk, and 34 should be considered for immediate vaccination. However, no one agreed to be vaccinated, and no HMPX cases occurred subsequently (Rao et al. 2022). Four months later, the United States again reported an imported case of HMPX, a 28-year-old male who returned from a trip to Nigeria. Therefore, the United States issued a Nigeria travel warning and determined that 40 exposed medical staff were at high risk of infection. They were not vaccinated and had no subsequent illness (Nguyen et al. 2021; Costello et al. 2022). From the aforementioned epidemiological history of HMPX, whether in African or non-African countries, HMPX cases tend to increase over time. Bunge et al. (2022) found that the number of HMPX cases increased significantly in DRC. The total number of suspected and confirmed cases reported in the 1980s and every ten years thereafter were 38, 343, 511, 10,027, and 18,788, respectively (Kumar et al. 2022). The evidence regarding the increment in the incidence of the HMPX virus was likewise factual in other African countries such as Nigeria, DRC, and the Central African Republic during the previous 40 years. The median age of reported cases has also increased over the past 50 years, from 4 years in the 1970s, 5 years in the 1980s, 10 years in the 2000s, and 21 years in the 2010s. The researchers believed that these epidemiological changes in HMPX might be correlated with the termination of smallpox vaccination and the weakening of cross-immune protection against MPXV, resulting in human-to-human transmission (de Sousa et al. 2022; Guarner et al. 2022; Rao et al. 2022; Zhu et al. 2022). Of interest, a Nigerian study shows that the population immunization rate dropped to 10.1% in 2016, and the level of immune protection has dropped from 65.6% in the 1970s to 2.6%; by 2018, the vaccine immunization rate was 9.3%, and the population immunity level was only 2.2% (Nguyen et al. 2021). The increased age of onset observed in Africa may be more related to increased awareness of child protection, avoidance of early exposure, and maintenance of susceptibility into adulthood, which is similar to adult infection cases in non-endemic countries. One should note that immunity gradually declines after childhood vaccination, and the phenomenon of increased morbidity in adolescents and adults is fundamentally different.
Between 2020 and 2022, HMPX cases were reported from countries in Africa’s central, northwestern, and southwestern regions. An outbreak of HMPX occurred in Cameroon in December 2021, and as of February 17, 2022, there were three confirmed cases, 25 suspected cases, and 2 deaths (Babkin et al. 2022). On March 14, 2022, the Central African Republic reported six confirmed cases of HMPX, including 2 deaths. From January 1 to April 17, 2022, the DRC reported 1,152 suspected cases with 55 deaths (4.8%), while only 138 suspected cases and 14 deaths (10.1%) were reported during the same period in 2021 (Adalja and Inglesby 2022; Quarleri et al. 2022). From January to April 2022, 46 suspected cases (of which 15 were confirmed) were reported in Nigeria (Guarner et al. 2022; Yao 2022). According to the latest WHO notification, from January to May 2022, a total of 44 confirmed cases and 1,408 suspected cases of HMPX were spotted in 7 endemic African nations; the number of suspected and confirmed cases was 1,294 in DRC and 87 in Nigeria; 66 patients died, 58 cases of DRC, 1 case in Nigeria (Bunge et al. 2022; Kumar et al. 2022). Countries which WHO has determined that HMPX is endemic based on previous case reports include Cameroon, Côte d’Ivoire, Central African Republic, DRC, Gabon, Nigeria, Liberia, DRC, Sierra Leone, and Ghana, as well as Benin and South Sudan, which have recorded imported cases (Guarner et al. 2022). Among them, the countries reporting MPXV infection cases in West Africa are mainly Cameroon and Nigeria (Adalja and Inglesby 2022). The MPXV infection cases in Central Africa are mainly in the Central African Republic and DRC. For the 2022 outbreak, WHO requires all countries except Cameroon, Central African Republic, DRC, and Nigeria to report new HMPX cases and requires that if Central African countries find HMPX cases caused by MPXV in West Africa, they should also report (Heskin et al. 2022). Since May 7, 2022, HMPX cases have been reported in non-African and non-endemic countries. On May 16, the United Kingdom reported 4 cases of male HMPX and pointed out that they were all homosexuals and/or bisexuals. Within a week afterward, 12 other countries reported confirmed cases of HMPX (Quarleri et al. 2022). Among the 53 cases of HMPX reported by 19 countries from May to June 2022, 2 (3.7%) patients were female, and 43 were male (81.1%); the authors did not clarify the sex of the other 8 cases (15.1%) (Bunge et al. 2022; Guarner et al. 2022). As of July 2022, reports of 2,027 HMPX patients have been published in 36 countries and regions around the world (Fig. 4). Recent travel history to Nigeria or West African countries was declared by 74 European afflicted cases (3.7%). Consistent with this finding, HMPX data gathered by non-endemic countries in 2022 revealed that the identified MPXV strains all belong to the West African branch (Kozlov 2022). Further, Isidro et al. (2022) performed whole-genome sequencing of 9 strains of MPXV isolated in Portugal and one strain of MPXV in the United States in May 2022. The analysis showed that all the sequences were clustered together, stipulating that the multi-country epidemics likely originate from the same source, most closely related to the strains exported from Nigeria to the UK, Israel, and Singapore in 2018 and 2019. Previous studies have hypothesized that this gene loss may be associated with human-to-human transmission (Sadeuh-Mba et al. 2019; Nguyen et al. 2021; Kozlov 2022). Another study also showed that MPXV isolated in Belgium on May 2022 was most closely related to the Portuguese strain detected in March 2022 and was closely related to the Nigerian export strain in 2018 and 2019 (Perez Duque et al. 2022).
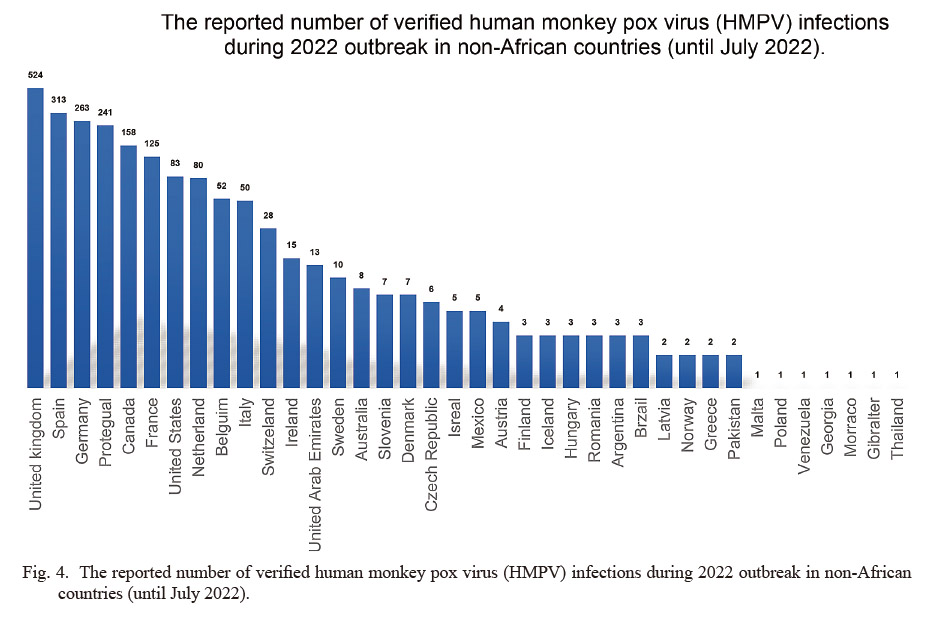
The reported number of verified human monkey pox virus (HMPV) infections during 2022 outbreak in non-African countries (until July 2022).
The number of new HMPX virus cases has increased sharply in a short period of time since 2022. In recent years, the epidemics in endemic countries of HMPX in Africa have occurred with a constant incidence and predictable spread, and there was no obvious sign of disease activity from December 2021 to May 2022. But in about a month since the first case was reported in the UK on May 7, 2022, the total number of confirmed cases of HMPX reported in non-endemic countries has exceeded 2,000. Accordingly, as shown in Fig. 2, the virus has spread worldwide, leading to the rapid upsurge of Monkeypox cases across the globe. This rapid increase in the number of new afflictions attracted attention with raised caution (Kozlov 2022). Notably, after the first case of HMPX was discovered in 1970, no HMPX case was reported in that region until 2003, and the total number of cases reported in the regions before the outbreak of HMPX in the United States in 2003 did not exceed 60 cases; however, the recently reported data far exceeds the worst-case scenario predicted by analytical models of WHO regarding 1980s outbreak (Heymann et al. 1998). On top of that, the WHO is also concerned that the reported cases may underestimate the prevalence of HMPX. Because some HMPX patients have relatively mild symptoms and self-limited disease course, they may not seek medical attention; globally, the clinical medical staff actually lack clinical knowledge of HMPX and does not have access to available diagnostic reagents and other supplies; it is more important to establish an effective monitoring system immediately difficult (Luo and Han 2022).
Affliction of non-endemic countriesHMPX broke out in many countries, and the non-endemic areas involved in the epidemic expanded significantly compared with the past. The public health risks associated with a single case of HMPX are extensive, so in a non-endemic country, one case of HMPX should be considered an outbreak (Adalja and Inglesby 2022; Quarleri et al. 2022). Before 2022, there were usually several thousand HMPX cases per year in West and Central Africa, with only a few cases outside Africa associated with the sojourn or import of infected animals in Africa (Kozlov 2022). From 2003 to this outbreak, only four non-endemic countries reported HMPX, and each outbreak involved only one country (Bunge et al. 2022). However, in this outbreak, over 40 countries and regions have reported confirmed cases of HMPX in one month, including Europe, Asia, America, Oceania, and African regions other than endemic countries. At the same time, the cases are clearly concentrated in Europe, suggesting that after the virus was brought out of Africa, the spread may have mainly occurred in Europe. The recent spreading pattern of HMPX in regions that were not previously reported indicates the importance of further investigations.
New transmission routesThe MPXV is conventionally transmitted by direct contact with fluids, lesions, and mucosa of the infected animals. Human-to-human transmission, which was not common in previous outbreaks, occurs due to contact with cutaneous ulcers, infected surfaces or objects, and particles of the respiratory tract, which mainly needs long-term and close face-to-face contact. HMPX virus could enter and replicate inside the oropharyngeal and respiratory mucosa, as well as involving the lymph nodes draining the mucosa. This spotlights the importance of preventive strategies specifically for caregivers upon conducting mechanical ventilation or masking due to high contact with potential infected droplets and mucosa. Moreover, even though there are still controversies regarding the airborne transmission route of the HMPX virus, the WHO emphasizes the role of repository droplets in spreading the virus and the potential role of taking masks in the management of disease in the epidemic regions. In the 2022 outbreak, a large number of cases of infection come from person-to-person transmission. Although human-to-human transmission of MPXV has long been demonstrated, outbreaks dominated by secondary human-to-human transmission have also occurred in endemic countries (Jamil et al. 2022; Quarleri et al. 2022). Although currently, there is no specific epidemiological report for this outbreak, information indicates that most cases have no direct epidemiological link with HMPX endemic countries (Vaughan et al. 2020; Heskin et al. 2022). In this epidemic, HMPX not only went out of Africa but also achieved international transmission between countries through human-to-human secondary transmission. There will likely be a transmission chain involving multiple generations and countries, which never happened before. Further, countries have successively reported cases with the same characteristics after the United Kingdom reported HMPX virus cases of gay and bisexual men who had intercourse with men on May 2022. Therefore, the outbreak cases were more concentrated in men aged 20 to 50 (Kozlov 2022; Luo and Han 2022). Although it has been reported that the age distribution of HMPX cases has gradually shifted from young children to adolescents and adults in the past 50 years, in general, children < 15 years old still consist of a considerable number of new cases. Taking into account the risk factors of human and animal infection, the HMPX outbreaks (Africa), mainly in animals, are often in children. Occupations associated with previously reported cases include traders, students, artisans, health workers, farmers, hunters, and transport workers (Bunge et al. 2022). Moreover, imported HMPX cases in non-endemic countries and regions are mainly adults, probably because of exposure to infection from overseas travel or work. This outbreak outside Africa is likely to start with imported cases in adults. The first reported case in the United Kingdom and a small number of cases with a history of travel in Nigeria or West Africa also provide clues to the origin. From the perspective of the entire epidemic, there must be a chain of transmission initiated by multiple imported cases. HMPX cases in non-specialized communities may not receive enough attention. Although uncommon, the route of sexual transmission of HMPX has long been described. When Ogoina et al. (2019) investigated the HMPX outbreak in Nigeria in 2017, they speculated that sexual transmission was a possible route of infection, assumed to be either direct skin contact or transmission through secretions. Needless to say, sexual activity in and of itself constitutes close contact, which per se is not beyond knowledge of the route of transmission of HMPX. The most likely explanation for this unexpected transmission mode is that MPXV was introduced into specific community groups and continued to spread (Kozlov 2022).
Unknown source of infection in many casesThe way that HMPX cases in non-endemic countries have been brought out of Africa is very clear, especially the cases brought out of Africa by people, and the first cases have a history of living in Africa (Luo and Han 2022). Although there are a small number of positive cases in this outbreak with a history of travel in Africa, these patients are not related to others. So, there is no clear and reliable evidence of epidemiological links between cases in various countries, which requires further investigation and research.
Novel presentations and clinical courseRecent reports identified some clinical manifestations of the HMPX infection, which were relatively rare in previous outbreaks. These findings, which may facilitate the diagnosis and lower the risk of misdiagnosis of the patients, are as follows; 1. Penile swelling and irritation as well as ambiguous pain in the perinea without primary classic lesions for which patients were referred to emergency departments (Patel and Balk 2012); 2. Perianal abscess as the mere sign of the disease treated by surgical interventions, while the typical rashes just began to spread through the genital skin a week after the patient’s discharge (Tarín-Vicente et al. 2022); 3. Systematic involvement such as sepsis and septic shock prior to cutaneous lesions (Patel and Balk 2012; Tarín-Vicente et al. 2022); 4. Focal involvement of skin and mucosa rather than total-body rashes considering solely tonsillar and perioral involvement, which could be misdiagnosed as bacterial tonsilitis (Tarín-Vicente et al. 2022); 5. Maculopapular lesions predominance compared with dominant vesiculopustular lesions in previous African region outbreaks (Patel and Balk 2012); and 6. Novel distribution pattern of lesions developing more in the face and perineal area and less in the trunk (Adler et al. 2022).
WHO assesses the current global overall public health risk as moderate because it is the first time that HMPX cases have been reported simultaneously in different countries and regions, and the cases are not clearly linked to endemic countries in West or Central Africa. Current epidemiological data suggest that the virus has begun widespread human-to-human transmission, possibly undetected for weeks or more. From previous outbreaks, deaths have occurred more in children and the immunocompromised, including those with poorly controlled HIV infection, who are infected with HMPX, have a higher individual health risk and may also result in higher public health risk. Notably, for the general population, the risk of infection with MPXV does not seem to be high (Reeves et al. 2011; Heskin et al. 2022; Yao 2022). Although HMPX could be transmitted between humans, this means of transmission of MPXV is limited. Human-to-human transmission requires close contact, and monkeypox is still mainly characterized by zoonotic infection. The favorable conditions for the occurrence of HMPX in Africa include endemic transmission of MPXV in agricultural areas and forest animals around human settlements; the custom of eating undercooked wild animals; and hunting, skinning, and human behavior in close contact with animals such as animal body specimens as toys (Reynolds et al. 2019). Of note, health workers are at risk of infection too if they do not wear appropriate personal protective equipment in their healthcare activities (Saied et al. 2022). There are also some small but unavoidable risks, such as infection in pregnant women and mother-to-child transmission. Recent studies spotlighted two factors that are more associated with HMPX infection: 1. Sharing rooms and beds of the same household and eating the same dish as human-to-human transmissions, and 2. Sleeping outdoors or on the ground, residing close to rainforests, and entering forests which elevate the risk of exposure to animal species. Surprisingly, assisted toileting, cleaning, and laundry were not significantly associated with MPXV infection (Nolen et al. 2015). This result may be related to the age distribution of the surveyed population. For adults in the foci, these behaviors are likely to be associated with early infection and later become protective factors. Persons from non-epidemic areas, regardless of age, should keep away from unprotected contact with wild animals, particularly those that are dead or sick, and should not eat wild animal meat from unknown sources that are not thoroughly cooked (Velavan and Meyer 2022). Smallpox vaccination can effectively prevent HMPX, and public health personnel can use a “ring vaccination” strategy to restrict the spread of the virus, which is to vaccinate close contacts of HMPX patients to cut off the transmission route. However, based on the current situation, it may be necessary to adopt prevention and control strategies other than ring vaccination to contain the current epidemic (Yinka-Ogunleye et al. 2018; Ogoina et al. 2019). The problem is that usually two-sided because the outbreak involves special populations, which may lead to neglect or underestimation of the risk of infection in the general population in this outbreak. The WHO believes that it is entirely possible that HMPX has been brought back to the community by particular populations and that, in addition to direct contact, MPXV could also spread through contact with contaminated objects (Yao 2022). Therefore, countries and regions with existing epidemics should take immediate action to control further transmission among high-risk groups and prevent transmission to the general population, so HMPX virus will not become a persistent clinical disease and public health problem in non-endemic countries (Costello et al. 2022). Until now, the animal reservoir of MPXV and how it continues to spread in animals has not been clearly understood, which also affects the assessment of outbreak development. While it is currently believed that the outbreak may not have been caused by infection of animals, HMPX cases may have brought the virus to local animals, and if reservoirs were established in animals, repeated transmission to humans could be possible, this may also happen even in countries with no known animal reservoirs (Diaz-Cánova et al. 2022). European health administrators firmly recommend that rodent pets, e.g., guinea pigs and hamsters, that belong to patients with HMPX, should be quarantined and watched or even euthanized to stop the spread of the virus (Heskin et al. 2022). Although the risk of such transmutation is low, it may be too late if it does occur, as animal monkeypox usually does not show clear symptoms like humans.
HMPX was initially revealed in African countries 52 years ago, and the transmission to humans was mainly due to infected animals. The scarce human-to-human transmission was reported 20 years ago, although it was not responsible for many outbreaks that occurred before. However, in the year 2022, not only African regions but also new countries are experiencing the soar of new cases with novel patterns of transmissions considering secondary affliction and sexual contacts. Further, all age groups are now involved compared with the previous children-predominant outbreaks. Concerning the pathogenic variants, the HMPX African branch seems to be more contagious, but it is limited to African countries, whilst the West African branch is mainly responsible for infections in countries outside Africa. Changes in human behavior, counteraction to the environment, and a decline in vast vaccination against smallpox may have critical impacts on the novel and atypical occurrence of HMPX outbreaks. More important, these outbreaks may reflect a broader range of zoonotic infections triggering and spreading during the interaction of pathogens with the natural environment and human behavior in the management of COVID-19 and its post-pandemic era.
The authors declare no conflict of interest.