2023 年 260 巻 2 号 p. 99-107
2023 年 260 巻 2 号 p. 99-107
Keratin-15 (KRT15) participates in the tumorigenesis of several cancers, especially in urinary tract carcinomas by regulating basal urothelial cell malignant proliferation and differentiation. This study intended to explore the association of KRT15 with tumor features and survival in renal cell carcinoma (RCC) patients. Totally, 210 RCC patients receiving surgical resection were retrospectively enrolled, and then, KRT15 was detected by immunohistochemistry (IHC) assay in the tumor and adjacent tissues. IHC score of KRT15 was increased in tumor tissues versus adjacent tissues (P < 0.001). Meanwhile, KRT15 was associated with RCC occurring in the left kidney (P = 0.024), tumor size > 10 cm (P = 0.035), higher N stage (P = 0.048), and higher tumor-node-metastasis (TNM) stage (P = 0.029). Additionally, high KRT15 (IHC score > 3) estimated poor disease-free survival (DFS) (P = 0.008) and overall survival (OS) (P = 0.011). In addition, multivariate regression analysis revealed that high KRT15 [hazard ratio (HR) = 1.719, P = 0.023], higher pathological grade (HR = 1.847, P < 0.001), and higher N stage (HR = 3.447, P < 0.001) were independently related to poor DFS; high KRT15 (HR = 1.796, P = 0.034), eastern cooperative oncology group performance status score (1 vs. 0) (HR = 1.734, P = 0.037), higher pathological grade (HR = 2.045, P < 0.001), and higher N stage (HR = 3.966, P < 0.001) were independently linked to unsatisfactory OS. Furthermore, data from Gene Expression Profiling Interactive Analysis suggested that KRT15 was linked to poor DFS (P = 0.037) and OS (P < 0.001); data from THE HUMAN PROTEIN ATLAS revealed that KRT15 was associated with shorter OS (P < 0.001) in RCC patients. In conclusion, KRT15 is increased in tumor tissues, and correlates with higher tumor stage and larger tumor size, along with poor prognosis for RCC.
Renal cell carcinoma (RCC) is a common and fatal renal tumor accounting for approximately 90% of all kidney cancers, and its risk factors include smoking, hypertension, obesity, chronic kidney disease, etc. (Hsieh et al. 2017; Capitanio et al. 2019; Saly et al. 2021). Globally, RCC contributes to about 2% of adult malignancies, and it is more common in males compared to females according to the GLOBOCAN2020 database (Sung et al. 2021). General management options for RCC patients include surgery, targeted therapy, immunotherapy, and radiation therapy, among which surgical resection is basically the preferred choice (Braun et al. 2021; Singh 2021; Motzer et al. 2022). However, the recurrence rate of RCC is still high, which contributes to the unsatisfactory 5-year recurrence-free survival rate of less than 90% in RCC patients (Shao et al. 2020; Abu-Ghanem et al. 2021). Thus, exploring novel biomarkers for RCC patients is necessary to better realize the stratified management of RCC patients.
Keratin-15 (KRT15) is a type I cytokeratin, which involves in the pathogenesis and progression of tumors in various ways (Tai et al. 2013; Giroux et al. 2018; Chen and Miao 2022). For instance, KRT15 regulates the β-catenin/matrix metalloproteinase-7 (MMP-7) pathway to accelerate the migration and invasion of colorectal cancer cells (Chen and Miao 2022). Importantly, a previous study reveals that elevated KRT15 contributes to malignant urothelial growth, indicating that KRT15 may participate in the tumorigenesis of the urinary system (Tai et al. 2013). Moreover, the clinical importance of KRT15 in patients with cancers has been revealed by several studies (Zhang et al. 2019; Galindo et al. 2020; Rao et al. 2020; Zhong et al. 2021). A study reports that KRT15 assists in discriminating lung squamous cell carcinoma from adenocarcinoma (Galindo et al. 2020). Besides, KRT15 is highly expressed in tumor tissues compared to non-tumor tissues, and its overexpression is related to poor T stage, differentiation, clinical stage, and the presence of lymph node metastasis in colorectal cancer patients (Rao et al. 2020). However, the clinical role of KRT15 in RCC patients remains unclear and requires further exploration.
Accordingly, this study enrolled 210 RCC patients with the aim to explore the dysregulation of KRT15 in tumor tissues versus adjacent tissues, as well as the correlation of KRT15 with tumor features and survival in these patients.
From January 2012 to December 2017, 210 RCC patients who came to our hospital for surgical treatment were included in this retrospective research. The main screening criteria were: (1) firstly diagnosed with primary RCC; (2) age > 18 years old; (3) no distant metastasis; (4) received surgical resection; (5) had formalin-fixed paraffin-embedded (FFPE) tumor tissue specimens from surgical resection that could be used in this study for detection; (6) had complete preoperative clinical features; (7) had at least one follow-up information that could be evaluated for survival. Patients with the following criteria were excluded: (1) had other primary cancers or hematologic malignancies in the past; (2) pregnant or lactating women. This research gained the approval of the Ethics Committee of First Affiliated Hospital of Baotou Medical College. Informed consents were also gained.
Data and specimensClinical features of RCC patients were collected, which contained age, sex, histological type, location, the eastern cooperative oncology group performance status (ECOG PS), pathological grade, tumor size, T stage, N stage, M stage, relapse status, survival status, etc. Tumor-node-metastasis (TNM) stage was re-evaluated referring to the 8th edition TNM staging (Amin et al. 2017). Besides, survival data were collected, then the disease-free survival (DFS)/overall survival (OS) rates were calculated according to it. RCC patients underwent a routine follow-up, with a median follow-up time of 7.0 months and a range from 1.1 to 10.4 months. The last follow-up data was on June 30th, 2022. Tumor tissue FFPE specimens from RCC patients were obtained after surgical resection for detection. The specimen repository was established after 2015 based on the research needs; thus, only 50 cases of adjacent tissues were obtained for the detection of KRT15 expression.
KRT15 expression detectionThe KRT15 expression was detected by IHC assay, and the experimental procedure was briefly summarized as the following steps: initial sample processing, preliminary test preparation, antibody incubation, and staining. The experiment procedure was established according to a previous study (Peng et al. 2020). The KRT15 rabbit monoclonal antibody (Beyotime, Shanghai, China) was used as primary antibody at 1:200 dilution, and the goat-anti-rabbit immunoglobulin G antibody (Abcam, Cambridge, UK) was applied as secondary antibody at 1:100 dilution. After IHC staining, the IHC score was calculated by multiplying the intensity of staining and the density of staining. The intensity of staining was scored as follows: 0 (negative); 1 (weak); 2 (moderate); and 3 (strong). The density of staining was defined as follows: 0 (0%); 1 (1%-25%); 2 (26%-50%); 3 (51%-75%); and 4 (> 75%) (Ye et al. 2020). The KRT15 expression (IHC score) was graded by two evaluators who were blinded to patients’ information, and the average of the two above was used as the final score. The tumor tissue IHC score consistency between the two evaluators was assessed through the Kappa coefficient test (Kappa = 0.860), and adjacent tissue IHC score consistency between the two evaluators was also assessed through the Kappa coefficient test (Kappa = 0.845). The KRT15 expression (IHC score) was divided into high and low expressions by the cutoff value 3.
StatisticsSPSS v.25.0 and GraphPad Prism v.7.01 were used for the data process and figure construction, respectively. The Kolmogorov-Smirnov test was used for normality test. All continuous variables were in abnormal distribution and described as median [interquartile range (IQR)]. The Wilcoxon signed-rank test was applied to compare KRT15 expression between the tumor and adjacent tissues. The receiver operating characteristics (ROC) curve was performed to show the distinguishing power of KRT15 expression. The Wilcoxon rank sum test was applied to analyze KRT15 expression between different groups. Kruskal-Wallis H rank sum test was applied to analyze KRT15 expression among different groups. The Spearman test was utilized to analyze the correlation of KRT15 expression with patients’ features. Kaplan-Meier curves were performed to show the divergence between high and low KRT15 expression in DFS/OS, for which a Log-rank test was used. Cox proportional hazards models (univariate and forward-multivariate) were used to analyze the risk factors related to DFS/OS. P < 0.05 was denoted statistically significant.
The median (IQR) age of enrolled RCC patients was 61.0 (52.0-71.0) years. Meanwhile, there were 74 (35.2%) females and 136 (64.8%) males. Regarding the ECOG PS score, there were 163 (77.6%) patients with a score of 0 and 47 (22.4%) patients with a score of 1. Additionally, 88 (41.9%) patients had a pathological grade of 1, 76 (36.2%) patients had a grade of 2, and 46 (21.9%) patients had a grade of 3. Furthermore, the median (IQR) tumor size was 5.0 (4.0-9.0) cm. In terms of the TNM stage, there were 127 (60.5%) patients at stage 1, 45 (21.4%) patients at stage 2, and 38 (18.1%) patients at stage 3. The specific information is exhibited in Table 1.
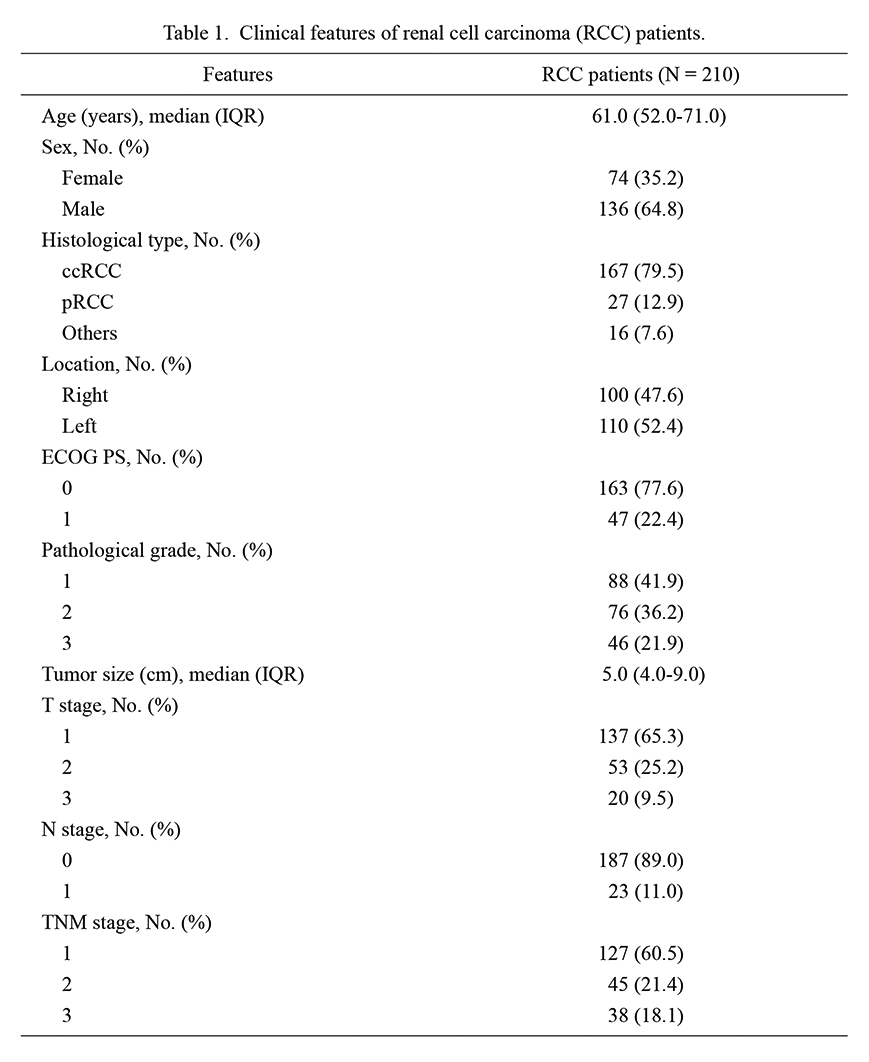
Clinical features of renal cell carcinoma (RCC) patients.
IQR, interquartile range; ccRCC, clear cell renal cell carcinoma; pRCC, papillary renal cell carcinoma; ECOG PS, eastern cooperative oncology group performance status; TNM, tumor-node-metastasis.
According to the Kappa coefficient test, the consistency between the two evaluators of tumor tissues’ IHC score (Kappa = 0.860) and adjacent tissues’ IHC score (Kappa = 0.845) was generally good. The IHC detected examples of KRT15 in tumor tissues and adjacent tissues were presented in Fig. 1A. KRT15 was increased in the tumor tissues [median (IQR) of IHC score: 4.0 (3.0-8.0)] compared to the adjacent tissues [median (IQR) IHC score: 2.5 (1.5-3.6)] (P < 0.001) (Fig. 1B). In addition, KRT15 held a good capacity to discriminate tumor tissues from adjacent tissues [area under the curve (AUC) (95% confidence interval, CI): 0.764 (0.673-0.855)] (Fig. 1C).
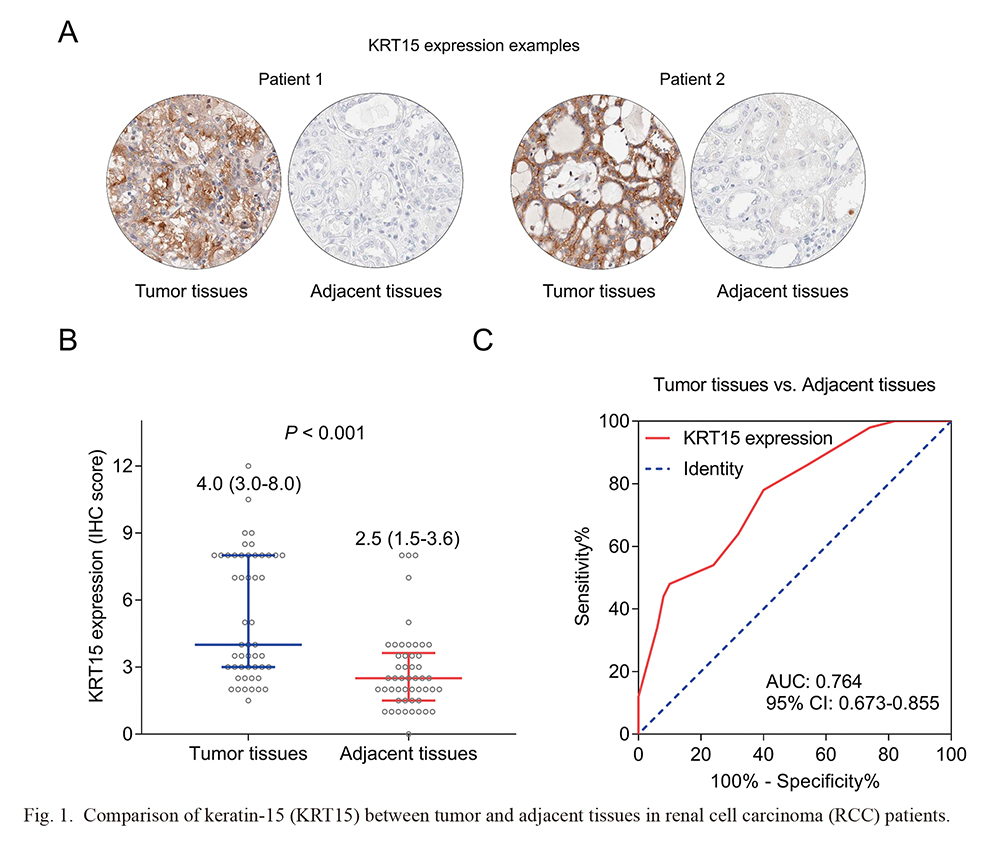
Comparison of keratin-15 (KRT15) between tumor and adjacent tissues in renal cell carcinoma (RCC) patients.
(A) Immunohistochemistry (IHC) images of KRT15 in tumor and adjacent tissues. Two representative cases are shown. (B) KRT15 was increased in tumor tissues versus adjacent tissues. Median (IQR) of IHC score is shown. (C) Receiver operating characteristic (ROC) curve for discriminating tumor tissues from adjacent tissues by KRT15 in RCC patients.
KRT15 was related to RCC occurring in the left kidney (P = 0.024), tumor size > 10 cm (P = 0.035), higher N stage (P = 0.048), and higher TNM stage (P = 0.029). However, KRT15 was not correlated with other clinical characteristics, including age, sex, histological type, ECOG PS score, pathological grade, tumor size (at cutoff values of 4 and 7 cm), and T stage (all P > 0.05) in RCC patients (Table 2).
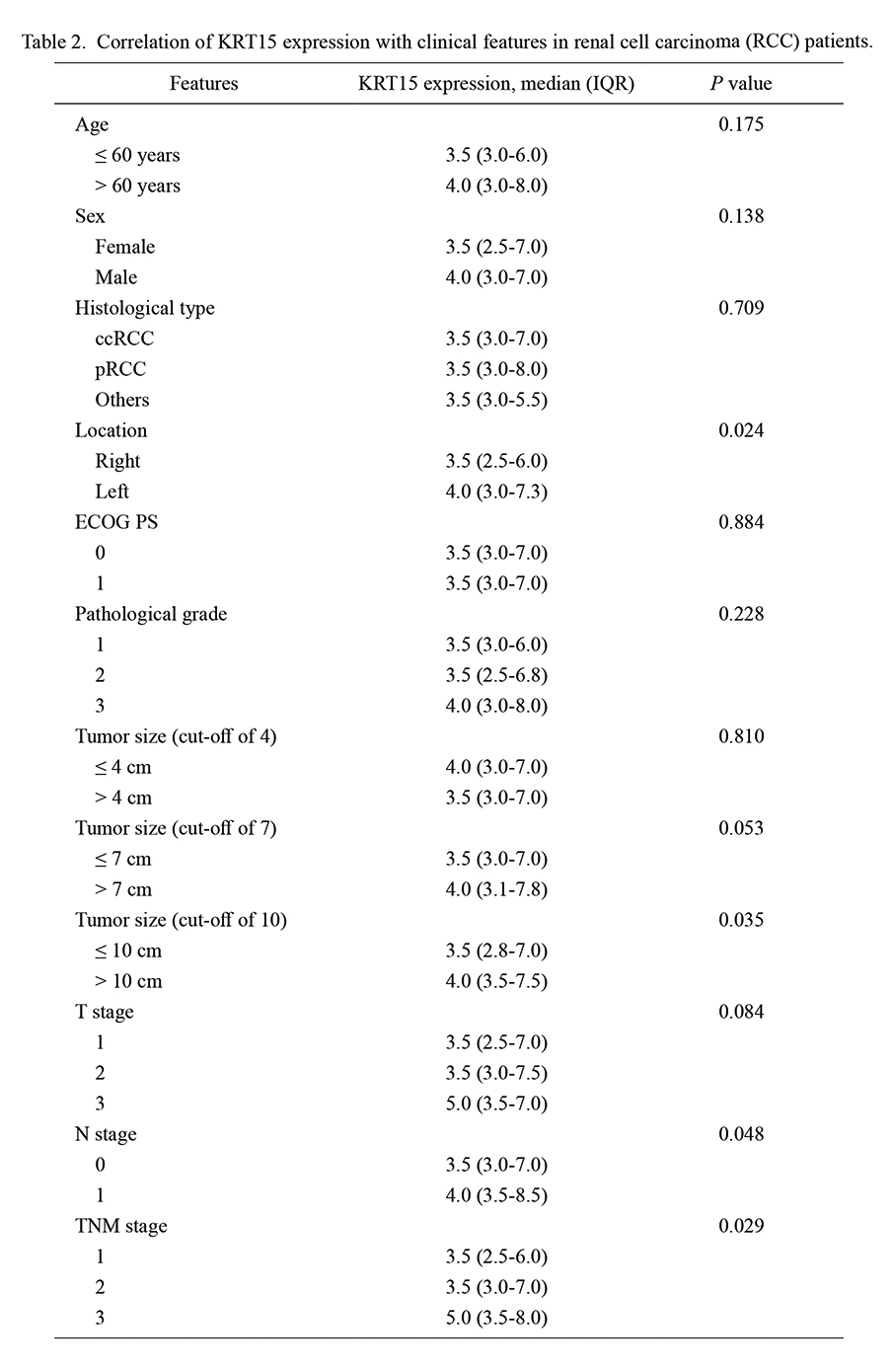
Correlation of KRT15 expression with clinical features in renal cell carcinoma (RCC) patients.
KRT15, keratin-15; IQR, interquartile range; ccRCC, clear cell renal cell carcinoma; pRCC, papillary renal cell carcinoma; ECOG PS, eastern cooperative oncology group performance status; TNM, tumor-node-metastasis.
High KRT15 (IHC score > 3) was associated with poor DFS in RCC patients (P = 0.008); in addition, the 3-year, 5-year, and 10-year DFS were 82.8%, 61.1%, and 47.4% in patients with high KRT15 (IHC score > 3), while they were 93.4%, 77.6%, and 64.3% in patients with low KRT15 (IHC score ≤ 3) (Fig. 2A). Besides, high KRT15 (IHC score > 3) was correlated with worse OS in RCC patients (P = 0.011); the 3-year, 5-year, and 10-year OS were 88.1%, 75.2%, and 57.4% in patients with high KRT15 (IHC score > 3), while they were 94.7%, 88.2%, and 73.2% in patients with low KRT15 (IHC score ≤ 3) (Fig. 2B).
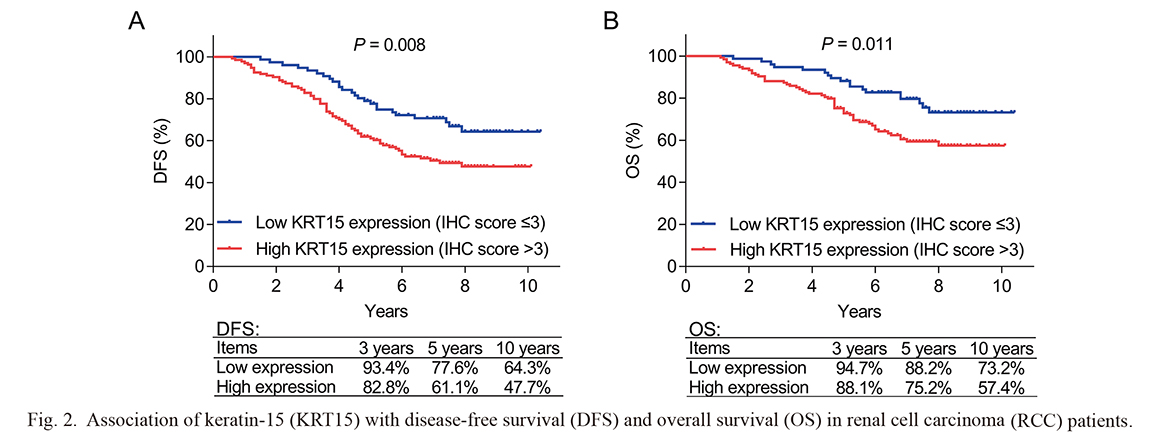
Association of keratin-15 (KRT15) with disease-free survival (DFS) and overall survival (OS) in renal cell carcinoma (RCC) patients.
High KRT15 was related to poor DFS (A) and OS (B) in RCC patients.
KRT15 (high vs. low) [hazard ratio (HR) = 1.850, P = 0.009], higher pathological grade (HR = 1.827, P < 0.001), higher T stage (HR = 1.923, P < 0.001), higher N stage (HR = 3.766, P < 0.001), and higher TNM stage (HR = 2.249, P < 0.001) were associated with shorter DFS in RCC patients (Fig. 3A). Further multivariate analysis revealed that KRT15 (high vs. low) (HR = 1.719, P = 0.023), higher pathological grade (HR = 1.847, P < 0.001), and higher N stage (HR = 3.447, P < 0.001) were independently correlated with poor DFS in RCC patients (Fig. 3B).
KRT15 (high vs. low) (HR = 1.975, P = 0.013), age (> 60 years vs. ≤ 60 years) (HR = 1.970, P = 0.007), ECOG PS score (1 vs. 0) (HR = 1.710, P = 0.040), higher pathological grade (HR = 1.943, P < 0.001), higher T stage (HR = 1.815, P < 0.001), higher N stage (HR = 4.356, P < 0.001), and higher TNM stage (HR = 2.175, P < 0.001) were related to worse OS in RCC patients (Fig. 4A). Further multivariate analysis disclosed that KRT15 (high vs. low) (HR = 1.796, P = 0.034), ECOG PS score (1 vs. 0) (HR = 1.734, P = 0.037), higher pathological grade (HR = 2.045, P < 0.001), and higher N stage (HR = 3.966, P < 0.001) were independently associated with shorter OS in RCC patients (Fig. 4B).
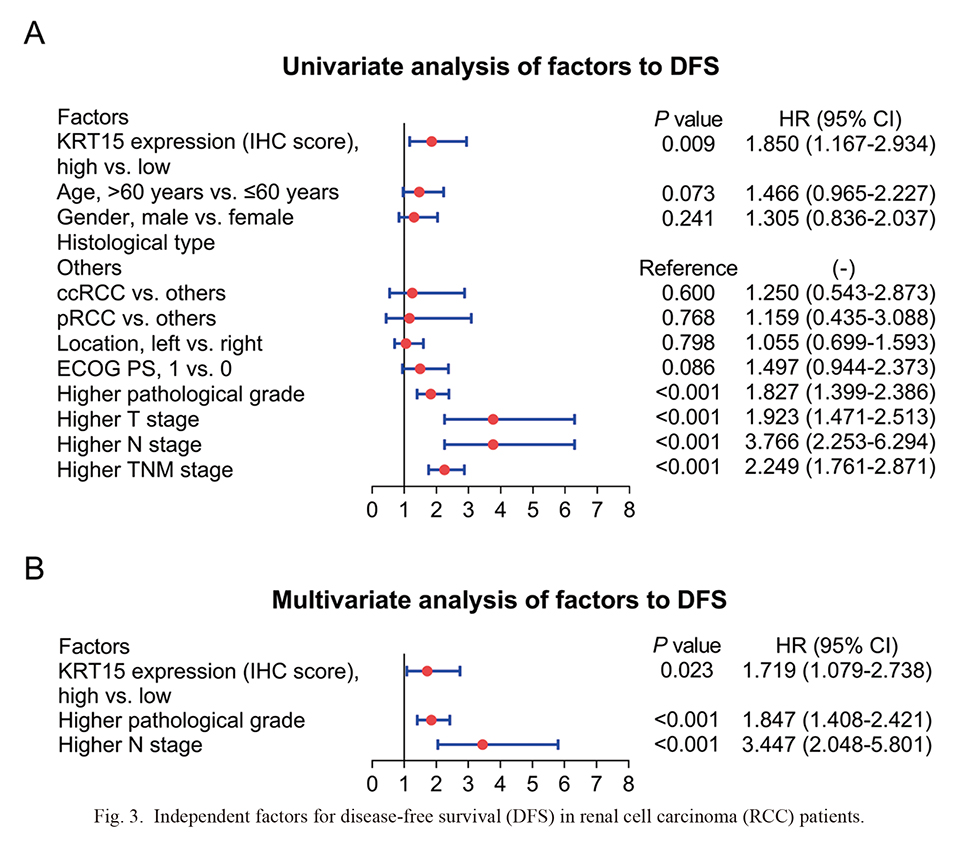
Independent factors for disease-free survival (DFS) in renal cell carcinoma (RCC) patients.
Univariate analysis (A) and multivariate analysis (B) for DFS are shown in RCC patients.
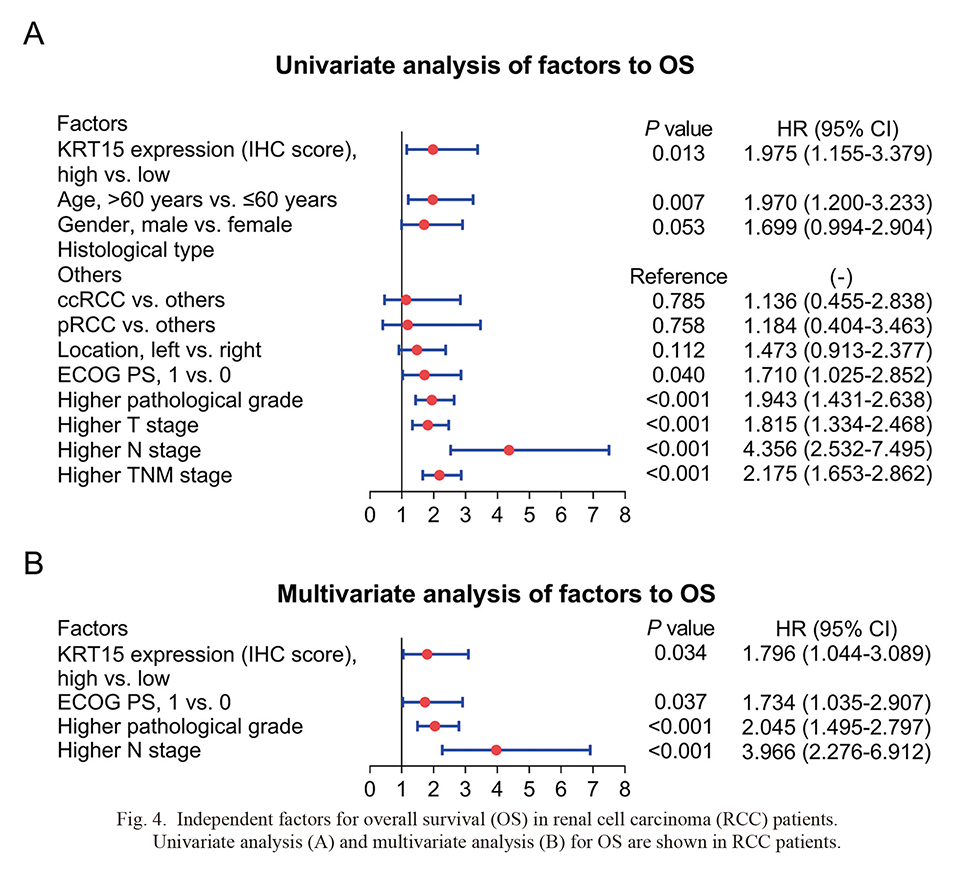
Independent factors for overall survival (OS) in renal cell carcinoma (RCC) patients.
Univariate analysis (A) and multivariate analysis (B) for OS are shown in RCC patients.
Data from the Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/) revealed that high KRT15 was related to poor DFS (P = 0.037) (Fig. 5A) and OS (P < 0.001) (Fig. 5B) in RCC patients. Meanwhile, data from THE HUMAN PROTEIN ATLAS (https://www.proteinatlas.org/) suggested that high KRT15 was associated with unsatisfactory OS in RCC patients (P < 0.001) (Fig. 5C).
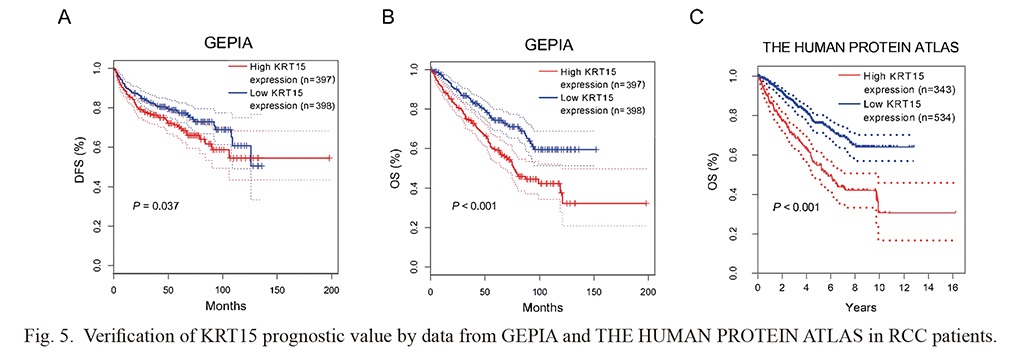
Verification of KRT15 prognostic value by data from GEPIA and THE HUMAN PROTEIN ATLAS in RCC patients.
Data from GEPIA revealed that high KRT15 was correlated with worse disease-free survival (DFS) (A) and overall survival (OS) (B); data from THE HUMAN PROTEIN ATLAS suggested that high KRT15 was associated with poor overall survival (OS) (C) in RCC patients.
The distribution of KRT15 IHC score was exhibited in 210 tumor tissues, and the median (IQR) IHC score of KRT15 in whole tumor tissues was 3.5 (3.0-7.0) (Supplementary Fig. S1). Additionally, KRT15 was not different between the paired tumor tissues [median (IQR) score: 4.0 (3.0-8.0)] and whole tumor tissues [median (IQR) IHC score: 3.5 (3.0-7.0)] (P = 0.280) (Supplementary Fig. S2).
KRT15 is dysregulated in patients with various cancers, including urinary tract carcinomas (Tai et al. 2013; Lin et al. 2020; Rao et al. 2020; Zhong et al. 2021; Yang et al. 2022). A previous study reveals that KRT15 is upregulated in a subset of invasive ureteric cancers and urinary bladder cancers (Tai et al. 2013). However, the dysregulation of KRT15 in RCC patients should be further investigated. The present study discovered that KRT15 was increased in tumor tissues compared to adjacent tissues in RCC patients. The potential reasons would be that: (1) KRT15 might be involved in the tumorigenesis of RCC (Tai et al. 2013); (2) KRT15 could accelerate the malignant proliferation of tumors; thus, it could reflect the malignant proliferation speed to some extent (Tai et al. 2013), while the malignant proliferation speed was faster in tumor tissues compared with adjacent tissues. Taken together, KRT15 was upregulated in the tumor tissues versus the adjacent tissues.
Regarding the correlation of KRT15 with tumor characteristics, several studies have explored this aspect in some cancers, such as colorectal, breast, and endometrial cancer (Rao et al. 2020; Zhong et al. 2021; Yang et al. 2022). For example, the KRT15 protein is related to the presence of lymphovascular invasion and higher International Federation of Gynecology and Obstetrics (FIGO) stage, while KRT15 mRNA is only correlated with unfavorable FIGO stage in endometrial cancer patients (Yang et al. 2022). Meanwhile, KRT15 is also correlated with the advanced T stage and TNM stage, as well as the existence of lymph node metastasis in esophageal carcinoma patients (Lin et al. 2020). The current study observed that KRT15 was associated with larger tumor size, higher N stage, and higher TNM stage in RCC patients. The possible argument would be that KRT15 could facilitate the proliferation, invasion, and migration of RCC; hence, KRT15 was linked to unfavorable tumor features (Tai et al. 2013; Chen and Miao 2022).
Importantly, KRT15 is related to poor survival profile in patients with esophageal, colorectal, and endometrial carcinoma according to previous research (Lin et al. 2020; Rao et al. 2020; Yang et al. 2022). In line with these studies, the current study disclosed that KRT15 was linked to shorter DFS and OS in RCC patients, which was confirmed by the multivariate regression analysis. To further confirm the prognostic implication of KRT15 in RCC patients, this study obtained data from GEPIA and THE HUMAN PROTEIN ATLAS, and it turned out that KRT15 could predict worse DFS and OS in RCC patients. The potential reason would be that KRT5 could regulate tumor cell proliferation, differentiation, invasion, and migration to exacerbate RCC, which would lead to poor survival in these patients (Tai et al. 2013; Chen and Miao 2022). Furthermore, higher pathological grade, N stage, and ECOG PS score were independently related to unsatisfactory survival in RCC patients. It could be explained by that higher pathological grade, N stage, and ECOG PS score reflected poor differentiation, the presence of lymph nodes, and unoptimistic health status (Dolan et al. 2020; Evens et al. 2021; Hu et al. 2021; Zhang et al. 2021); thus, patients with these features usually bore higher disease burden, which further contributed to worse prognosis in RCC patients. Notably, KRT15 appeared to be an anti-oncogene in breast cancer according to previous studies, and these studies indicated that KRT15 was related to prolonged survival in breast cancer patients, which conflicted with the results of this study (Xu et al. 2020; Zhong et al. 2021; Barron-Gallardo et al. 2022). A possible reason would be that breast cancer might be regulated by hormones (Fang et al. 2022), and KRT15 might have a certain correlation with hormones; meanwhile, in other cancers (such as RCC), the effect of hormones would be unobvious. Thus, KRT15 had different prognostic implication in breast cancer compared to other cancers, including RCC. However, this hypothesis was required further verification.
Although several interesting findings were discovered in this study, some limitations should still be mentioned: (1) the specimen repository was established independently until 2015 in our hospital, since then the adjacent specimens were obtained and stored; thus, only the recent 50 adjacent specimens were collected, which was relatively fewer compared to tumor specimens and might further interfere with the results; (2) this study only enrolled resectable RCC patients; however, the clinical role of KRT15 in unresectable RCC patients still required exploration; (3) the detailed mechanism of KRT15 involved in the pathogenesis of RCC was not explored in the current study, which could be further investigated; (4) further studies could consider detecting KRT15 mRNA to validate the prognostic implication of KRT15 in RCC patients; (5) patients were retrieved during 2012 to 2017, and this period was long; thus, the treatment strategies would be changed for some patients, which might possibly affect their prognosis.
In conclusion, KRT15 is increased in tumor specimens, and associates with RCC occurring in the left kidney, larger tumor size, higher N stage, and higher TNM stage, but not with other clinical characteristics; in addition, KRT15 also predicts poor DFS and OS in RCC patients according to the Kaplan-Meier curve analysis, multivariate analysis, as well as data from GEPIA and THE HUMAN PROTEIN ATLAS. Clinically, the findings of this study suggest that KRT15 may function as a potential biomarker to assist in reflecting the poor prognosis of RCC patients, which may better stratify these patients. However, further verification is warranted.
This study was supported by Youth Science and Technology Talent Development Plan of Baotou Medical College (No. BYJJ-QNGG 2022012).
The authors declare no conflict of interest.