2022 Volume 258 Issue 4 Pages 287-301
2022 Volume 258 Issue 4 Pages 287-301
We report three cases of Waterhouse-Friderichsen syndrome (WFS) that were confirmed during forensic autopsies. Case 1 involved a man in his 50s post-splenectomy. Bacteriological examination revealed Streptococcus pneumoniae (S. pneumonia). The patient was considered to have died of asphyxiation after aspirating vomit. Case 2 involved a man in his 40s. Bacteriological examination again revealed S. pneumoniae. Histopathological examination showed hypoplasia of the spleen. This patient was considered to have died of multiple-organ failure due to sepsis, disseminated intravascular coagulation, and WFS. Case 3 involved a post-splenectomy woman in her 60s with a history of systemic lupus erythematosus. Bacteriological examination revealed Streptococcus oralis. This patient was considered to have died of multiple-organ failure due to sepsis, disseminated intravascular coagulation, and WFS. These three cases were included among forensic autopsies conducted in the last 5 years. WFS has been considered a rare disease, but may be more frequent than previously assumed. If a mildly ill patient displays a sudden change in status and dies within a short period of time, we consider it necessary to perform not only bacteriological examinations, but also histopathological examination of the spleen during autopsy.
Waterhouse-Friderichsen syndrome (WFS) is a rare disease that occurs as a result of sepsis, causing acute hemorrhagic necrosis of bilateral adrenal glands (acute adrenal insufficiency), dot hemorrhages of the skin and shock, and often resulting in death within 24 h (Fox 1971; Hamilton et al. 2004; Wu et al. 2020). The prevalence of this disease is unclear, but bilateral adrenal hemorrhage is seen in approximately 1% of routine autopsies (Xarli et al. 1978; Karki et al. 2021). Although the mechanism of hemorrhage is unknown, increased capillary resistance may facilitate the development of adrenal hemorrhage, and stress-induced increases in adrenocorticotropic hormone (ACTH) and catecholamines may cause vasoconstriction and platelet aggregation, contributing to subsequent hemorrhage (Kovacs et al. 2001; Dhawan et al. 2015; Kumar et al. 2015; Karwacka et al. 2018).
The most frequent chief complaint in the adrenal hemorrhage is pain, such as upper abdominal pain, lateral abdominal pain, and back pain, followed by fever, hypotension, and nausea (Rao et al. 1989; Matsuzawa et al. 2015). Disseminated intravascular coagulation (DIC) symptoms also occur in patients with WFS, and skin hemorrhages, as a somewhat specific finding in WFS among many nonspecific symptoms, has been suggested to be caused by DIC (Margaretten and McAdams 1958; Fox 1971). However, whether adrenal hemorrhage is caused by DIC or endotoxemia is unclear (Fox 1971; Karki et al. 2021). The main bacterium detected in WFS cases is Neisseria meningitidis, but many cases caused by other bacteria [e.g., Streptococcus pneumoniae (S. pneumoniae)] have also been reported (Fox 1971; Hamilton et al. 2004; Hata et al. 2015; Karki et al. 2021). Some cases also show splenic dysfunction, which is considered an important risk factor for WFS (Hata et al. 2015). Computed tomography (CT) is useful for detecting adrenal hemorrhage, which appears as focal heterogeneous high-density lesions on imaging (Karwacka et al. 2018).
We report three cases of non-meningococcal WFS in adults with hypoplastic spleen or post-splenectomy status that were confirmed in forensic autopsies during a 5-year period. All showed findings suggestive of sudden death, such as petechiae. The characteristics of each case are shown in Table 1.
The presentation of this case report was approved by the Ethics Committee of the Faculty of Medicine, University of Yamanashi (No. 2605).
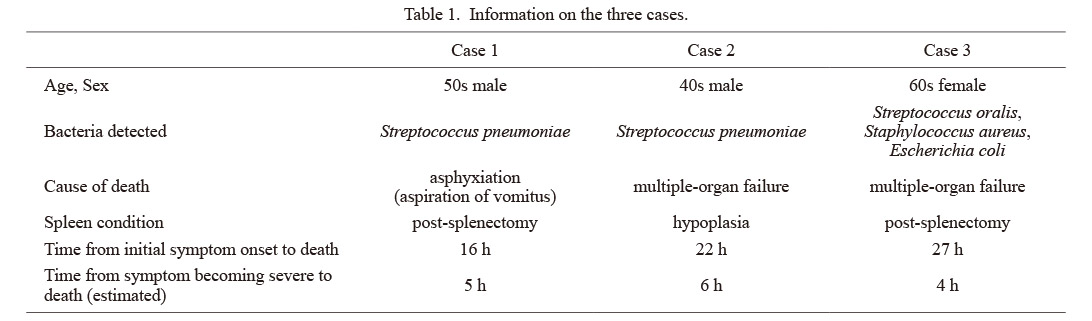
Information on the three cases.
Medical history: The patient was a man in his 50s. His spleen had been removed in childhood following a traffic accident. After having dinner with his family around 19:00, he developed chills and fever around 21:00. Vomiting, diarrhea, and pain in the lower limbs started from around 23:00 and worsened, so he visited the hospital around 02:00 the next day. His temperature at that time was 39.8°C. After undergoing blood examination and placement of an intravenous drip, he was prescribed antibiotics and returned home around 05:00. However, vomiting recurred and pain in the lower limbs did not improve. Pain in the lower limbs was alleviated after he took an antipyretic analgesic, and he went to bed around 09:00. Around 13:00, his family found him unconscious in the toilet and called an ambulance. His heart was in a state of pulseless electrical activity when the ambulance team arrived, and purpura-like rash was also seen on the face. Chest compressions were performed, but no resumption of heartbeat was achieved. Death was confirmed at 13:55.
Blood examination data from the hospital the patient had visited at 02:00 are shown in Table 2. The vaccination history of the patient was unknown. Rectal temperature at the time of investigation by the police about 2 h after death was 39°C.
Forensic autopsy and examination findings, and cause of death: Autopsy was performed approximately 48 h after death. The patient was 162 cm tall and weighed 60.8 kg. His face was highly congested. Erythemas were found in the face, thoracoabdominal region, and left and right inguinal regions (Fig. 1). Severe hemorrhage was found in almost all of the bilateral adrenal glands (Fig. 2a, b). In addition, swelling of the cervical and hilar lymph nodes, enlarged tonsils, and severe redness of the pharynx and larynx (Fig. 2c) were found. A large amount of food residue was present in the airways, almost completely obstructing the right and left main bronchi (Fig. 2c). A 3 × 2-cm decomposing discoloration was also identified in the right lower abdomen.
In the histopathological examination, bilateral adrenal glands were highly hemorrhagic or necrotic, mainly in the cortex (Fig. 3). The lungs showed relatively severe congestive edema, and some bronchi were filled with food residue. Hemophagocytic macrophages were identified in the bone marrow.
In the bacteriological examination, a small number of S. pneumoniae and Escherichia coli (E. coli) were detected in swabs of the subarachnoid space, respectively. Blood culture examination was not performed.
Based on all of the above findings, the patient was considered to have developed WFS after developing sepsis. The patient was considered to have died of asphyxiation due to aspiration of vomitus by impaired consciousness caused by WFS.

Blood examination results for Case 1.
*reference values in male.
CRP, C-reactive protein; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; γ-GTP, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; UA, uric acid; CK, creatinine kinase.

Erythematous or purpura-like rash spots on the skin in Case 1.
a) Thoracoabdominal region. b) Inguinal area. c) Enlargement of the left inguinal area in b. d) Enlargement of the right inguinal area in b.

Macroscopic findings in Case 1.
a) Split surface of the left adrenal gland. b) Split surface of the right adrenal gland. c) Severe redness of the pharynx and larynx.
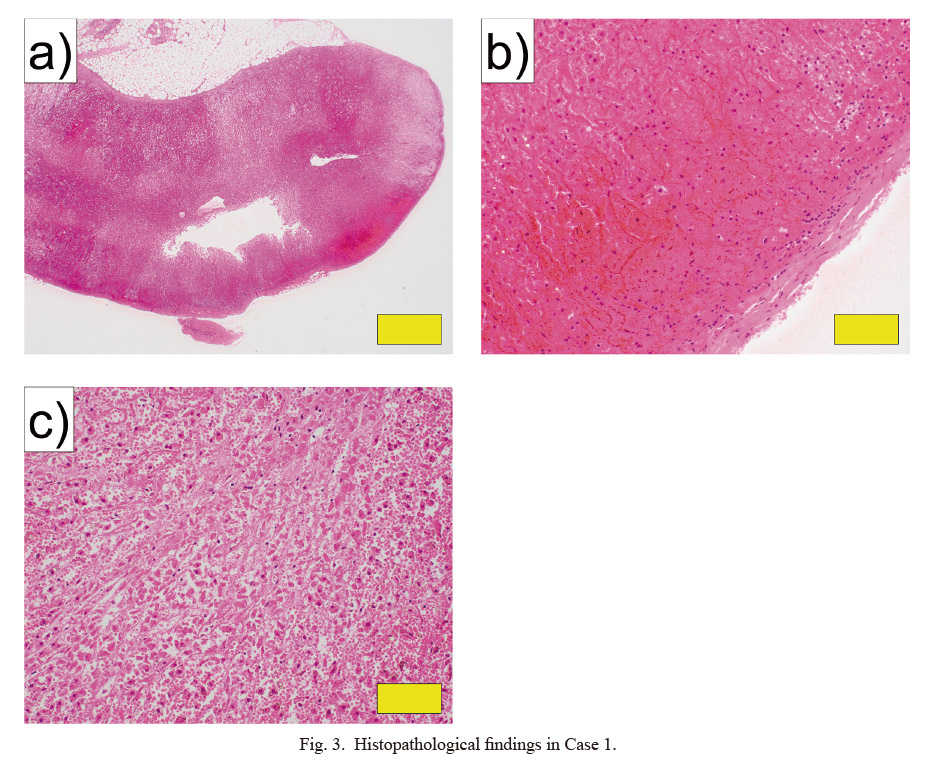
Histopathological findings in Case 1.
a, b) Adrenal gland (HE stain: a, × 20; b, × 200). c) Necrosis of the adrenal gland (HE stain: × 200). Yellow bars indicate 1 mm in a, and 100 μm in b and c.
HE, hematoxylin eosin.
Medical history: The patient was a male in his 40s with a history of gout. He was fishing with his friend from around 23:00 (minimum temperature, approximately 28°C). He continued fishing without taking a break, and drank 1,500 ml of beer while fishing. He took a break from around 02:00 the next day, and vomited around 04:00, so he and his friend returned home. Headache, lower limb cramps, and hand convulsions appeared and worsened, so he went to the general hospital around 08:00. The patient showed fever (40.5°C) with chills and shivers. Heatstroke was suspected, so fluid replacement and antipyretic analgesic were administered, but symptoms remained unimproved and he was hospitalized. Around 15:00 on the same day, he was able to talk and defecate on his own, and CT showed no adrenal hemorrhage. After hospitalization, he complained of right lower back pain, and cyanosis, hypoglycemia, decreased urine volume, and bloody stool appeared after 20:00. Body temperature was 35.2℃ at 21:00. Blood glucose level increased to 171 mg/dL after glucose administration. However, SpO2 decreased to 80% around 22:00, then became unmeasurable, and blood glucose level decreased to 31 mg/dL again. Liver failure, renal failure, and DIC were suspected. Around midnight, he was transferred to another hospital for dialysis. However, a few minutes after starting the transfer, he entered cardiopulmonary arrest (CPA), and cardiac rhythm could not be restored. Death was confirmed at 01:39, shortly after arrival at the receiving hospital.
Table 3 shows blood examination data from the general hospitals. Value 1 data were obtained at 09:00 (immediately after visiting hospital), and Value 2 data were obtained at 21:00 (at the time of the sudden change). The vaccination history of the patient was unknown.
Forensic autopsy and examination findings, and cause of death: Autopsy was performed approximately 48 h after death. The patient was 168 cm tall and weighed 76.5 kg. The skin of the whole body appeared highly congested, with many skin hemorrhages (Fig. 4). Strong hemorrhage was noted on the split surface of bilateral adrenal glands (Fig. 5a, b). The spleen weighed 38 g (Fig. 5c, d). In addition, both the pharynx and larynx showed severe redness.
In the histopathological examination, bilateral adrenal glands showed necrosis and strong hemorrhage, mainly in the cortex (Fig. 6a-c). In the kidneys, thrombi were evident inside most glomeruli (Fig. 6d). In addition, hemorrhage found in the heart was attributed to DIC. The spleen was hypoplastic, with about half the normal amount of white pulp and a small number of CD20-positive cells in the white pulp (Figs. 6e, f and 7). Furthermore, numbers of CD3-, CD5-, CD21-, and Ki67-positive cells were also reduced (Fig. 8). More than moderate infiltration of neutrophils and lymphocytes was found in the lungs and small intestine. Hemophagocytic macrophages were found in the bone marrow.
In the bacteriological examination, blood culture examination at the time of initial hospitalization detected S. pneumoniae and Fusobacterium mortiferum.
Based on all of the above findings, the patient was considered to have developed WFS and DIC after developing sepsis. The patient was considered to have died of multiple-organ failure due to sepsis, DIC, and WFS.
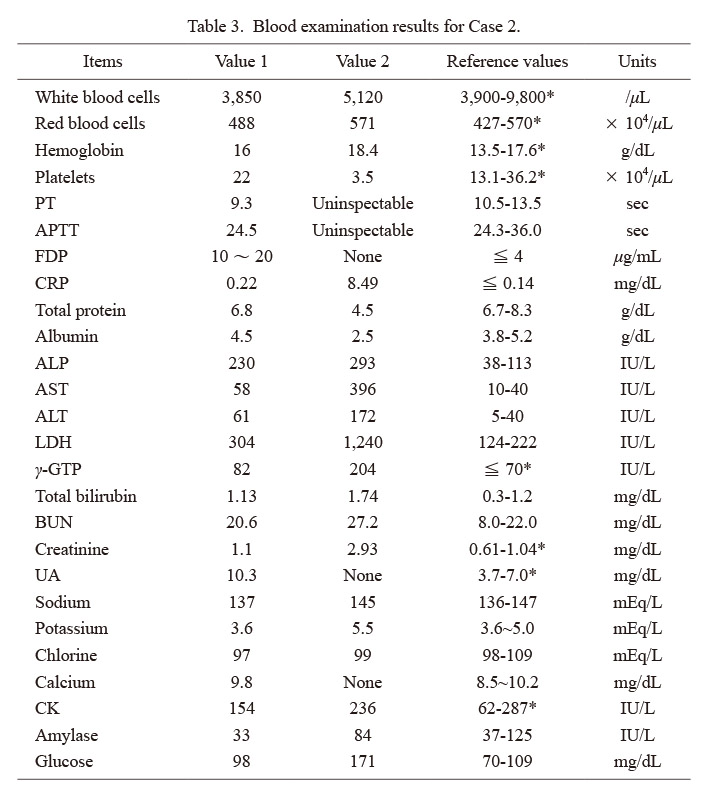
Blood examination results for Case 2.
*reference value in male.
Values 1 and 2 were measured at 09:00 and 21:00, respectively.
PT, prothrombin time; APTT, activated partial thromboplastin time; FDP, fibrin degradation product; CRP, C-reactive protein; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; γ-GTP, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; UA, uric acid; CK, creatine kinase.
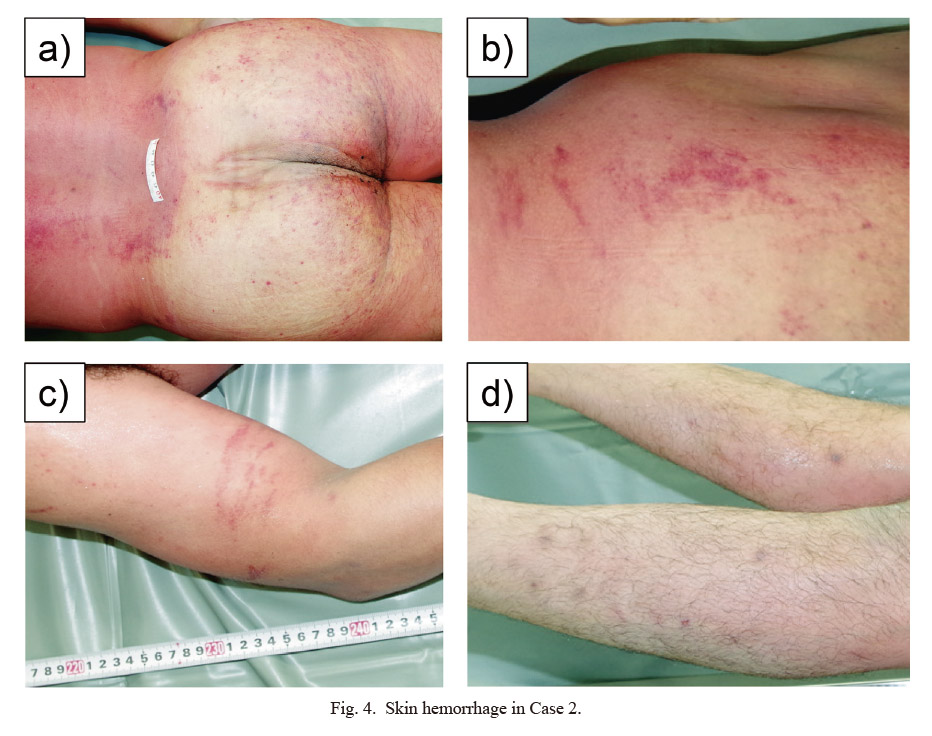
Skin hemorrhage in Case 2.
a) Gluteal region. b) Right waist region. c) Right upper arm. d) Bilateral lower legs.
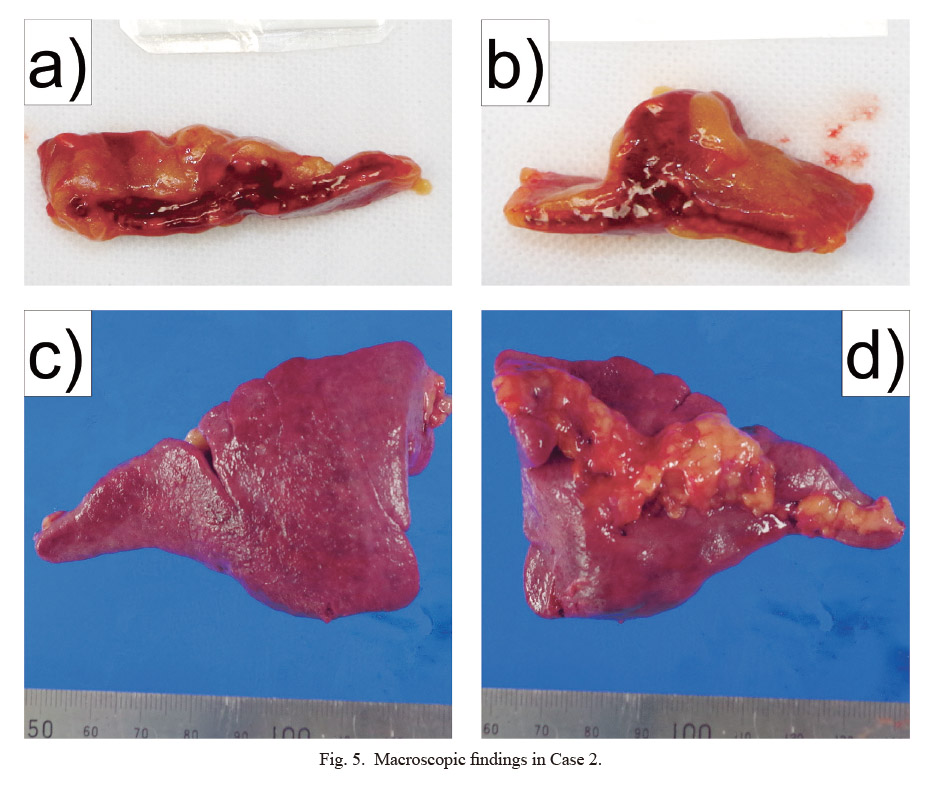
Macroscopic findings in Case 2.
a) Split surface of the left adrenal gland. b) Split surface of the right adrenal gland. c) Anterior view of the spleen. d) Posterior view of the spleen.
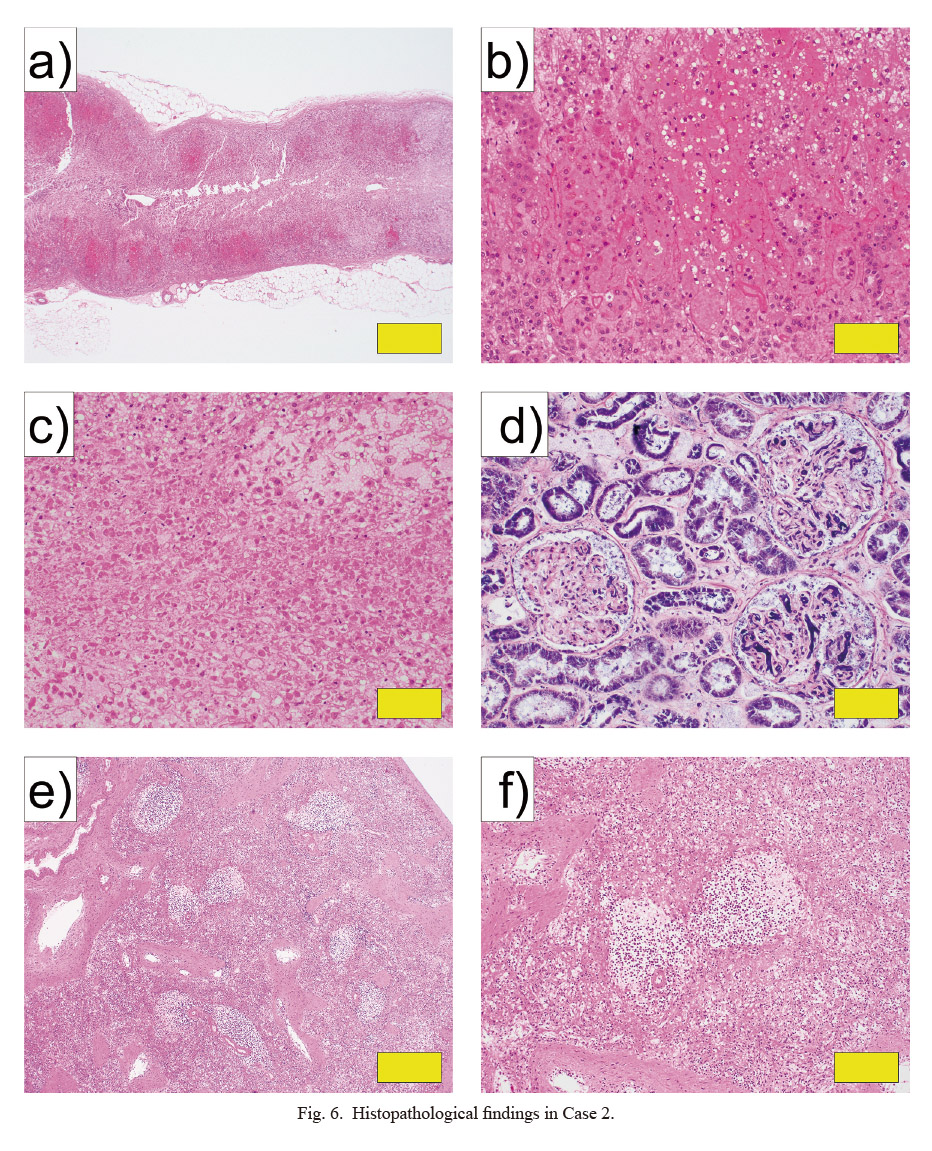
Histopathological findings in Case 2.
a, b) Adrenal gland (HE stain: a, × 20; b, × 200). c) Necrosis of the adrenal gland (HE stain: × 200). d) Kidney (PTAH stain: × 200). e, f) Spleen (HE stain: g, × 40; h, × 100). Yellow bars indicate 1 mm in a, 100 μm in b-d, 500 μm in e, 200 μm in f.
HE, hematoxylin eosin; PTAH, phosphotungstic acid hematoxylin.
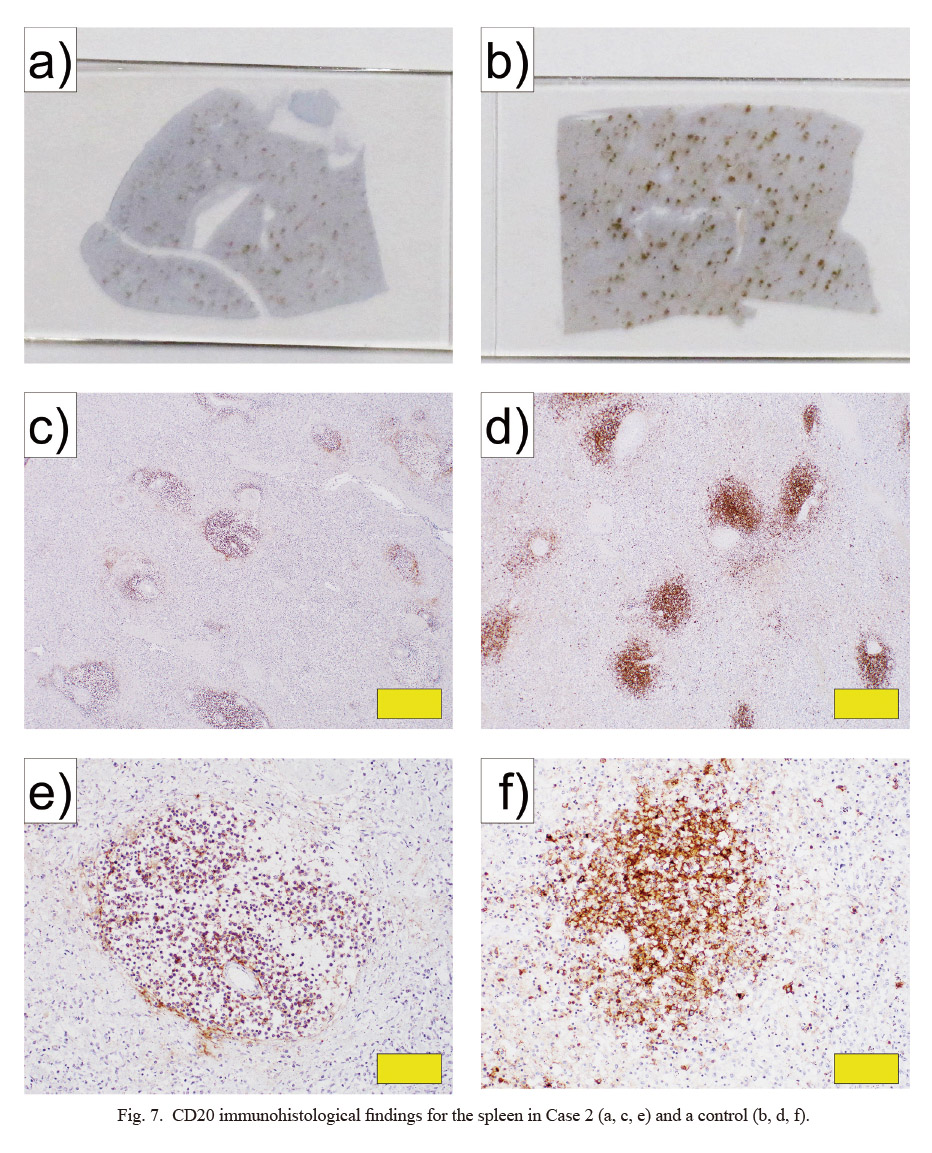
CD20 immunohistological findings for the spleen in Case 2 (a, c, e) and a control (b, d, f).
a, b) Appearance of specimen slides. c-f) Pathological tissues (c, d: × 40; e, f: × 200). a, c, e) Spleen from Case 2. b, d, f) Normal spleen of a male in his 60s (control). Yellow bars indicate 500 μm in c and d, and 100 μm in e and f.
CD, cluster of differentiation.
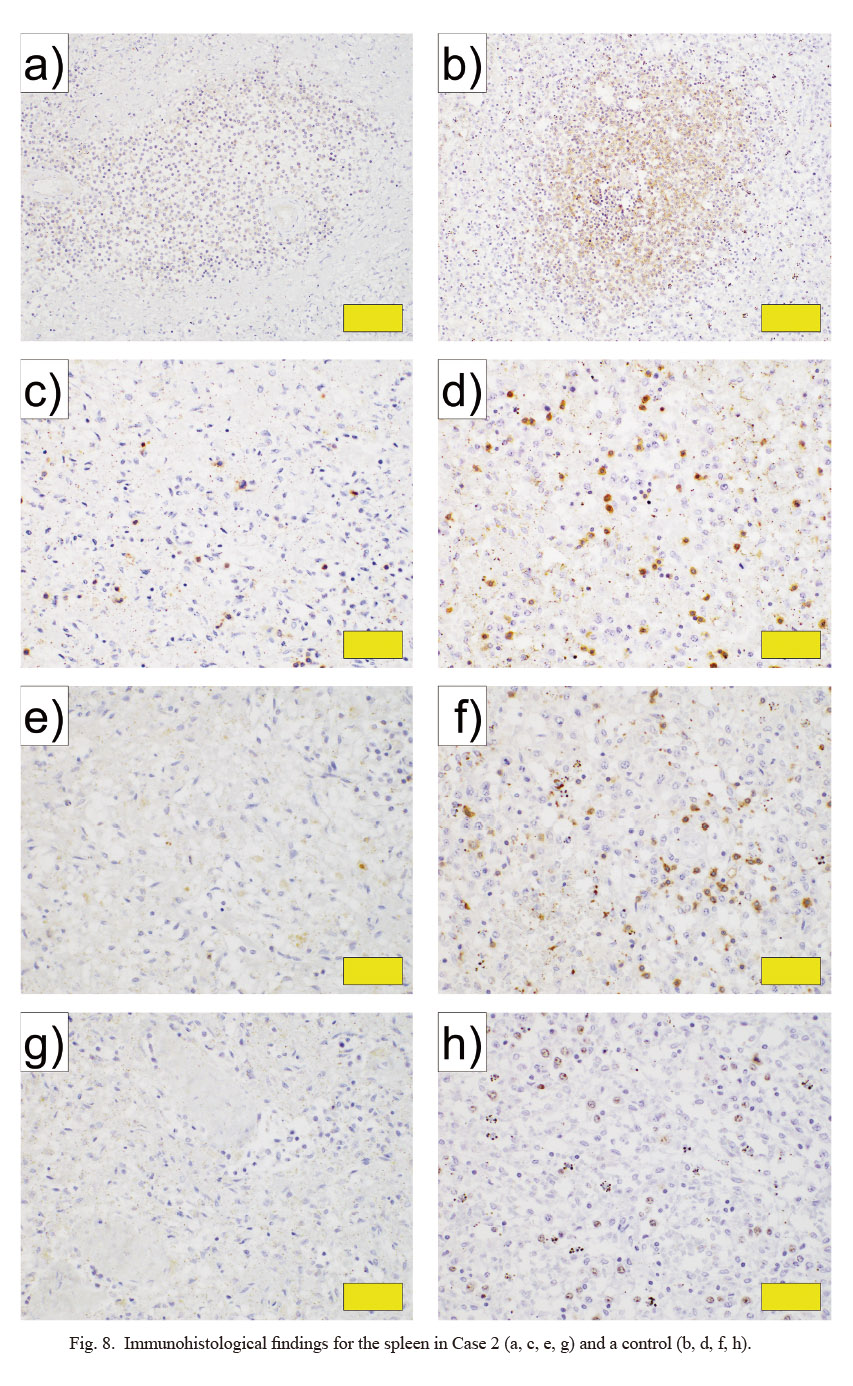
Immunohistological findings for the spleen in Case 2 (a, c, e, g) and a control (b, d, f, h).
a, b) CD21 (white pulp, × 200). c, d) CD3 (red pulp, × 400). e, f) CD5 (red pulp, × 400). g, h) Ki67 (red pulp, × 400). a, c, e, g) Spleen from Case 2. b, d, f, h) Normal spleen of a male in his 60s (control). Yellow bars indicate 100 μm in a and b, and 50 μm in c-h.
CD, cluster of differentiation.
Medical history: The patient was a woman in her 60s who had developed systemic lupus erythematosus (SLE) at 15 years old. She and her father received influenza vaccinations from a visiting doctor around noon. She noted numbness in the lower limbs, difficulty with body movement, and appearance of dysarthria around 16:00. The next day, symptoms had not improved and her father found her lying down but conscious at around 15:00. She called for an ambulance on her own, but was in CPA on arrival at the hospital. Her heartbeat resumed after chest compressions and administration of adrenaline, but ventricular fibrillation occurred several minutes later. Chest compressions were performed again, and her heartbeat resumed. However, her pulse decreased from around 18:50 and she did not respond to administration of adrenaline. Death was confirmed at 19:00.
Blood examination data from the family physician (Value 1: 3 years and 9 months before death; Value 2: 2 months before death) and the hospital to which the patient was transported at the time of death (Value 3) are shown in Table 4. The patient also had autoimmune hemolytic anemia (AIHA) and thrombocytopenic purpura at about the same time as the onset of SLE, and her spleen had been removed for treatment. These diseases had been in relative remission in recent years, but steroid administration was continued as replacement therapy due to adrenal insufficiency. The patient had also received vaccination for S. pneumoniae about a year before death. Her medication compliance appeared good. The medications she had been taking were prednisolone, atorvastatin, alendronate, and loxoprofen.
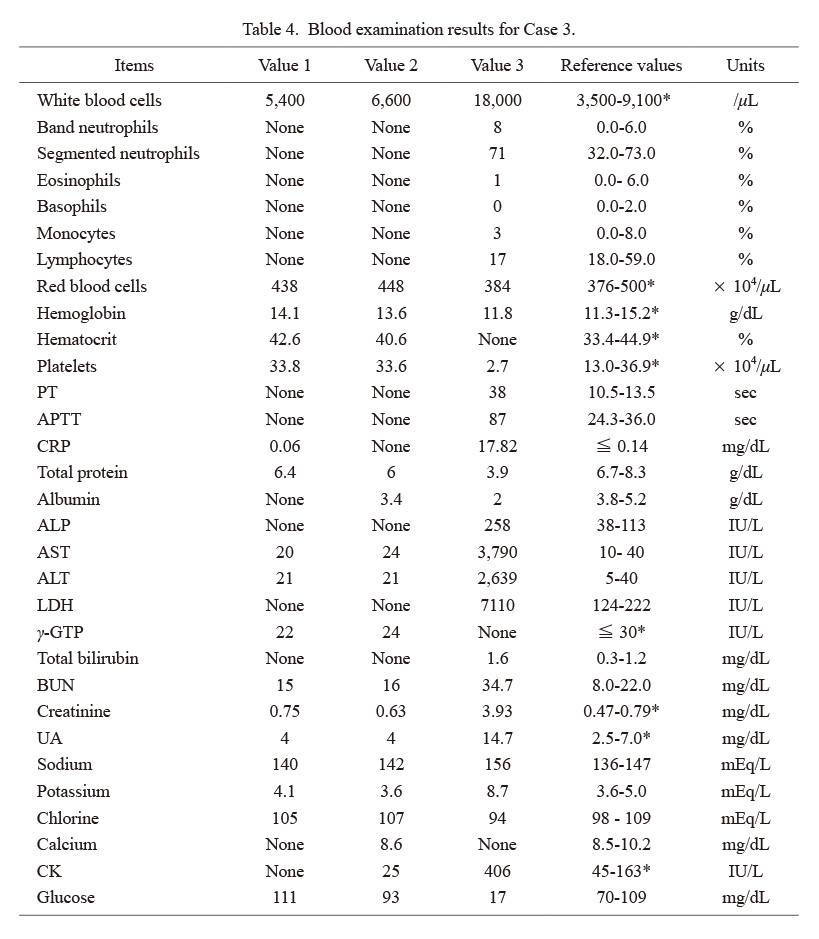
Forensic autopsy and examination findings, and cause of death: Autopsy was performed approximately 38 h after death. She was 153 cm tall and weighed 54.7 kg. The skin over the entire body was highly congested with many egg-sized or smaller hemorrhagic spots on the skin surface (Fig. 9). An 18 × 1-cm old scar was apparent in the left hypochondriac region. Bilateral adrenal glands were highly atrophic, and rather severe hemorrhage was evident over the entire split surface (Fig. 10a, b). The spleen was absent. Moderate numbers of hemorrhages of 2 × 2 cm or smaller were scattered throughout the greater omentum, mesentery, and retroperitoneum. Bilateral ovarian hemorrhages were also found (Fig. 10c). Many hemorrhages of 0.3 × 0.3 cm or smaller were apparent in the posterior part of the right occipital lobe of the brain (Fig. 10d). These hemorrhages were attributed to DIC. In addition, redness of the pharynx and larynx was found.
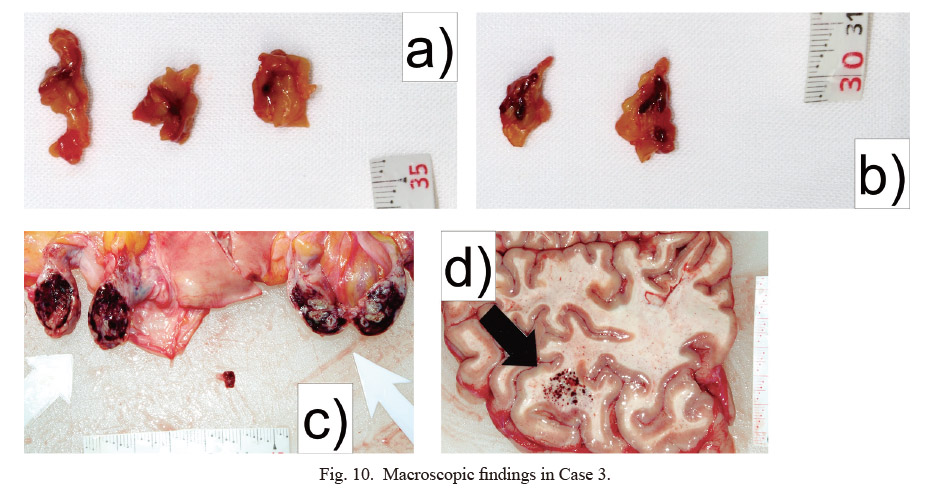
In the histopathological examination, bilateral adrenal glands were highly atrophic, with necrosis and strong hemorrhage centered on the cortex (Fig. 11a-c). Thrombi were found in most glomeruli in the kidneys (Fig. 11d), and hemorrhage evident in the heart, lungs, liver, and kidneys was attributed to DIC. Hemophagocytic macrophages were found in the bone marrow.
In the bacteriological examination, blood culture examinations identified E. coli, Staphylococcus aureus, and Streptococcus oralis. In addition, E. coli and Staphylococcus aureus were detected from pharyngeal swabs.
Based on all of the above findings, the patient was considered to have developed WFS and DIC after developing sepsis. The patient was considered to have died of multiple-organ failure due to sepsis, DIC, and WFS.
Blood examination results for Case 3.
*reference value in female
Values 1 and 2 were measured by the family physician treating SLE, approximately 3 years 9 months and 2 months before death, respectively. Value 3 was measured at the hospital to which the patient was transported at the time of death.
PT, prothrombin time; APTT, activated partial thromboplastin time; CRP, C-reactive protein; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; γ-GTP, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; UA, uric acid; CK, creatine kinase.

Hemorrhagic spots in Case 3.
a) Right buttock. b) Medial side of bilateral thighs. c) Enlargement of the medial side of the left thigh from b. d) Enlargement of the medial side of the right thigh from b.
Macroscopic findings in Case 3.
a) Split surface of the left adrenal gland. b) Split surface of the right adrenal gland. c) Bilateral ovarian hemorrhage. d) Brain (right occipital lobe) hemorrhage.
Histopathological findings in Case 3.
a, b) Adrenal gland (HE stain: a, × 40; b, × 200). c) Necrosis of the adrenal gland (HE stain: × 200). d) Kidney (PTAH stain: × 200). Yellow bars indicate 500 μm in a, and 100 μm in b-d.
HE, hematoxylin eosin; PTAH, phosphotungstic acid hematoxylin.
The spleen has a phagocytic filtering function, removing blood-derived microorganisms and producing antibodies (Di Sabatino et al. 2011). Most bacteria are first opsonized and then removed by macrophages in the spleen and liver. However, for encapsulated bacteria such as S. pneumoniae, opsonization is insufficient because the capsule prevents complement binding. Removal of these bacteria requires immunoglobulin (Ig)M, produced by IgM memory B cells in the spleen, which can facilitate phagocytosis either directly or via complement deposition on the capsule (Kruetzmann et al. 2003; Di Sabatino et al. 2011). Therefore, in hyposplenism (as either splenic dysfunction or a post-splenectomy state), removal of these bacteria does not proceed and the risk of developing sepsis is elevated (Davidson and Wall 2001). The aggravation of infectious diseases after splenectomy is called “overwhelming post-splenectomy infection” (OPSI), and requires timely response (Davidson and Wall 2001; Di Sabatino et al. 2011). In addition, spleen atrophy and dysfunction can occur in a variety of conditions, including alcoholism, and autoimmune diseases such as SLE (William and Corazza 2007; Di Sabatino et al. 2011; Kirkineska et al. 2014). If a patient suspected adrenal insufficiency visits the outpatient departments, taking abdominal CT is necessary to confirm the presence of not only adrenal hemorrhage but also hypoplastic spleen (or post-splenectomy). In addition, if an atrophic spleen remains, immunohistochemical specimens can show obscuration of the germinal center and reduction of marginal zone macrophages (Hata et al. 2015). In the present series, Case 1 and 3 had no spleens. The patient in Case 2 was considered to have hyposplenism based on autopsy and histopathological findings.
Several case reports have described WFS with SLE (Vachtenheim 1971; Guertler and Carter 1996; Friedl et al. 2017). SLE carries a high risk of death from infectious diseases because the disease causes an immunocompromised state due to factors such as decreased phagocytosis by immune cells and reduction in serum complement levels (Cuchacovich and Gedalia 2009; Mitander et al. 2018). As mentioned earlier, complement is involved in the removal of encapsulated bacteria, so reductions in complement are considered to make the removal of them difficult. In addition, the use of steroids and immunosuppressive drugs in the treatment of SLE also promotes an immunocompromised state. Further, prolonged administration of steroids can suppress ACTH secretion with negative feedback, resulting in atrophy of the adrenal cortex (Dinsen et al. 2013; Raff et al. 2014; Güven 2020). When this more vulnerable adrenal gland is exposed to septic stress, WFS may be more likely to develop and may prove more lethal than the state without adrenal atrophy. SLE can also be associated with diseases in which splenectomy may be chosen as the treatment, such as AIHA and thrombocytopenia (Fayyaz et al. 2015; Justiz Vaillant et al. 2022). Functional asplenia (autosplenectomy) occurs in approximately 5% of SLE patients, and anatomical asplenia is also found in a small number of cases (Dillon et al. 1982; Santilli et al. 2003; Leipe et al. 2007). In particular, some reports have found no correlation between SLE activity and hyposplenism (Neilan and Berney 1983; Di Sabatino et al. 2011), so SLE patients should be careful about infection by encapsulated bacteria, regardless of severity. In addition to SLE, WFS cases associated with autoimmune diseases such as rheumatoid arthritis have also been reported (Guertler and Carter 1996; Hamilton et al. 2004), and these diseases are considered to be associated with splenic dysfunction. Case 3 was considered likely to have become fatal with the onset of adrenal hemorrhage due to the history of SLE, adrenal atrophy, and splenectomy. SLE should thus be considered a risk factor for WFS in terms of both pathophysiology and treatment.
To summarize the clinical course of the three presented cases, all cases were considered to involve an immunocompromised state (hypoplastic spleen or post-splenectomy). The cause of pain in the lower limbs in Case 1 is unknown, but this symptom is often observed in WFS cases (Ferguson and Chapman 1948; Emori et al. 2016; Ikeda et al. 2019). Although an adult in their 50s asphyxiating due to aspiration of vomitus is rare, his family confirmed that the patient had been vomiting in the toilet before falling unconscious. The patient was therefore considered to have died of asphyxiation due to aspiration of vomitus under a state of impaired consciousness caused by WFS. The hyperglycemia at 21:00 in Case 2 was the result of correcting the hypoglycemia with glucose administration. However, blood glucose levels subsequently decreased again to 31 mg/dL around 22:00. Thus, the adrenal insufficiency may be present if the blood glucose level decreases immediately after glucose administration. Although the cause of hypoplastic spleen was unknown, the patient had a history of gout, suggesting the possibility of alcoholic spleen hypoplasia. In all three cases, there were few specific findings or examination results that could clearly diagnose sepsis. However, considering not only the multiple findings or examination results observed at clinical courses and autopsy but also the presence of WFS, it seems reasonable to conclude that all three cases had sepsis.
The times at which symptoms were estimated to have become more severe in each case may also be relevant. According to Table 1, the time from first symptom appearance to death in Case 1-3 was in the range of 16-27 h, roughly one day. In addition, assuming that the estimated times of worsening symptoms were 09:00, 20:00, and 15:00 in Case 1-3, respectively, the time from symptoms becoming severe to death was in the range of approximately 4-6 h. Therefore, WFS may be a disease in which the time of mild disease is relatively long, on the other hand, the time from sudden change to death is short. Furthermore, not only sepsis and DIC, but also adrenal hemorrhage and adrenal insufficiency due to WFS must be treated promptly within that short window. If the presence of WFS is not kept in mind, adrenal insufficiency may go unnoticed and the patient will then be at high risk of death. This sudden change can lead to confusion or misunderstandings among the family members of the affected patients. In fact, one report has already described a case in which the family of the patient suspected medical malpractice due to delayed clinical diagnosis in a WFS autopsy case for a patient that died about an hour after the sudden change (Mascolo et al. 2021).
Vaccination histories were unknown in Case 1 and 2. However, in Case 3, the patient had been vaccinated against S. pneumoniae. Prevention of WFS includes reducing the risk of preceding severe infections. Centers for Disease Control and Prevention and some reports recommends vaccination against S. pneumoniae and other pathogens for congenital or acquired asplenia (Buzelé et al. 2016; Centers for Disease Control and Prevention 2022). In fact, reports have described WFS in post-splenectomy patients who were not properly vaccinated (Hale et al. 2016), and also due to bacteria of other serotypes even in patients with vaccination (Lindblad and Lindblad 1990; Friedl et al. 2017).
The above three cases were autopsied within a 5-year period. WFS is considered a rare disease, but may be more frequent than previously assumed. This pathology is difficult to manage, so the degree of recognition needs to be increased, especially in outpatient departments. If an apparently mildly ill patient suddenly deteriorates and dies within a short period of time, bacterial tests (preferably blood cultures) and autopsy are necessary. At autopsy, evaluation of splenic function by histopathological examination is also important, even in the absence of macroscopic splenic hypoplasia.
In conclusion, three patients with WFS were autopsied within a 5-year period. All the patients had hyposplenism and were considered immunocompromised, especially with regard to encapsulated bacteria. Knowing in advance the functional status of the spleen, the history of SLE and other such factors will quicken the response to not only sepsis and DIC, but also WFS causing adrenal insufficiency due to adrenal hemorrhage.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We are grateful to Dr. Satoshi Furuya, Dr. Haruo Yamashita, Dr. Hideaki Onda, Dr. Satoru Kojika and Dr. Tetsu Yamane for providing detailed clinical data.
The authors declare no conflict of interest.