Abstract
Nucleos(t)ide analogues (NAs) suppress hepatitis B virus (HBV) replication, but the risk of hepatocellular carcinoma still remains. The presence of detectable HBV DNA in the serum during NA therapies for chronic hepatitis B patients has been reported to be associated with the risk of hepatocellular carcinoma. In this study, we investigated the antiviral effect of switching from entecavir (ETV) to tenofovir alafenamide fumarate (TAF) in chronic hepatitis B patients who had detectable HBV DNA in the serum at least once within a year. Among a total of 77 cases in 7 hospitals that switched NAs from ETV to TAF, 23 patients with detectable HBV DNA in a year before switching were analyzed. When the detection frequencies of HBV DNA in the 1st and 2nd years after switching to TAF were analyzed, they were significantly lower than those in the year before switching (68.8% vs. 34.1% for the 1st year and 21.3% for the 2nd year, P < 0.001 for both). The HBsAg decline tended to be larger after switching than before (−2.5% vs. −3.0% for 1st year and −3.1% for 2nd year), but the difference was not significant. One patient died of a cardiovascular event 11 months after the treatment switch, but no adverse effects due to TAF including renal function were observed. In conclusion, it was suggested that switching from ETV to TAF might be effective to suppress the HBV DNA level further in patients whose HBV DNA is detectable, even if at a very low level.
Introduction
According to an estimation of the World Health Organization, 296 million people were infected with hepatitis B virus (HBV) chronically, which resulted in 820,000 deaths, mostly from cirrhosis and hepatocellular carcinoma (HCC) in 2019. Nucleos(t)ide analogs (NAs) are widely used for the treatment of chronic hepatitis B (CHB) because of their potent antiviral effects and few side effects. NAs inhibit reverse transcriptase of HBV and efficiently suppress HBV DNA in the serum, reduce liver inflammation, and lower the risk of developing liver fibrosis and HCC (Marcellin et al. 2003; Liaw et al. 2004; Singal et al. 2013; Papatheodoridis et al. 2015). However, the effect of NAs on hepatitis B surface antigen (HBsAg) in the serum is limited, and the risk of HCC still remains (Inoue et al. 2019, 2022). The antiviral effect may sometimes be insufficient due to virus resistance or other factors. It has been reported that, if HBV DNA is detected during the administration of NAs, there is a risk of developing HCC (Sinn et al. 2015; Kim et al. 2017; Kaneko et al. 2020), and strong suppression of HBV is necessary.
The current guidelines recommend entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide fumarate (TAF) as the first-line drugs for NAs for CHB (Sarin et al. 2016; European Association for the Study of the Liver 2017; Terrault et al. 2018). The effects of HBV DNA suppression among these drugs are mostly similar, but there are reports that the suppression of HBsAg is stronger with TDF than with ETV (Koike et al. 2018). We reported that HBeAg-positive patients showed larger declines of HBsAg than HBeAg-negative patients after the treatment switch from ETV to TDF (Inoue et al. 2021). TAF is a new prodrug of tenofovir that is as effective as TDF for HBV suppression. Also, because TAF has greater stability in plasma than TDF, TAF is able to deliver the active metabolite more efficiently to target cells at a substantially lower dose with fewer effects on bone and kidney function (Buti et al. 2016; Chan et al. 2016; Agarwal et al. 2018). TAF has been reported to suppress ETV-resistant HBV efficiently (Yamashige et al. 2021) and switching from ETV to TAF was reported to obtain a better virological response in patients with low-level viremia of serum HBV DNA > 20 IU/ml and < 2,000 IU/ml (Li et al. 2021). However, it is still unclear whether the benefits of TAF can be obtained in ETV-treated patients with very low-level viremia, including those whose serum HBV DNA was detectable only occasionally. In this study, we aimed to verify the antiviral effects of switching to TAF in patients with detectable HBV DNA at least once in a year, regardless of the value, during ETV administration.
Materials and Methods
Study design and patients
This study was a retrospective observational study conducted at 7 of the facilities participating in the Tohoku Hepatology Research Meeting (THERME), which consists of Tohoku University Hospital and related facilities. Among CHB patients from 2018 to 2020 who had been taking ETV orally and switched to TAF, those who fulfilled the following criteria were analyzed. Inclusion criteria were as follows: i) ETV 0.5 mg/day had been administered for > 2 years continuously before switching to TAF 25 mg/day; ii) HBV DNA was detected at least once, regardless of the value, in a year just prior to switching to TAF; iii) patients ≥ 20 years old; iv) they had no history of decompensated liver cirrhosis. The exclusion criteria were as follows: i) patients receiving interferon or immunosuppressive therapies; ii) coinfection with hepatitis C virus or human immunodeficiency virus.
Determination of serological HBV markers
The HBV DNA levels in the serum were quantified using quantitative PCR assays with Cobas TaqMan HBV Auto, according to the manufacturer’s protocol (Roche Diagnostics, Tokyo, Japan). The detection limit is 1.0 log IU/ml, but even though the HBV DNA level is below the limit, a positive signal is reported. A persistent complete virological response (pCVR) was evaluated every year as undetectable HBV DNA in all tests that were performed at least 3 times a year. The serum levels of HBsAg were quantified using a chemiluminescent enzyme immunoassay (CLEIA) with LUMIPULSE HBsAg-HQ (Fujirebio, Tokyo, Japan). HBV genotypes were determined with an IMMUNIS HBV genotype EIA kit (Institute of Immunology, Tokyo, Japan).
Evaluation of antiviral effects and safety of the Nucleos(t)ide analogs
Using serum samples, HBV DNA detection frequency, HBsAg, liver and renal functions, and inorganic phosphorus (IP) were measured from 12 months before drug switching to 24 months after drug switching every 3 months. As for the score for evaluating liver fibrosis, the Fibrosis-4 (FIB-4) index was calculated as follows: FIB-4 = age (years) × aspartate aminotransferase (AST, U/L)/[platelet counts (PLT, 103/μL) × √alanine aminotransferase (ALT, U/L)] (Sterling et al. 2006).
Statistical analysis
Statistical analysis was performed using JMP version 15.0 (SAS Institute Inc., Cary, NC, USA). Statistical comparisons were performed using a t-test for comparison of the frequencies of the HBV DNA detection or the continuous variables between two groups. A paired t-test was used for the evaluation of parameter changes over time. P < 0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics of the enrolled patients
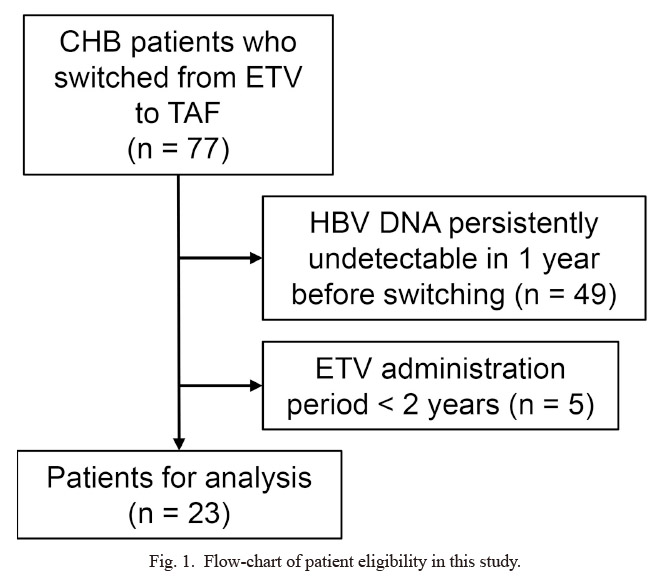
In 7 hospitals, a total of 77 cases that switched NAs for CHB from ETV to TAF were screened. After excluding 5 cases in which the ETV administration period was 2 years or less and 49 cases in which HBV DNA was persistently undetectable within 1 year before the drug switching, 23 cases were included in the analysis (Fig. 1). Table 1 shows the clinical parameters of the included patients at the time of the drug switching. The median age was 62 years old [interquartile range (IQR), 48‐70 years] and the patients consisted of 13 males (57%) and 10 females (43%). The median serum ALT level was 20 U/l (IQR, 18-29 U/l), the estimated glomerular filtration rate (eGFR) was 72.3 ml/min/1.73 m2 (IQR, 58.9-80.4 ml/min/1.73 m2), and the IP level was 3.2 mg/dl (IQR, 3.0-3.5 mg/dl). There were 14 (60.9%) cases whose serum HBV DNA was detectable, with a median level of less than 1.0 log IU/ml. The median serum HBsAg level was 811 IU/ml (IQR, 245-3,976 IU/ml). The median duration of ETV administration before the drug switching was 4.1 years (IQR, 3.4-5.9 years).
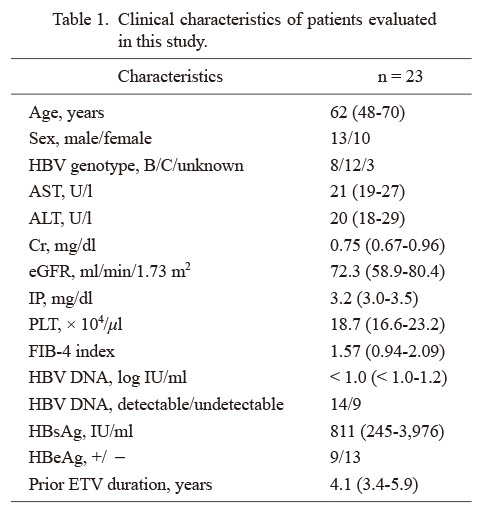
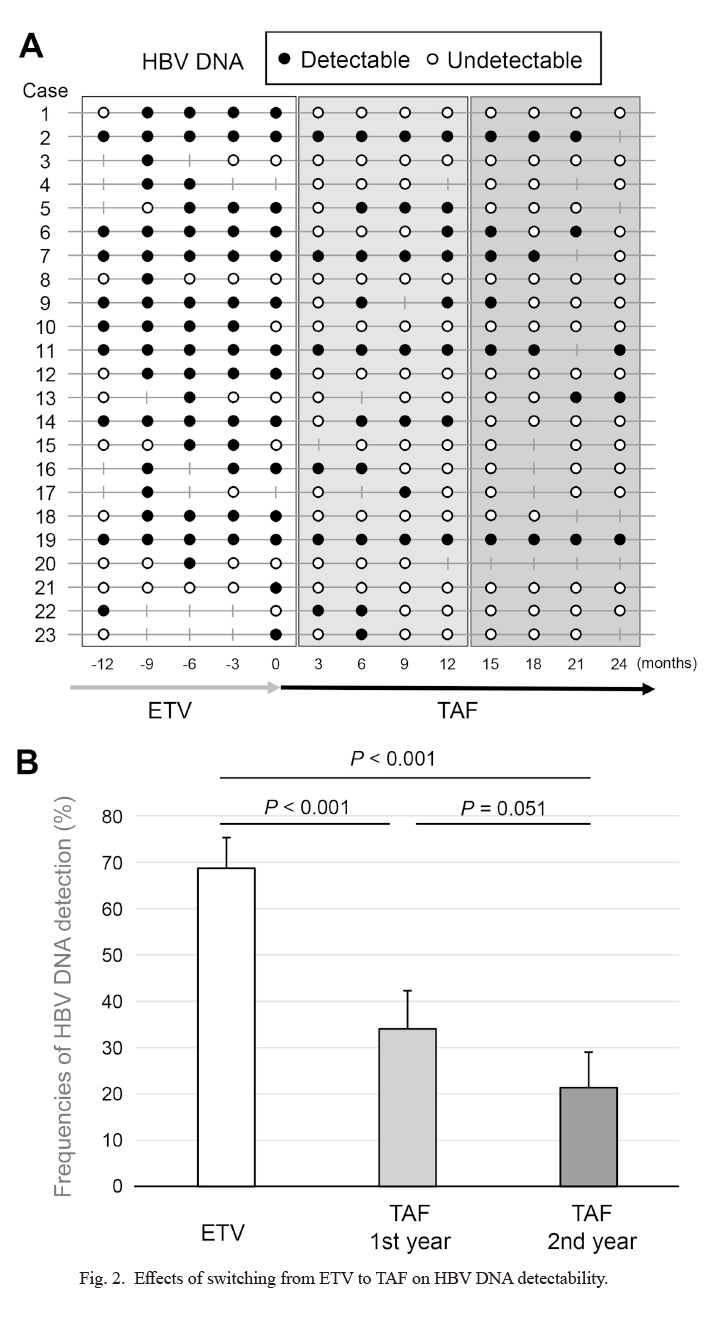
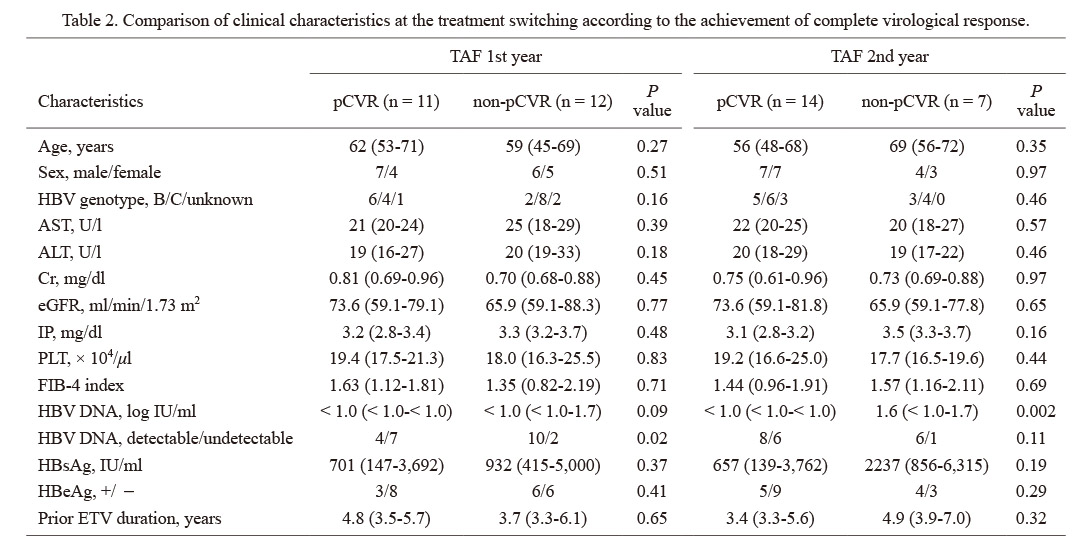
The results of HBV DNA, which was basically measured at every 3 months, are shown in Fig. 2A as black dots for those that were detectable and white dots for those undetectable in each case. Some of the HBV DNA data were missing, and case no. 20 died of acute myocardial infarction, which was considered not to be related to TAF administration by the physician, at 11 months after the drug switching. As shown in the inclusion criteria, all the 23 patients showed detectable HBV DNA at least once in a year just before switching to TAF. Among patients whose HBV DNA was tested at least 3 times a year, 11/23 (47.8%) patients achieved pCVR in the 1st year of TAF and 14/21 (66.7%) did in the 2nd year. The frequencies of HBV DNA detection in a year before NA switching from ETV to TAF (5 time points at every 3 months including data at the time of switching), in the 1st year after switching to TAF (4 time points), and in the 2nd year after switching (4 time points) were calculated for each case and the mean values were compared (Fig. 2B). It was significantly lower after switching than before (68.8% vs. 34.1% in the 1st year and 21.3% in the 2nd year, P < 0.001 for both). The background was compared between pCVR and non-pCVR cases at the 1st or 2nd year after switching to TAF (Table 2). The pCVR group in the 1st year was significantly more likely to have undetectable HBV DNA at the time of switching (63.6% vs. 16.7%, P = 0.02). In the 2nd year, the same tendency was observed (42.9% vs. 14.3%, P = 0.11). The serum HBsAg levels tended to be lower in the pCVR group in both the 1st and 2nd years (701 IU/ml vs. 932 IU/ml, P = 0.37 for 1st year; 657 IU/ml vs. 2,237 IU/ml, P = 0.19 for 2nd year). In addition, the proportion of patients negative for HBeAg in pCVR was higher (72.7% vs. 50.0%, P = 0.41 for 1st year; 64.3% vs. 42.9%, P = 0.29 for 2nd year), although the difference was not significant. There were 3 patients whose HBV DNA was detected in all tests even after switching to TAF (cases 2, 11 and 19 in Fig. 2A). No characteristic change of HBsAg was observed, but they were all infected with genotype C HBV and positive for HBeAg during the study period.
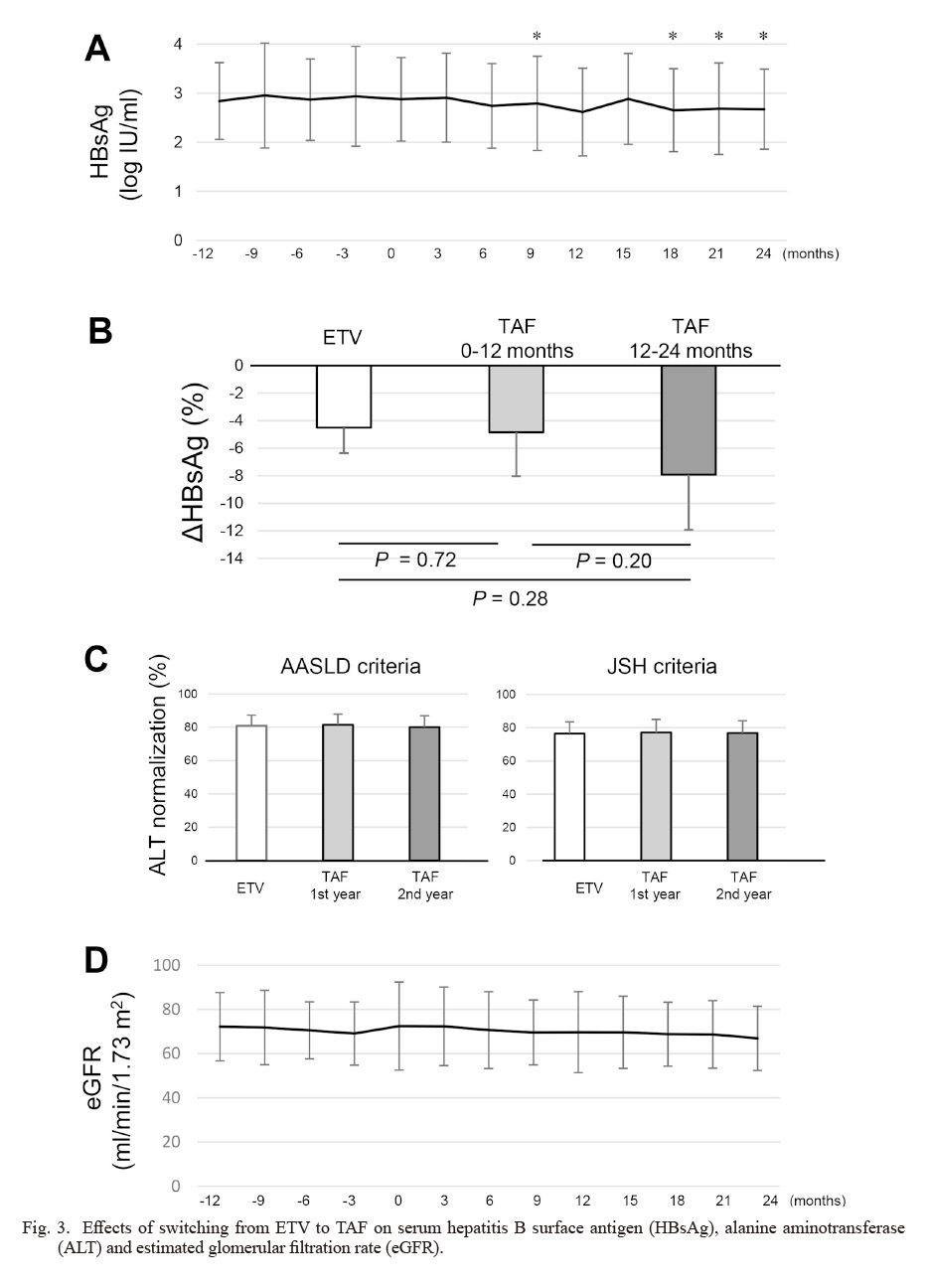
The changes in the mean value of the serum HBsAg levels from 12 months before switching to 24 months after switching are shown in Fig. 3A. It seems to have decreased gradually after the drug switching and, in comparison with 0 months, there were significant differences at 9, 18, 21, 24 months (2.87 log IU/ml vs. 2.79 log IU/ml, P = 0.01 for 9 months; vs. 2.75 log IU/ml, P = 0.003 for 18 months; vs. 2.69 log IU/ml, P = 0.04 for 21 months; vs. 2.68 log IU/ml, P = 0.02 for 24 months). In addition, the mean value of the serum HBsAg change during the 12 months of ETV administration, that in the 0-12 months after switching, and that in the 12-24 months after switching were expressed as %ΔHBsAg, which was calculated as the percentage of change from the beginning of each duration (Fig. 3B). The decreases in the serum HBsAg levels were comparable in the 12 months before and after switching (−4.5% vs. −4.8% log IU/ml, P = 0.72). A little larger decrease was obtained for 12-24 months after switching to TAF, but there was still no significant difference (−4.5% vs.−7.9% log IU/ml, P = 0.28).
The percentage of ALT normalization was compared among 1 year of ETV administration, the 1st year after TAF switching, and the 2nd year after TAF switching (Fig. 3C). The ALT normalization was evaluated using the American Association for the Study of Liver Diseases (AASLD) criteria (male ≤ 35 U/l, female ≤ 25 U/l) and the Japan Society of Hepatology (JSH) criteria (≤ 30 U/l). There was no significant difference in the normalization rate of ALT between the duration of ETV and that of TAF in the two sets of criteria (AASLD criteria; ETV vs. TAF-1st year vs. TAF-2nd year, 80.9% vs. 81.5% vs. 80.1%, JSH criteria; ETV vs. TAF-1st year vs. TAF-2nd year, 76.5% vs. 77.2% vs. 76.8%).
Factors associated with HBsAg decline
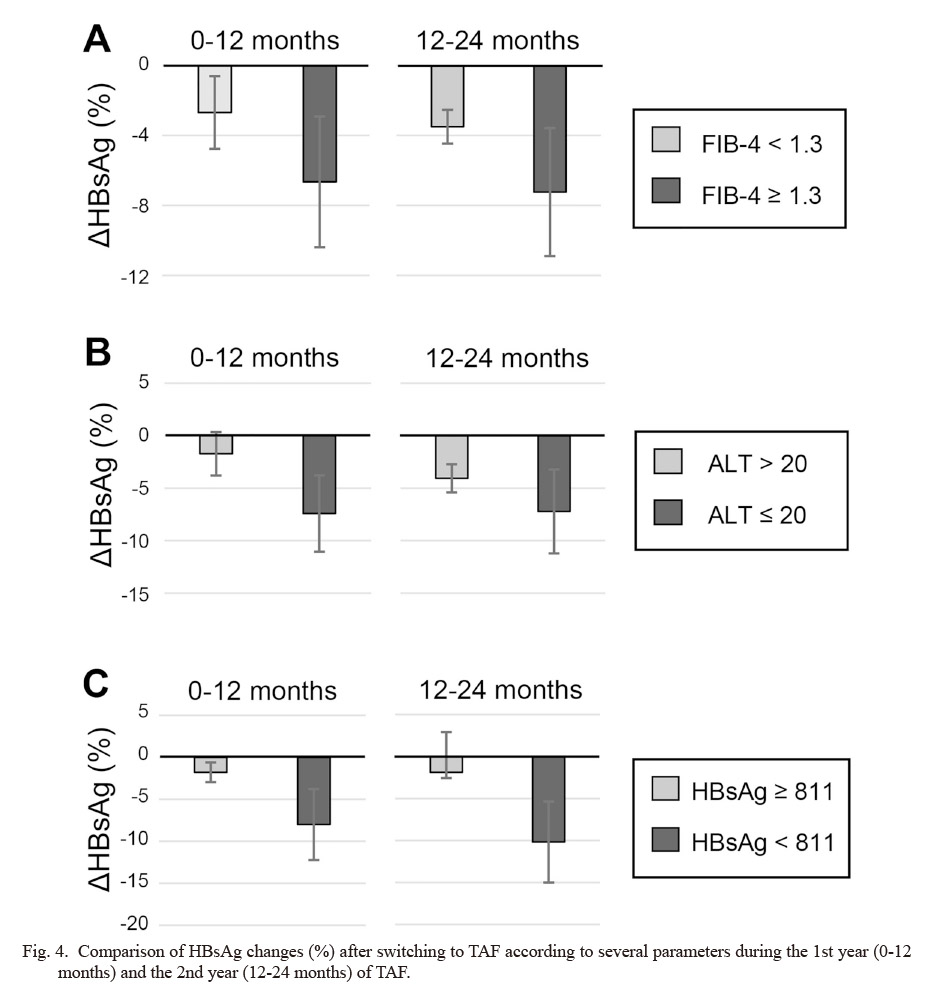
Sub-analyses were performed on the amount of decrease in HBsAg after NA switching from ETV to TAF according to the clinical characteristics. When the cut-off value of FIB-4 index was set at 1.3 as previously reported (McPherson et al. 2010), the group with a higher FIB-4 index tended to have a larger %ΔHBsAg than the group with a lower FIB-4 index (−6.65% vs. −2.69% for 0-12 months, P = 0.95; −7.24% vs. −3.50% for 12-24 months, P = 0.32) (Fig. 4A). The %ΔHBsAg tended to decrease in the group with serum ALT levels of 20 U/l or less than in the group with higher than 20 U/l at drug switching (−7.42% vs. −1.73% for 0-12 months, P = 0.27; −7.21% vs. −4.07% for 12-24 months, P = 0.52) (Fig. 4B). When the viral factors were examined, the %ΔHBsAg tended to decrease in the group with serum HBsAg levels of 811 IU/ml or less at the time of switching than in the higher HBsAg group (−8.00% vs. −1.78% for 0-12 months, P = 0.24; −10.18% vs. −1.89% for 12-24 months, P = 0.15) (Fig. 4C). However, no significant difference was found in comparison of parameters.
The changes in the mean values of the serum eGFR levels are shown in Fig. 3D. There was no significant change of eGFR during the study period. When the %ΔeGFR was evaluated, it was larger in the 1st year of TAF (ETV vs. TAF-1st year vs. TAF-2nd year, −0.41 % vs. −2.96 % vs. −1.12%), but there was no significant difference. No other adverse effects including hypophosphatemia were observed.
Discussion
Previous reports have shown that the serum HBV DNA was detectable in 20-30% of patients receiving ETV therapy (Kim et al. 2017; Sun et al. 2020). It is speculated that one of the reasons for this is that ETV may have insufficient bioavailability because its blood concentration decreases when taken orally after meals (Jung et al. 2018; Li et al. 2021). Sun et al. (2020) reported that the detection rate of HBV DNA 78 weeks after the start of ETV was correlated with the progression of liver fibrosis in CHB patients undergoing ETV therapy. In addition, some reports have shown that low-level viremia (LLV; HBV DNA between 20 and 2,000 IU/mL) during NAs therapy is a predictor of HCC development (Sinn et al. 2015; Kaneko et al. 2020; Li et al. 2021). According to the AASLD guidelines, LLV patients undergoing NAs therapy are recommended to switch to or add other NAs. In the present study, we aimed to evaluate the benefit of NA switching from ETV to TAF in patients with very-low-level viremia during ETV, including those whose HBV DNA was detectable at least once a year, and showed that HBV DNA detection frequency was significantly reduced after switching to TAF.
Several studies of TAF switching have been reported in LLV patients on ETV therapy. Ogawa et al. (2022) reported that 94.9% of 39 patients with LLV had a complete virological response (CVR), which was defined as HBV DNA below the detection limit including a positive signal, 144 weeks after switching from ETV to TAF. Nguyen et al. (2021) reported that CVR was obtained in about half of 34 LLV patients receiving ETV after 48 weeks of switching to TAF and in more than 80% after 96 weeks. In addition, Li et al. (2021) conducted a prospective study in which LLV patients receiving ETV were aligned by propensity score matching to compare the TAF switching group and ETV continuation group (n = 75 each). The percentage of CVR at 24 weeks was significantly higher in the TAF group (62.7% vs. 9.3%) (Li et al. 2021). These results suggest that switching to TAF may be important for obtaining CVR for LLV patients undergoing ETV therapy. Differing from the previous studies, we compared the HBV DNA detectable frequency every year to evaluate the presence of very-low-level viremia in the present study, and showed that TAF suppressed HBV DNA further in such patients.
In this study, we focused on host factors and viral factors to evaluate the factors related to HBsAg reduction after TAF switching. Previous reports showed that the amount of HBsAg decline was larger after TAF switching than during ETV administration (Uchida et al. 2022). The results in the present study showed a similar tendency but they were not significant. This might be due to the small number of patients in this study. Previously, we performed a randomized controlled study switching from ETV to TAF and reported that HBsAg decreased significantly in the TAF group in patients with advanced fibrosis (Sato et al. 2022). In the present study as well, although there was no significant difference, the amount of the %HBsAg decline tended to be larger in the group with a high FIB-4 index. In addition, it was observed that the serum HBsAg levels tended to decrease more in the group with low ALT, low HBsAg at the time of switching. The present study suggests that TAF switching might have a stronger effect on HBsAg decline in patients with reduced viral activity at the time of switching. Murata et al. (2020) reported additional immunological effects of acyclic nucleotide phosphonates, adefovir dipivoxil (ADV), TDF and TAF in comparison with lamivudine or ETV. It was suggested that TAF may contribute to the reduction of HBsAg by not only the reverse transcriptase inhibitory effect, but also mediating the immune system. Moreover, LLV may be associated with the incomplete ETV medication adherence or the development of drug resistance to ETV. ETV should be taken on an empty stomach, but TAF can be administered independently of meal timing. It has been reported that switching from ETV to TAF associated with fewer missed doses (Uchida et al. 2020), which may contribute to stronger antiviral effects. However, we did not obtain data on this point in the present study.
There are several limitations in this study. First, the number of patients analyzed in the study was relatively small. Although HBV DNA detection frequency was significantly reduced after NA switching to TAF, there was no significant difference in the amount of change in HBsAg. This point should be evaluated in a larger cohort. Second, this is a retrospective study and there is no control group. Third, the observation period was only 2 years and there were no data on the progression of fibrosis or the development of HCC.
In conclusion, it is suggested that switching from ETV to TAF may provide further viral suppression in patients whose serum HBV DNA was detectable, even if not in all tests, during ETV therapy. A study in a larger cohort with a longer observation period is required to evaluate the actual merit of NA switching from ETV to TAF.
Conflict of Interest
Jun Inoue received research funding from AbbVie. The other authors declare no conflict of interest.
References
-
Agarwal,
K.,
Brunetto,
M.,
Seto,
W.K.,
Lim,
Y.S.,
Fung,
S.,
Marcellin,
P.,
Ahn,
S.H.,
Izumi,
N.,
Chuang,
W.L.,
Bae,
H.,
Sharma,
M.,
Janssen,
H.L.A.,
Pan,
C.Q.,
Çelen,
M.K.,
Furusyo,
N.,
et al. (2018) 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J. Hepatol., 68, 672-681.
-
Buti,
M.,
Gane,
E.,
Seto,
W.K.,
Chan,
H.L.,
Chuang,
W.L.,
Stepanova,
T.,
Hui,
A.J.,
Lim,
Y.S.,
Mehta,
R.,
Janssen,
H.L.,
Acharya,
S.K.,
Flaherty,
J.F.,
Massetto,
B.,
Cathcart,
A.L.,
Kim,
K.,
et al. (2016) Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol., 1, 196-206.
-
Chan,
H.L.,
Fung,
S.,
Seto,
W.K.,
Chuang,
W.L.,
Chen,
C.Y.,
Kim,
H.J.,
Hui,
A.J.,
Janssen,
H.L.,
Chowdhury,
A.,
Tsang,
T.Y.,
Mehta,
R.,
Gane,
E.,
Flaherty,
J.F.,
Massetto,
B.,
Gaggar,
A.,
et al. (2016) Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol., 1, 185-195.
-
European Association for the Study of the Liver
(2017) EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol., 67, 370-398.
-
Inoue,
J.,
Akahane,
T.,
Kobayashi,
T.,
Obara,
N.,
Umetsu,
T.,
Kakazu,
E.,
Ninomiya,
M.,
Iwata,
T.,
Sano,
A.,
Tsuruoka,
M.,
Sato,
K. &
Masamune,
A.
(2021) Switching to tenofovir disoproxil fumarate in entecavir-treated chronic hepatitis B patients: a pilot randomized controlled study. Biomed. Rep., 14, 20.
-
Inoue,
J.,
Akahane,
T.,
Nakayama,
H.,
Kimura,
O.,
Kobayashi,
T.,
Kisara,
N.,
Sato,
T.,
Morosawa,
T.,
Izuma,
M.,
Kakazu,
E.,
Ninomiya,
M.,
Iwata,
T.,
Takai,
S.,
Nakamura,
T.,
Sano,
A.,
et al. (2019) Comparison of hepatitis B virus genotypes B and C among chronically hepatitis B virus-infected patients who received nucleos(t)ide analogs: a multicenter retrospective study. Hepatol. Res., 49, 1263-1274.
-
Inoue,
J.,
Kobayashi,
T.,
Akahane,
T.,
Kimura,
O.,
Sato,
K.,
Ninomiya,
M.,
Iwata,
T.,
Takai,
S.,
Kisara,
N.,
Sato,
T.,
Nagasaki,
F.,
Miura,
M.,
Nakamura,
T.,
Umetsu,
T.,
Sano,
A.,
et al. (2022) Non-achievement of alanine aminotransferase normalization associated with the risk of hepatocellular carcinoma during nucleos(t)ide analogue therapies: a multicenter retrospective study. J. Clin. Med., 11, 2354.
-
Jung,
H.J.,
Ho,
M.J.,
Ahn,
S.,
Han,
Y.T. &
Kang,
M.J.
(2018) Synthesis and physicochemical evaluation of entecavir-fatty acid conjugates in reducing food effect on intestinal absorption. Molecules, 23, 731.
-
Kaneko,
S.,
Kurosaki,
M.,
Joko,
K.,
Marusawa,
H.,
Kondo,
M.,
Kojima,
Y.,
Uchida,
Y.,
Kimura,
H.,
Tsuji,
K.,
Yagisawa,
H.,
Kusakabe,
A.,
Kobashi,
H.,
Akahane,
T.,
Tamaki,
N.,
Kirino,
S.,
et al. (2020) Detectable HBV DNA during nucleos(t)ide analogues stratifies predictive hepatocellular carcinoma risk score. Sci. Rep., 10, 13021.
-
Kim,
J.H.,
Sinn,
D.H.,
Kang,
W.,
Gwak,
G.Y.,
Paik,
Y.H.,
Choi,
M.S.,
Lee,
J.H.,
Koh,
K.C. &
Paik,
S.W.
(2017) Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology, 66, 335-343.
-
Koike,
K.,
Suyama,
K.,
Ito,
H.,
Itoh,
H. &
Sugiura,
W.
(2018) Randomized prospective study showing the non-inferiority of tenofovir to entecavir in treatment-naïve chronic hepatitis B patients. Hepatol. Res., 48, 59-68.
-
Li,
Z.B.,
Li,
L.,
Niu,
X.X.,
Chen,
S.H.,
Fu,
Y.M.,
Wang,
C.Y.,
Liu,
Y.,
Shao,
Q.,
Chen,
G. &
Ji,
D.
(2021) Switching from entecavir to tenofovir alafenamide for chronic hepatitis B patients with low-level viraemia. Liver Int., 41, 1254-1264.
-
Liaw,
Y.F.,
Sung,
J.J.,
Chow,
W.C.,
Farrell,
G.,
Lee,
C.Z.,
Yuen,
H.,
Tanwandee,
T.,
Tao,
Q.M.,
Shue,
K.,
Keene,
O.N.,
Dixon,
J.S.,
Gray, D.F. & Sabbat, J.; Cirrhosis Asian Lamivudine Multicentre Study Group
(2004) Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med., 351, 1521-1531.
-
Marcellin,
P.,
Chang,
T.T.,
Lim,
S.G.,
Tong,
M.J.,
Sievert,
W.,
Shiffman,
M.L.,
Jeffers,
L.,
Goodman,
Z.,
Wulfsohn,
M.S.,
Xiong,
S.,
Fry, J. & Brosgart, C.L.; Adefovir Dipivoxil 437 Study Group
(2003) Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med., 348, 808-816.
-
McPherson,
S.,
Stewart,
S.F.,
Henderson,
E.,
Burt,
A.D. &
Day,
C.P.
(2010) Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut, 59, 1265-1269.
-
Murata,
K.,
Tsukuda,
S.,
Suizu,
F.,
Kimura,
A.,
Sugiyama,
M.,
Watashi,
K.,
Noguchi,
M. &
Mizokami,
M.
(2020) Immunomodulatory mechanism of acyclic nucleoside phosphates in treatment of hepatitis B virus infection. Hepatology, 71, 1533-1545.
-
Nguyen,
M.H.,
Atsukawa,
M.,
Ishikawa,
T.,
Yasuda,
S.,
Yokohama,
K.,
Trinh,
H.N.,
Arai,
T.,
Fukunishi,
S.,
Ogawa,
E.,
Hsu,
Y.C.,
Maeda,
M.,
Dang,
H.,
Tseng,
C.H.,
Takahashi,
H.,
Jun,
D.W.,
et al. (2021) Outcomes of sequential therapy with tenofovir alafenamide after long-term entecavir. Am. J. Gastroenterol., 116, 1264-1273.
-
Ogawa,
E.,
Nakamuta,
M.,
Koyanagi,
T.,
Ooho,
A.,
Furusyo,
N.,
Kajiwara,
E.,
Dohmen,
K.,
Kawano,
A.,
Satoh,
T.,
Takahashi,
K.,
Azuma,
K.,
Yamashita,
N.,
Yamashita,
N.,
Sugimoto,
R.,
Amagase,
H.,
et al. (2022) Switching to tenofovir alafenamide for nucleos(t)ide analogue-experienced patients with chronic hepatitis B: week 144 results from a real-world, multicentre cohort study. Aliment. Pharmacol. Ther., 56, 713-722.
-
Papatheodoridis,
G.V.,
Chan,
H.L.,
Hansen,
B.E.,
Janssen,
H.L. &
Lampertico,
P.
(2015) Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J. Hepatol., 62, 956-967.
-
Sarin,
S.K.,
Kumar,
M.,
Lau,
G.K.,
Abbas,
Z.,
Chan,
H.L.,
Chen,
C.J.,
Chen,
D.S.,
Chen,
H.L.,
Chen,
P.J.,
Chien,
R.N.,
Dokmeci,
A.K.,
Gane,
E.,
Hou,
J.L.,
Jafri,
W.,
Jia,
J.,
et al. (2016) Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int., 10, 1-98.
-
Sato,
K.,
Inoue,
J.,
Akahane,
T.,
Kobayashi,
T.,
Sato,
S.,
Kisara,
N.,
Ninomiya,
M.,
Iwata,
T.,
Sano,
A.,
Tsuruoka,
M.,
Onuki,
M. &
Masamune,
A.
(2022) Switching to tenofovir alafenamide versus continued therapy in chronic hepatitis B patients who were treated with entecavir: a prospective, multicenter, randomized controlled study. Medicine (Baltimore), 101, e30630.
-
Singal,
A.K.,
Salameh,
H.,
Kuo,
Y.F. &
Fontana,
R.J.
(2013) Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment. Pharmacol. Ther., 38, 98-106.
-
Sinn,
D.H.,
Lee,
J.,
Goo,
J.,
Kim,
K.,
Gwak,
G.Y.,
Paik,
Y.H.,
Choi,
M.S.,
Lee,
J.H.,
Koh,
K.C.,
Yoo,
B.C. &
Paik,
S.W.
(2015) Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology, 62, 694-701.
-
Sterling,
R.K.,
Lissen,
E.,
Clumeck,
N.,
Sola,
R.,
Correa,
M.C.,
Montaner,
J.,
Sulkowski M.S Torriani,
F.J.,
Dieterich,
D.T.,
Thomas,
D.L.,
Messinger, D. & Nelson, M.; APRICOT Clinical Investigators
(2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology, 43, 1317-1325.
-
Sun,
Y.,
Wu,
X.,
Zhou,
J.,
Meng,
T.,
Wang,
B.,
Chen,
S.,
Liu,
H.,
Wang,
T.,
Zhao,
X.,
Wu,
S.,
Kong,
Y.,
Ou,
X.,
Wee,
A.,
Theise,
N.D.,
Qiu,
C.,
et al. (2020) Persistent low level of hepatitis B virus promotes fibrosis progression during therapy. Clin. Gastroenterol. Hepatol., 18, 2582-2591.e2586.
-
Terrault,
N.A.,
Lok,
A.S.F.,
McMahon,
B.J.,
Chang,
K.M.,
Hwang,
J.P.,
Jonas,
M.M.,
Brown,
R.S. Jr.,
Bzowej,
N.H. &
Wong,
J.B.
(2018) Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology, 67, 1560-1599.
-
Uchida,
Y.,
Nakao,
M.,
Tsuji,
S.,
Uemura,
H.,
Kouyama,
J.I.,
Naiki,
K.,
Motoya,
D.,
Sugawara,
K.,
Nakayama,
N.,
Imai,
Y.,
Tomiya,
T. &
Mochida,
S.
(2020) Significance of switching of the nucleos(t)ide analog used to treat Japanese patients with chronic hepatitis B virus infection from entecavir to tenofovir alafenamide fumarate. J. Med. Virol., 92, 329-338.
-
Uchida,
Y.,
Nakao,
M.,
Yamada,
S.,
Tsuji,
S.,
Uemura,
H.,
Kouyama,
J.I.,
Naiki,
K.,
Sugawara,
K.,
Nakayama,
N.,
Imai,
Y.,
Tomiya,
T. &
Mochida,
S.
(2022) Superiority of tenofovir alafenamide fumarate over entecavir for serum HBsAg level reduction in patients with chronic HBV infection: a 144-week outcome study after switching of the nucleos(t)ide analog. PLoS One, 17, e0262764.
-
Yamashige,
D.,
Hosaka,
T.,
Suzuki,
F.,
Fujiyama,
S.,
Kawamura,
Y.,
Sezaki,
H.,
Akuta,
N.,
Kobayashi,
M.,
Suzuki,
Y.,
Saitoh,
S.,
Arase,
Y.,
Ikeda,
K.,
Kobayashi,
M. &
Kumada,
H.
(2021) Effectiveness of tenofovir alafenamide for chronic hepatitis B patients with a poor response to the previously used nucleos(t)ide analogs. J. Gastroenterol., 56, 1008-1021.