2023 Volume 259 Issue 2 Pages 127-133
2023 Volume 259 Issue 2 Pages 127-133
Laparoscopic adrenalectomy is currently the standard treatment modality for unilateral aldosterone-producing adenoma (APA); however, a less-invasive treatment is needed for its treatment. A new bipolar ablation system that poses a lower risk of complications has been recently developed. This study aimed to evaluate the safety and performance of a novel bipolar radiofrequency ablation (RFA) system for the treatment of APAs. Ablations were performed in an ex vivo study using bovine adrenal glands [group A: n = 6, single-probe; group B: n = 6, two probes, interprobe distance (ID) = 12 mm; group C: n = 6, two probes, ID = 20 mm]. The in vivo study was conducted in groups A and B (n = 2 each) using porcine adrenal glands. For the ex vivo study, the mean vertical diameter (Dv) of the coagulative necrosis area and the mean transverse diameter (Dt) values were 11.99 mm and 10.96 mm for group A, 12.66 mm and 10.0 mm for group B, and 23.37 mm and 22.10 mm for group C, respectively. For the in vivo study, the mean Dv and Dt values were 12.23 mm and 9.03 mm for group A, and 16.38 mm and 9.52 mm for group B, respectively. No heat-induced damage to the adjacent organs was observed. To our best knowledge, this is the first study to evaluate the performance of the bipolar system in RFA of the adrenal gland. RFA using the new bipolar ablation system is safe and produces a sufficient coagulation area to treat APAs.
Primary aldosteronism is the most common form of secondary hypertension, with a prevalence of approximately 10% in hypertension patients (Young 2007). Unilateral aldosterone-producing adenomas (APA) and bilateral idiopathic hyperaldosteronism are the two most common subtypes of primary aldosteronism (Young 2007). Laparoscopic adrenalectomy is currently the standard treatment modality for unilateral APAs (Assalia and Gagner 2004), but considering that APAs are benign tumors, minimally invasive treatment should be applied. In this regard, computed tomography (CT)-guided percutaneous radiofrequency ablation (RFA) has received increasing attention as a minimally invasive treatment for various types of tumors (Liu et al. 2010). RFA uses high-frequency alternating electric current to induce ionic agitation, which creates frictional heating around the electrode. In addition to frictional heat, thermal conduction transfers heat to a more distant area around the electrode, thereby creating an ablated area (Goldberg 2001; Brace 2009).
Commonly available RFA systems for adrenal tumors are monopolar systems, which require grounding pads to complete the electrical circuit. However, a new bipolar ablation system has recently been developed and employed clinically. Nevertheless, to conduct RFA using the newly developed bipolar ablation system for the treatment of APAs, further studies are required to confirm the size of the coagulation area produced with the bipolar system using various combinations of the insertion patterns, including one or two radiofrequency probes, power levels, and input energy levels. In our preliminary investigation (unpublished data), 97% of APAs (n = 106) in humans had a diameter of less than 25 mm; thus, the maximum size of coagulation required for RFA would be 25 mm.
To our best knowledge, no studies have evaluated the performance of the bipolar system in adrenal RFA. This study aimed to evaluate the safety and performance of a novel bipolar RFA system for the treatment of APAs and to determine the appropriate positioning of the radiofrequency probes. Towards this goal, we estimated the size of the coagulation area and heat-induced damage to adjacent organs using a newly developed bipolar ablation system and examined the influence of insertion patterns of the radiofrequency probes on coagulation size.
All ablations were performed using a 470 KHz radiofrequency generator (CelonLab POWER; OLYMPUS WINTER & IBE GMBH Berlin Facility, Teltow, Germany) that provided a maximum power output of 250 W and included 16 G internally cooled bipolar applicators (CelonPro Surge; OLYMPUS WINTER & IBE GMBH Berlin Facility).
Ex vivo studyEx vivo studies were conducted using resected bovine adrenal glands. Six excised bovine adrenal glands were used in each of the three groups. Groups were determined according to the number of probes and insertion depth as follows. The probe is shown in Fig. 1a. In group A, a single probe was inserted into the adrenal glands (Fig. 1b). The entire insulated part and distal end of the electrode were inserted into the adrenal gland tissue, and the proximal electrode was only partially inserted. In groups B and C, two probes were inserted in parallel orientation (Fig. 1c, d). The insertion depth and distance between the two probes differed between groups B and C. In group B, the entire distal electrode was placed in the adrenal tissue, whereas the insulated part was only partially inserted. In group C, the entire distal electrode, insulated part, and distal end of the proximal electrode were inserted into the adrenal gland. The inter-probe distances in groups B and C were 12 mm and 20 mm, respectively. Layers of adipose tissue were placed to interpose the adrenal gland to simulate an anatomical situation of the adrenal gland, mostly surrounded by fat (Figs. 1b and 2).
The radiofrequency power was gradually increased and maintained at 20 W for group A and at 40 W for groups B and C. The radiofrequency power was set at 1 W per 1 mm of the length of the electrode, a standard for RFA of liver tumors performed using the RFA generator used in this study. The procedure was terminated when the generator automatically stopped because of increased impedance or when the input energy level reached 4 kJ for group A and 6 kJ for groups B and C, which we set based on our preliminary investigation (unpublished data).

Three patterns of probe insertion.
(a) The schematic representation of a probe. In group A, a single probe was inserted into the adrenal glands (b). The entire insulated part and distal end of the electrode were inserted into the adrenal gland tissue, and the proximal electrode was only partially inserted. In groups B and C, two probes were inserted in parallel orientation (c, d). The insertion depth and distance between the two probes differed between groups B and C.

Arrangement of the adrenal gland and the adipose tissue layer in the ex vivo study.
Layers of adipose tissue were placed to interpose the adrenal gland to simulate an anatomical situation of the adrenal gland, mostly surrounded by fat.
Two 3-month-old, castrated male domestic pigs weighing 40-60 kg (San-S Breeding Co., Ltd., Funabashi, Japan) for each group A and B were used in the in vivo study. All procedures were approved by the Animal Care and Use Committee of the Olympus Medical Systems Corp. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
The heart rate, percutaneous oxygen saturation (SpO2), and invasive arterial blood pressure under general anesthesia were continuously monitored throughout the procedure. A midline laparotomy incision was made, and the bilateral adrenal glands were gently exposed to precisely position the radiofrequency electrodes. Two ablations were performed for each group. Probes were inserted as described for the ex vivo studies for groups A and B, but the probes could not be inserted in group C because the porcine adrenal glands were too small for this pattern. Layers of adipose tissue were placed to interpose the adrenal gland (Fig. 3). RFA was applied at the same amount of power as that used in the ex vivo studies.
Immediately after the in vivo experiments, the pigs were euthanized and the bilateral adrenal glands and adjacent organs were resected. In both the ex vivo and in vivo experiments, tissue specimens were fixed, sectioned, and stained with nicotinamide adenine dinucleotide diaphorase and hematoxylin/eosin. The areas of coagulative necrosis were measured in two perpendicular directions. The longest diameter along the axis of the needles was established as the vertical diameter (Dv), and the diameter perpendicular to Dv was established as the transverse diameter (Dt) (Fig. 4).

Arrangement of the adrenal gland and the adipose tissue layer in the in vivo study.
Layers of adipose tissue were placed to interpose the adrenal gland.
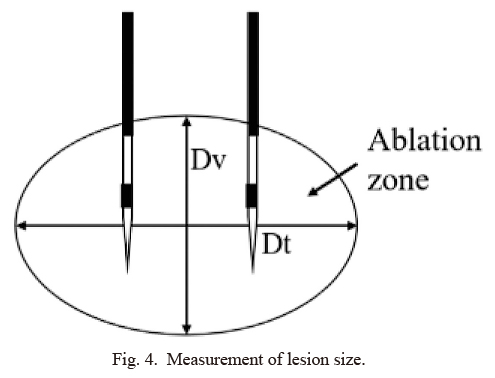
Measurement of lesion size.
The longest diameter along the axis of the needles was established as the vertical diameter (Dv), and the diameter perpendicular to Dv was established as the transverse diameter (Dt).
The diameters of the areas of coagulative necrosis are presented as the mean ± standard deviation (SD). Differences in the Dv and Dt values between the three groups in the ex vivo studies were evaluated using the Kruskal-Wallis H-test, followed by the Mann-Whitney U-test with Bonferroni correction. All statistical analyses were performed using JMP® Pro statistical software (version 11.0.0; SAS Institute, Cary, NC, USA). Results with a probability (p) < 0.0167 (α/n = 0.0167, for α = 0.05, and n = 3) were considered statistically significant.
All the procedures were performed in accordance with the protocol (Fig. 5). In groups B and C, all adrenal glands were ablated in almost the entire area. All adrenal glands shrank after the procedure. The mean Dv values were 11.99 ± 1.16 mm, 12.66 ± 2.40 mm, and 23.37 ±1.95 mm, whereas the mean Dt values of the radiofrequency-induced coagulation zones were 10.96 ± 1.50 mm, 10.0 ± 1.65 mm, and 22.10 ± 2.00 mm for groups A, B, and C, respectively (Table 1). The results of the Kruskal-Wallis H-test showed significant differences in the Dv (p = 0.0028) and Dt (p = 0.0028) values among the three groups. The Mann-Whitney U-test with Bonferroni correction showed that there were significant differences in both Dv and Dt values between groups C and A (p < 0.0167), Dv value between groups C and A (p = 0.0051), Dt value between groups C and A (p = 0.0051), Dv value between groups C and B (p = 0.0051), and Dt value between groups C and B (p = 0.0051). Meanwhile, there were no significant differences in either the Dv or Dt values between groups A and B (Dv: p = 0.9361; Dt: p = 0.3785).

Cut section of the specimen stained with nicotinamide adenine dinucleotide diaphorase in group A.
The area surrounded by a red broken line is the area of coagulative necrosis. Dv, vertical diameter; Dt, transverse diameter.

Mean diameter of radiofrequency-induced coagulation areas (ex vivo study).
Data are shown as mean ± SD.
†There are significant differences between groups A and C (p = 0.0051) and between groups B and C (p = 0.0051).
*There are significant differences between groups A and C (p = 0.0051) and between groups B and C (p = 0.0051).
Three of the four procedures were performed in accordance with the protocol. In one procedure in group B, ablation was terminated at 5 kJ because the impedance was too high. All adrenal glands were ablated in almost the entire area and were slightly shrunken (Fig. 6). The mean Dv values were 12.23 mm and 16.38 mm, and the mean Dt values of the radiofrequency-induced coagulation zones were 9.03 mm and 9.52 mm for groups A and B, respectively (Table 2). Owing to the small number of samples, statistical analysis of the RFA lesions created in the in vivo experiments was not possible. Regarding biological reactions during ablation, in the first case, when ablation was performed without the administration of antihypertensive drugs, the blood pressure increased sharply approximately 1 minute after the start of ablation, reaching a maximum systolic blood pressure of 280 mmHg. In the second and subsequent cases, ablation was performed with the administration of α-blockers or calcium channel blockers before and during ablation. Both drugs suppressed the increase in blood pressure to ≤ 160 mmHg.

Cut section of the specimen stained with nicotinamide adenine dinucleotide diaphorase in group B in the in vivo study.
The area surrounded by a red broken line is the area of coagulative necrosis. Dv, vertical diameter; Dt, transverse diameter.

Mean diameter of radiofrequency-induced coagulation areas (in vivo study).
*In one case, ablation was terminated at 5 kJ because the impedance was too high.
In the in vivo experiment, the cauterization area (Dv × Dt) was 12 mm × 9 mm using one probe and 16 mm × 10 mm using two probes. From the results, adrenal adenomas less than approximately 10 mm could be cauterized using one probe, and tumors up to 15 mm could be ablated using two probes, considering the safety margin. Furthermore, if the probe insertion pattern of group C in ex vivo experiment is clinically feasible, it is speculated that even larger adenomas can be ablated. Yamakado et al. (2011) examined the extent of adrenal adenoma cauterization using a monopolar probe. They inserted a probe approximately 5 cm into the adrenal gland in the long axis direction, and complete cauterization was achieved in 5/6 cases (Yamakado et al. 2011). However, the extent of cauterization with respect to the circumference of the long axis of the probe has not yet been examined. Comparisons of bipolar and monopolar probes in RFA for hepatic tumors have been reported, and the bipolar probe showed a larger cauterization area than did the monopolar system (Yoon et al. 2015; Chang et al. 2017). To our best knowledge, this is the first study to evaluate the performance of the bipolar system in RFA of the adrenal gland.
According to previous studies, the average diameter of APAs is 16 mm (Liu et al. 2010), and in our preliminary investigation (unpublished data), 97% of APAs (n = 106) in humans had a diameter of less than 25 mm. Therefore, most APAs can be successfully treated using the proposed bipolar RFA device.
In the ex vivo experiment, no significant differences were found in either the Dv or Dt values between groups A and B. There are two possible reasons for this. First, in group B, only the distal electrode was in the adrenal gland, and the proximal electrode was completely in the adipose tissue layer. To create a larger coagulation area, both the distal and proximal electrodes must be in the adrenal gland. The access route for RFA of APA is considered to be the posterior retroperitoneal approach. In group B, it is assumed that inserting a radiofrequency probe deeply is not feasible because of its close proximity to critical organs, such as the pancreas or small intestine, on the deep side of the probe. Second, in group B, all adrenal glands were ablated in almost the entire area. Therefore, it is assumed that the ablation area is underestimated in group B.
The coagulation areas were expected to be smaller in the in vivo experiments than in the ex vivo experiments because of the heat sink effect of the blood flow. However, in our study, the sizes of the coagulation areas were similar between the in vivo and ex vivo experiments. In vivo, the adrenal glands are held in place by the surrounding connective tissue, and it may shrink ex vivo when resected, with the shrinkage being more significant than that in vivo. Continuous arterial pressure was measured in the in vivo experiments to assess blood pressure changes during ablation. In the first case, when ablation was performed without the administration of antihypertensive drugs, blood pressure increased sharply and reached a maximum systolic blood pressure of 280 mmHg. The cause was direct puncture and ablation of the normal adrenal gland without any treatment. This indicates that intraoperative blood pressure control with drugs is essential. Thus, subsequent ablation was performed by administering an α-blocker or calcium channel blocker before and during ablation in the second and subsequent cases. Both drugs suppressed the increase in blood pressure to ≤ 160 mmHg, confirming that safe blood pressure control during ablation could be achieved with the administration of antihypertensive drugs.
Primary aldosteronism is the most common cause of secondary hypertension, while unilateral APA and bilateral idiopathic hyperaldosteronism are the two most common subtypes of PA (Young 2007). Laparoscopic adrenalectomy is currently recognized as the standard treatment for unilateral APA (Assalia and Gagner 2004). However, CT-guided percutaneous RFA has been widely used for the treatment of adrenal adenomas in recent years. To date, only monopolar systems are available for RFA of adrenal adenomas. In the monopolar RFA system, only one probe can be used for energization. Therefore, to cauterize a large lesion, the probe must be reinserted several times, and multiple energizations must be performed. Meanwhile, the bipolar RFA system can energize multiple probes (up to three at present) simultaneously and can cauterize a larger area than when each probe is energized separately as the current is applied sequentially between multiple electrodes.
Consequently, it can be inferred that the bipolar RFA system can cauterize a similar volume of lesions with fewer punctures than with the monopolar RFA system. The monopolar RFA system can also cauterize relatively large lesions by using a probe with an expandable tip, but it is difficult to use an expandable-type probe for a target such as the adrenal gland, which is located in a narrow area and surrounded by important tissues and organs. The higher the number of punctures, the higher the risk of accidental puncture of surrounding tissues such as blood vessels and nerves or nearby organs other than the intended one, and, consequently, the higher the frequency of complications. Thus, it is suggested that the bipolar RFA system can cauterize lesions more safely.
In addition, monopolar RFA systems pose a risk of collateral damage to adjacent organs, burning of the skin under the grounding pads, and uncontrollable energy flow (Goldberg et al. 2000; Rhim et al. 2004). In contrast, bipolar systems do not require a grounding pad because the electric current flows between two electrodes, and these systems place two electrodes on one applicator. Therefore, bipolar systems pose a lower risk of collateral damage to adjacent organs and burning of the skin and can overcome uncontrollable energy flow (Goldberg 2001; Brace 2009). Based on our preliminary investigation, we estimated the size of the coagulation areas with certain probe insertion patterns, power levels, and input energy levels in the current study. Both ex vivo and in vivo experiments were then performed to confirm the estimated coagulation areas with different combinations of the three insertion patterns of the radiofrequency probes, power levels, and input energy levels using a newly developed bipolar ablation system.
This study is conducted on the assumption that clinical application of this treatment would be limited to cases in which there is an interposition of adipose tissue of ≥ 5 mm between the target adrenal adenoma and critical organs, such as the pancreas and intestine. Small adenomas (< 10 mm) were assumed to be treated by inserting a single probe. In this case, the adenoma was ablated by the current flowing between the two electrodes in one probe. The part of the electrode at the probe tip was 8 mm long, with a 4-mm insulation part proximal to it and another 8-mm electrode part proximally (Fig. 7a). To position the two electrodes of a single probe within a 10-mm adenoma, assuming that the insulation part is positioned at the center of the tumor, the proximal 3 mm of the tip electrode should be within the tumor and the distal 5 mm should be located outside the tumor (Fig. 7b). When blood vessels or other organs behind the tumor limit the space and prevent advancement of the probe to place two electrodes within the tumor, it is possible to ablate the tumor between each distal electrode of the two probes placed within or on the tumor (Fig. 7c, d). The probe insertion pattern performed in Group B assumed such cases. Given that clinical use assumes a puncture from the back, in the above case, the proximal electrode would be located in the retroperitoneal adipose tissue and would not be a relevant cause of adverse events. It must be ensured that the position of the proximal electrodes would not include ablation of the important organs.
A recent multicenter prospective clinical study validated the clinical efficacy and safety of RFA using bipolar electrodes in patients with primary aldosteronism (Oguro et al. 2022). APAs were adequately ablated by percutaneous CT-guided RFA, and the same bipolar device was used in our study. The study revealed that plasma aldosterone concentration or aldosterone-renin ratio was normalized to 87% on day 84, comparable to that in surgical adrenalectomy.
This study has some limitations. First, all ablations were performed on normal bovine and porcine adrenal glands. Furthermore, the in vivo study was performed under laparotomy, which is different from image-guided ablation performed in real clinical practice. Therefore, whether the bipolar system can successfully treat APAs in humans remains unknown. Second, statistical analysis could not be conducted in the in vivo experiments because of the small number of RFA lesions. Third, endocrinological assessments were not performed in this study. Finally, the safety verification was insufficient. Theoretically, bipolar probes can be expected to pose a lower risk of systemic electrical complications because there is no current flow through the systemic tissue between the probe and the grounding pad. Adrenal tissue, which is a more vascular organ than the liver, is theoretically less conductive than liver tissue; therefore, the risk of complications due to overcauterization should be low (Matsumoto et al. 1982). However, this study did not evaluate the effect of electrification on the whole body when the liver tissue or adrenal glands were ablated; thus, it is not an experimental verification but only a theoretical prediction.
In conclusion, RFA using the new bipolar ablation system is safe and produces a sufficient coagulation area to treat APAs. In addition, an appropriate puncture pattern of the radiofrequency probe according to the size of the aldosterone-producing adenoma has been established.

Insertion patterns of probes assumed when the tumor is small or when there is a dangerous organ behind the tumor.
The part of the electrode at the probe tip was 8 mm long, with a 4-mm insulation part proximal to it and another 8-mm electrode part proximally (a). To position the two electrodes of a single probe within a 10-mm adenoma, assuming that the insulation part is positioned at the center of the tumor, the proximal 3 mm of the tip electrode should be within the tumor and the distal 5 mm should be located outside the tumor (b). When blood vessels or other organs behind the tumor limit the space and prevent advancement of the probe to place two electrodes within the tumor, it is possible to ablate the tumor between each distal electrode of the two probes placed within or on the tumor (c, d).
The authors wish to thank all individuals involved in this study for their support. The authors are also grateful to Makoto Inaba and his colleagues (Olympus Medical Systems Corp., Tokyo, Japan) for their contribution. This research was supported by AMED under Grant Number JP20hk0102060.
The authors declare no conflict of interest.