Abstract
The coronavirus disease 2019 (COVID-19) pandemic remains a global public health concern. The clinical course and risk of developing severe illness among patients with COVID-19 who are at low-risk of severe COVID-19 remain uncertain. This retrospective cohort study from an isolation facility for low-risk COVID-19 patients in Japan evaluated the potential risks for severe disease with hypoxia (SpO2 ≤ 93%) or experiencing prolonged isolation period longer than 14 days with persistent acute symptoms. The study was performed before the spread of the alpha variant in the country and before the start of a nationwide mass vaccination campaign against COVID-19. Among the 929 participants with reliable outcome data regarding the development of hypoxia, 63 (6.8%) developed severe disease with hypoxia during their stays at the facility. Higher age [adjusted odds ratio (aOR), 1.08; 95% confidence interval (CI), 1.06-1.10] and male sex (aOR, 4.70; 95% CI, 2.39-9.22) were associated with this outcome. As for the experience of prolonged isolation period, higher age (aOR, 1.02; 95% CI, 1.01-1.04), atopic diseases (aOR, 1.69, 95% CI, 1.09-2.64), presence of cough at onset (aOR, 1.64; 95% CI, 1.09-2.48), and prescription of oral antibiotics before positive test results for COVID-19 (aOR, 2.37; 95% CI, 1.33-4.22) were associated with this outcome. In summary, 5-10% of low-risk COVID-19 patients later develop hypoxia. Older age and male sex were associated with both the development of hypoxia and prolonged acute symptoms. The unnecessary prescription of antibiotics before COVID-19 diagnosis may prolong COVID-19 symptoms.
Introduction
Pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), known as coronavirus disease 2019 (COVID-19) emerged in December 2019 in Wuhan, China (WHO, World Health Organization 2022b). COVID-19 has rapidly spread worldwide and has critically impacted public health and social activities (Moeti et al. 2022). Although effective vaccines and therapeutic drugs against SARS-CoV-2 have been developed, the constant emergence of variants strains and its strong infectiousness hamper the end of the pandemic. As of July, 2022, over 567 million confirmed cases and 6.3 million deaths have been reported globally (WHO 2022a). During the early phase of the COVID-19 pandemic, 14-19% of patients with COVID-19 became severe, and 2-5% died, whereas most patients recover only with mild-to-moderate clinical manifestations (Stokes et al. 2020; Wu and McGoogan 2020). Several clinical, radiographic, and laboratory findings have been identified as potential risk factors for severe COVID-19 (Guan et al. 2020; Shi et al. 2020; Malik et al. 2021). Moreover, several backgrounds before the COVID-19 infection, such as higher age, male sex, larger numbers of comorbidities, obesity, and diabetes mellitus have been identified as predictors for later developing severe illness (Fan et al. 2020; Knight et al. 2020; Liang et al. 2020; Yamada et al. 2021). Importantly, some patients who are initially considered to be at lower risks for severe illness can develop severe conditions afterward, which is among the important issues in managing clinically mild-to-moderate patients. These patients may experience some delays in seeking appropriate medical interventions as they usually stay at home or in designated non-hospital isolation facilities. To date, few studies have focused on the clinical course and risks of later becoming severe among clinically mild-to-moderate COVID-19 patients at low-risk of developing severe illness. In this study, we aimed to elucidate the characteristics and risk factors of patients initially judged to be at a lower risk of developing severe disease but later developed hypoxia, using data from a governmental isolation facility in Japan for low-risk COVID-19 patients. We also investigated the risk factors for prolong isolation period caused by persistent acute symptoms.
Materials and Methods
Data source and study period
This retrospective cohort study evaluated records regarding the demographic data, past medical histories, and disease courses of COVID-19 from individuals infected by COVID-19 admitted to a governmental isolation facility in Sendai City for the isolation and acute care of patients with COVID-19. All admitted individuals who were discharged from the facility between December 7, 2020, and February 21, 2021, were enrolled in this study. The study period was before the detection of the first case of SARS-CoV-2 variants of concern or interest in the prefecture. Furthermore, the study period occurred before the start of the mass vaccination campaign against COVID-19 for citizens of Japan (Akaishi et al. 2022), and none of the evaluated patients were vaccinated against COVID-19. This isolation facility was only for patients with COVID-19 who were judged by the local public health center staff to be at low risk for developing severe disease based on their demographic data and past medical histories. Other patients with COVID-19 judged to be at high risk of later developing severe disease or who were already hypoxic were hospitalized and not admitted to the isolation facility.
Eligibility for admission to the isolation facility
The isolation facility where this study was conducted has been collaboratively managed by the Miyagi Prefectural Government and Tohoku University Hospital since 2020. SARS-CoV-2 infections in the evaluated patients were confirmed by polymerase chain reaction (PCR) or antigen quantification tests using nasopharyngeal swabs or saliva samples. Since the first case of COVID-19 was confirmed in Japan, all patients with SARS-CoV-2 infection were hospitalized for appropriate treatment and isolation in the first several months. However, as the number of patients infected with SARS-CoV-2 increased in Miyagi Prefecture, patients with COVID-19 at low risk of developing severe disease were admitted to an existing hotel for exclusive use by patients with COVID-19 as an isolation facility. Doctors of the Miyagi COVID-19 Response Team played a central role in managing the medical resources for patients with COVID-19 and determined whether each patient was hospitalized or admitted to the isolation facility using the algorithm described later to stratify the necessity for hospitalization. The admitted individuals to the isolation facility included asymptomatic or mild-to-moderate patients with COVID-19. Admission to the isolation facility was implemented after obtaining informed consent from all patients.
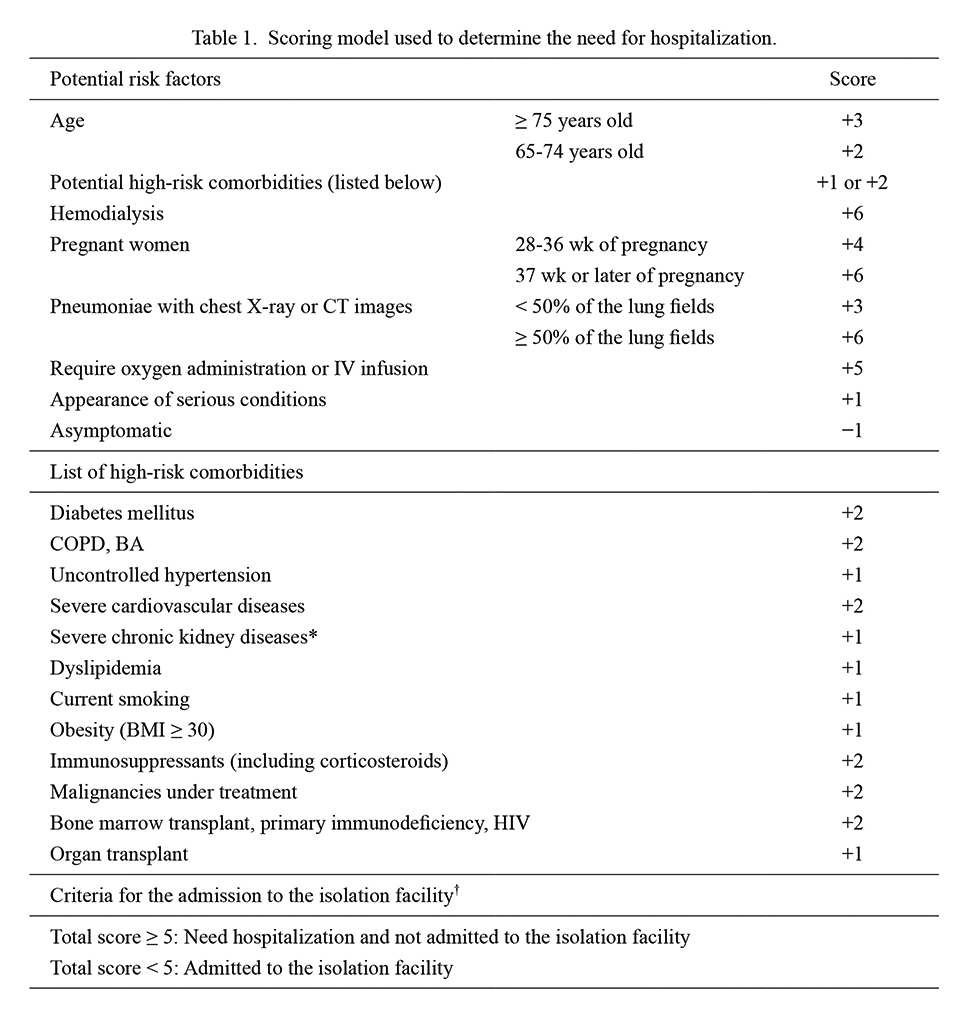
Algorithms for determining the need for hospitalization before admission to the isolation facility
The criteria used to determine whether to hospitalize or admit each patient to the isolation facility (Table 1) were developed by modifying the original version of the risk stratification model developed by the Kanagawa Prefectural Government, Japan (2020). In the original and modified risk stratification models, scores ranging from −1 to +6 were assigned to each subgroup in the abovementioned variables. Individuals with total scores of ≥ 5 were judged to be at high risk for developing severe COVID-19 with hypoxic conditions and were not admitted to the isolation facility. In the modified version of the risk stratification model used in this study, patients with at least one of the following information were automatically categorized as high-risk and were not admitted to the isolation facility: pregnant women at 37 weeks or later of gestation, those undergoing hemodialysis, pneumonia involving ≥ 50% of the lung fields, and already hypoxic patients requiring oxygen administration or intravenous drip infusions. Patients who were not assessed by chest radiography or computed tomography (CT) were assigned a score of 0 for the involved range of pneumonia based on these imaging studies. The clinical decision for each patient by the doctors of the COVID-19 Response Team prioritized automated risk stratification based on this scoring model.
During their stay at the isolation facility, the COVID-19 severity was evaluated according to the clinical management guidelines for patients with COVID-19 in Japan, which adopted the criteria of the globally accepted guidelines from the National Institute of Health in the US (Kato 2021; National Institute of Health 2022). The severity of each patient’s condition was classified into the following four categories every day: Stage I [asymptomatic or mild; blood oxygen saturation (SpO2) ≥ 96%, no shortness of breath, and no acute pneumonia], Stage IIa (moderate without hypoxia; SpO2 94-95% and the presence of shortness of breath or acute pneumonia), Stage IIb [moderate with hypoxia; SpO2 ≤ 93%, which corresponded to the “severe” type in the Centers for Disease Control and Prevention (CDC) guideline (CDC 2022)], and Stage III (severe; admission to an intensive care unit or requirement of a mechanical ventilator) (National Institute of Health 2022). Only patients in Stages I and IIa were allowed to remain in the isolation facility; those who developed hypoxic conditions matching Stage IIb during their stay at the isolation facility were immediately considered to be transferred to designated hospitals by emergency ambulance.
Healthcare workers stayed at the facility for 24 hours and checked the condition of the patients staying in the facility. The patients’ body temperatures and SpO2 were measured daily using instruments provided at the beginning of their stay. The attending doctors ordered blood tests and/or chest radiography as needed and prescribed symptomatic medications according to patient complaints (Takayama et al. 2020, 2021; Kikuchi et al. 2022). Patients who presented with severe COVID-19-related symptoms (e.g., SpO2 ≤ 93%, severe shortness of breath, or severe dehydration) or other urgent complications were immediately considered for transfer to designated hospitals. Medications brought to the facility were continued, but oral antibiotics for COVID-19, which are considered inappropriate medications, were discontinued upon admission unless there was evidence of bacterial coinfection.
The completion of isolation for each patient was decided by healthcare workers according to the following criteria based on the presence of COVID-19-related symptoms. For symptomatic patients, those who fulfilled both (1) 10 days after the day of onset and (2) 72 hours after the resolution of fever without any antipyretic or respiratory symptoms were allowed to leave the facility. Body temperature < 37.0℃ was defined as the criterion for deciding the resolution of fever. The intensity of each symptom was evaluated with a numeric rating scale (0-10), and symptom resolution was determined based on improvement in the scale. For asymptomatic patients, 10 days after the day of test sampling for SARS-CoV-2 was set as the provisional time for completing isolation (Kato 2021).
Outcomes
This study evaluated the following two outcomes, which were considered to have clinically important implications: (1) occurrence of hypoxia (SpO2 ≤ 93%) among the overall participants and (2) prolonged isolation for > 14 days from the onset of COVID-19 symptoms among symptomatic and presymptomatic (transient) patients. The occurrence of hypoxia has been accepted worldwide as a marker of severe COVID-19 (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team 2020; National Institute of Health 2022), and has been used as an objective criterion for hospitalization. Patients who reported abnormally low SpO2 levels inconsistent with their COVID-19-related symptoms or general condition were excluded from the analysis as clinically irrelevant measurements (Chan et al. 2013). An isolation duration of > 14 days were considered longer than expected among the overall patients with mild-to-moderate COVID-19. Prolonged isolations mostly resulted from prolonged fever (body temperature ≥ 37.0℃) or respiratory symptoms (i.e., cough, sputum, or dyspnea). Individuals with the following criteria were excluded from the analysis using prolonged isolation as the outcome: those who were asymptomatic throughout their clinical courses, those who were transferred to hospitals because of the development of hypoxia before completing their designated isolation periods, those who were discharged from the facility because they refused to continue staying, and those who stayed for > 14 days because of unknown reasons unrelated to their clinical conditions.
Predictors
The available demographic and background data from each patient included age, sex, nationality, comorbidities, medications, current smoking status (smoking/non-smoking), onset day, test day for SARS-CoV-2, medical-seeking behavior before admission to the facility, prescribed medications before positive test results for SARS-CoV-2, and presence of COVID-19-related symptoms. For asymptomatic individuals, the day of onset was substituted with the SARS-CoV-2 testing date. The presence of COVID-19-related symptoms was categorized as symptomatic (i.e., with symptoms already present at the diagnosis of COVID-19), presymptomatic/transient (i.e., symptoms developed after the test of SARS-CoV-2), or asymptomatic (i.e., symptoms did not develop during their isolation). Data on the comorbidities associated with the development of severe COVID-19 were also collected. The evaluated comorbidities included hypertension, diabetes mellitus, heart disease, cerebrovascular disease, history of cancer, liver disease, bronchial asthma, chronic obstructive pulmonary disease (COPD), and psychiatric disease, according to previous reports (National Center for Immunization and Respiratory Disease, Division of Viral Diseases 2020). As less than half of the cases reported body weight, we excluded body mass index from the analyses. The analyzed symptoms and vital signs included the body temperature, pulse rate, SpO2, cough, sputum, dyspnea, chest pain, fatigue, headache, runny nose/nasal obstruction, sore throat, myalgia/arthralgia, diarrhea, nausea/vomiting, dysgeusia, dysosmia, poor appetite, anxiety, sleep disorders, and any other symptoms.
Statistical analysis
Categorical variables are presented as counts with percentages, and continuous variables are presented as medians with interquartile ranges (IQR). We used chi-square or Fisher’s exact tests for categorical variables, as appropriate, and the Mann−Whitney U test for continuous variables. Multiple logistic regression models were used to identify the risk factors associated with hypoxia and prolonged isolation > 14 days. We included all variables with p < 0.10 in the univariate analyses into the multiple logistic regression models. However, there were concerns about overfitting in the multivariate logistic regression models if the number of independent variables exceeded one per 10 outcome events. Then, we constructed logistic regression models only with age and sex as independent variables. The presence of fever, cough, sputum or dyspnea at presentation was considered a potential confounding factor for prescriptions, particularly for the prescription of oral antibiotics before the positive result of the SARS-CoV-2 test, and resolution of those symptoms was also necessary for ending the isolation. Therefore, we added these symptoms in the multiple logistic regression model to analyze prolonged isolation. The results of the multiple logistic regression models were presented as adjusted odds ratios (aORs) and 95% confidence intervals (CIs). We confirmed that the variance inflation factors were sufficiently low to increase the risk of multicollinearity. Two-tailed p < 0.05 was considered significant in all statistical analyses. All statistical analyses were performed using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) and its graphical user interface EZR (Kanda 2013).
Ethics
This study adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Tohoku University Graduate School of Medicine (approval number: 2020-1-807). Approval for the use of the participants’ data was also obtained from the Miyagi Prefectural Government. The requirement for informed consent was waived due to the retrospective nature of the study, and an opt-out approach was adopted.
Results
Demographics and clinical data
This study enrolled a total of 996 patients discharged from the isolation facility between December 7, 2020, and February 21, 2021. Among these, 52 patients were excluded due to missing records and unavailability for univariate and multivariate analyses (Fig. 1). Among the 944 patients included in the study, the median age was 38 years (IQR, 25-52 years; full range, 2-95 years); 412 (43.6%) were female, and 919 (97.4%) were Japanese (Table 2). The median interval between testing for SARS-CoV-2 and admission to the isolation facility was 3 days (IQR, 2-4 days) and that between the onset and admission to the isolation facility was 5 days (IQR, 4-7 days). In this study, 255 (27.0%) patients showed at least one comorbidity, most frequently atopic disease (including asthma, atopic dermatitis, or allergic rhinitis) (22.5%), followed by hypertension (13.9%), and dyslipidemia (8.8%). At the test of SARS-CoV-2, 164 (17.4%) patients were asymptomatic and the other 780 (82.6%) patients were with COVID-19 symptoms. At the time of admission to the isolation facility, 92 (9.7%) patients were asymptomatic, 823 (87.2%) were with mild symptoms, and 29 (3.1%) were with moderate symptoms. Among the 92 patients who were asymptomatic at admission, 67 patients remained asymptomatic and 25 patients later developed COVID-19 symptoms during their stay at the isolation facility. A total of 303 (32.1%) patients were prescribed any drug before positive SARS-CoV-2 test results by practitioners or clinicians in the outpatient departments of hospitals, and approximately one-third of these patients were administered oral antibiotics. The frequencies of symptoms at onset (i.e., symptoms occurring within 48 hours of onset), 10 days after clinical onset, and throughout the disease are shown in Fig. 2. Fever (≥ 37.5℃) and cough were more frequent at onset (43.3% and 33.5%, respectively). In contrast, dysosmia, runny nose/nasal obstruction, dysgeusia, and coughing were more frequent on day 10 (33.2%, 30.8%, 25.2%, and 21.7%, respectively). Dysosmia and dysgeusia were not resolved in most patients, whereas the other symptoms were almost resolved. The median duration of stay at the isolation facility was 7 days (IQR, 5-9 days). A total of 63 (6.7%) patients were eventually transferred to hospitals while staying at the isolation facility, 50 and 13 of whom were transferred because of progressive respiratory conditions and other reasons (e.g., persistent fever, worsening underlying medical conditions, or severe dehydration), respectively. None of the patients presented critical conditions during their stay in the facility.


The analysis of the risk factors associated with the development of hypoxia (SpO2 ≤ 93%) included a total of 929 patients. Among these, 63 patients developed hypoxia with a median duration of 8 days (IQR, 6-10 days) from onset to hypoxia. The factors identified as covariate candidates for multivariate analysis were older age, male sex, number of comorbidities, hypertension, dyslipidemia, hyperuricemia, heart disease, cerebrovascular disease, liver disease, COPD, and not being a current smoker (Table 3). Regarding the occurrence of hypoxia, the number of independent variables exceeded one per 10 outcome events. Therefore, a logistic regression model with only age and sex as independent variables was used. In calculating aORs for age and sex, only the other variable was used as the covariate. The results of the multiple logistic regression analysis adjusted for age and sex are shown in Fig. 3. A higher age (aOR for 1 year, 1.08; 95% CI, 1.06-1.10) and male sex (aOR, 4.70; 95% CI, 2.39-9.22) were statistically significant risks for developing hypoxia among patients with low-risk COVID-19. Meanwhile, the evaluated past medical histories, adjusted for age and sex, were not statistically significant predictors of hypoxia development.

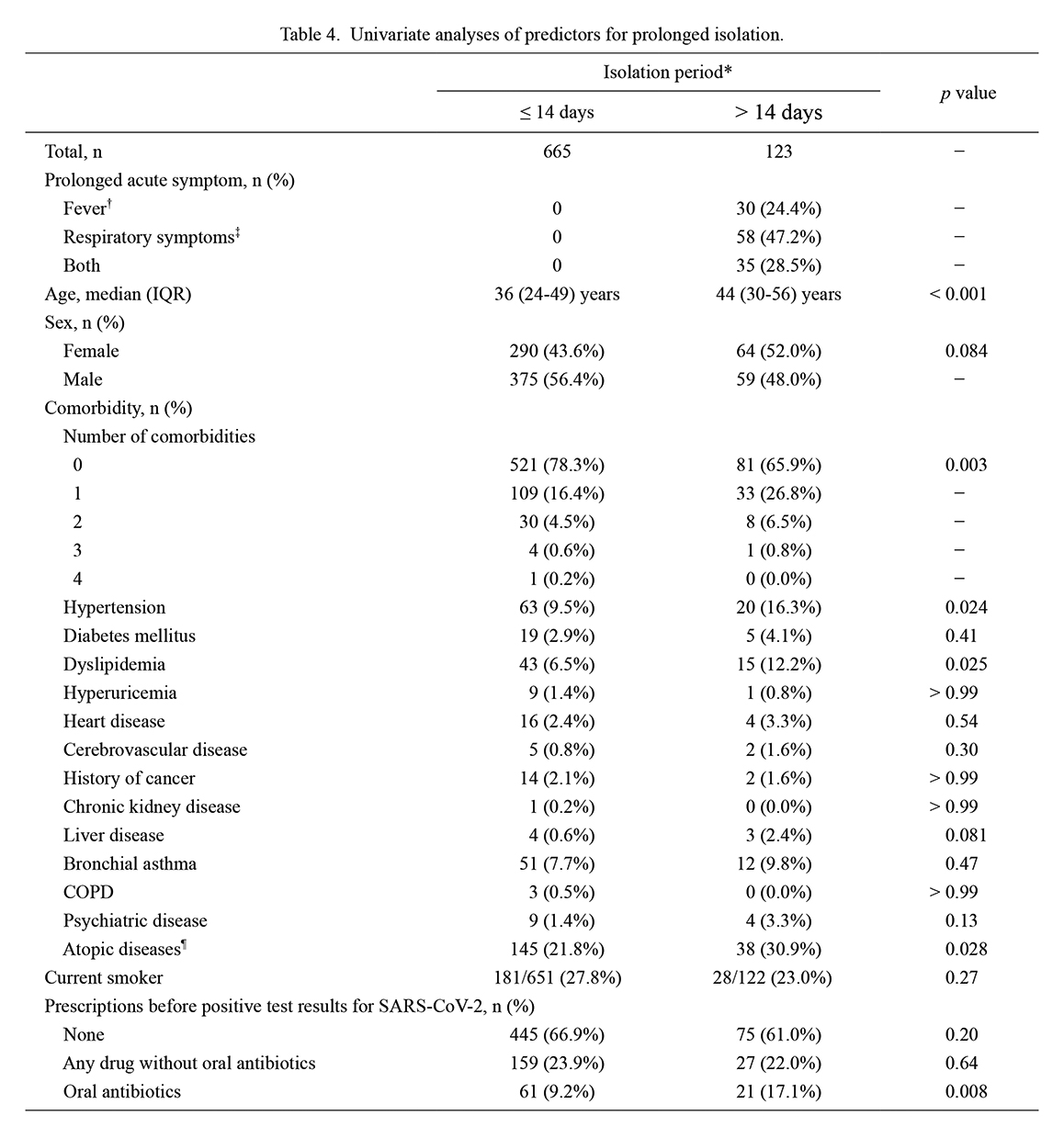
The analysis of the risk factors associated with prolonged isolation for > 14 days from onset included a total of 788 patients. Among these, 123 patients had prolonged isolation based on prolonged acute symptoms, such as fever (≥ 37.0℃) or respiratory symptoms (cough, sputum, or dyspnea). The longest isolation period was 32 days. The factors identified as covariate candidates for multivariate analysis were increasing age, female sex, number of comorbidities, hypertension, dyslipidemia, liver disease, atopic disease, and prescription of oral antibiotics before positive test results for SARS-CoV-2 (Table 4). Regarding the occurrence of prolonged isolation, the number of independent variables did not exceed one per 10 outcome events. Therefore, all variables with p < 0.10 in the univariate analyses were included as covariates in the following multiple logistic regression analysis. The results showed that higher age (aOR, 1.02; 95% CI, 1.01-1.04), atopic disease (aOR, 1.69, 95% CI, 1.09-2.64), cough at onset (aOR, 1.64; 95% CI, 1.09-2.48), and prescription of oral antibiotics before COVID-19 diagnosis (aOR, 2.37; 95% CI, 1.33-4.22) were statistically significant predictors of prolonged isolation (Fig. 4).
Finally, to estimate the validity of the risk stratification score for determining the need for hospitalization before admission to the isolation facility, the occurrence rates of the two evaluated outcomes (hypoxia and prolonged isolation) were investigated, using data of the risk stratification scores upon admission to the isolation facility and the occurrence of the outcomes. The occurrence rates of the two outcomes stratified by the calculated risk scores are listed in Table 5. The results demonstrated that the calculated risk score of ≥ +5 upon admission was a significant predictor for the subsequent hypoxia development (unadjusted OR, 6.05; 95% CI, 2.95-12.40), whereas it was not a significant predictor for the occurrence of prolonged isolation period based on persistent acute symptoms (unadjusted OR, 1.06; 95% CI, 0.36-3.10).
Discussion
This study evaluated the potential risk for developing hypoxia (SpO2 ≤ 93%) and prolonged isolation period > 14 days among patients with asymptomatic or mild-to-moderate COVID-19 without critical risks such as end-stage renal diseases requiring hemodialysis or full-term pregnancy. By now, few studies have evaluated the potential risks for hypoxia and prolonged acute symptoms among patients at lower-risk of developing severe illness. Hypoxia was used as the criterion for severe COVID-19 in this study, whereas several previous reports set hospitalization as the outcome (Gottlieb et al. 2020; Ioannou et al. 2020; Killerby et al. 2020; Petrilli et al. 2020). This was because we thought that it is important to specify the reasons of hospitalization and hypoxia would be a more objective measure than hospitalization. The obtained results in this study demonstrated that higher ages and male sex increased the risk of hypoxia. The estimated rate of developing hypoxia among the participants was lower than that in previous reports (Stokes et al. 2020; Wu and McGoogan 2020). This suggests that the risk stratification scoring model used before admission to the facility effectively identified higher-risk patients. Hypertension and dyslipidemia were suggested to be significant risk of hypoxia with the univariate analysis, but they were not statistically significant after adjusting for age and sex. This finding is consistent with those of previous studies (Arons et al. 2020; Gottlieb et al. 2020; Ioannou et al. 2020; Killerby et al. 2020; Petrilli et al. 2020; Zheng et al. 2020; Terada et al. 2021). Regarding the outcome of prolonged isolation, higher ages, atopic diseases, presence of cough at onset, and prescription of oral antibiotics before COVID-19 diagnosis were suggested to be significant predictors.
Among the 164 patients who were asymptomatic at testing for SARS-CoV-2, 97 (59.1%) patients later developed one or more COVID-19-related symptoms before or during their stay in the facility (i.e., transient or presymptomatic), whereas the other 67 (40.9%) remained asymptomatic throughout their disease courses. The proportions of presymptomatic individuals (i.e., initially asymptomatic at the time of COVID-19 diagnosis and later developing related symptoms) varied extensively in previous reports, and factors associated with remaining asymptomatic or presenting with any symptoms have not been identified (Arons et al. 2020; Sakurai et al. 2020). This wide variation could be due to the heterogeneous backgrounds and reasons for having COVID-19 testings in the absence of related symptoms. Although we evaluated possible factors associated with asymptomatic disease course, none of the evaluated factors were statistically significant predictors (Supplementary Table S1).
As another important finding of this study, the prescription of oral antibiotics before COVID-19 diagnosis was among the significant risk factors of prolonged isolation period with persistent acute symptoms. The Supplementary Fig. S1 shows the classification of administered oral antibiotics. Antibiotics may be used empirically because most PCR test results were available to clinicians the day after the test during the study period (Ishii et al. 2021). Vaughn et al. (2021) highlighted that unnecessary antibacterial therapy should not be used in COVID-19 treatment because 56.6% of hospitalized patients with COVID-19 were administered early empirical antibacterial drugs, whereas only 3.5% were confirmed to have bacterial co-infections. Similar results were reported in another study (Karami et al. 2021). Furthermore, CDC (2022) reported that the threat of antimicrobial resistance is growing during the COVID-19 pandemic owing to the increased use of antibacterial agents. From the perspective of human homeostatic microbiota, a recent study reported that COVID-19 provoked dysbiosis in the gut microbiota, which was associated with COVID-19 severity and was affected by antibiotics (Yeoh et al. 2021). The gut microbiota is closely associated with systemic immunopathology, including the respiratory immune system, and potentially enhances antiviral immunity (Round et al. 2011; Furusawa et al. 2013; He et al. 2020). Moreover, angiotensin-converting enzyme 2 (ACE2), which is the target receptor for infection by SARS-CoV-2, is associated with dysbiosis caused by interactions between SARS-CoV-2 infection and gastrointestinal metabolism (Hashimoto et al. 2012; Zhao et al. 2018; He et al. 2020). Furthermore, among recent studies on lung microbiota, Dumas et al. (2018) reviewed the associations between respiratory diseases (e.g., infectious disease, COPD, and asthma) and immunopathology related to lung microbiota as well as the immunopathological interactions between gut microbiota and lung microbiota, the so-called “gut-lung axis.” Considering these reports, we hypothesized that antibiotics might disturb immune responses to SARS-CoV-2 infection by impairing the gut and lung microbiota, leading to prolonged fever and respiratory symptoms. However, this hypothesis requires verification through further experimental and comparative studies. Consequently, we should consider the possibility of adverse, although not critical, effects of antibiotics on patients with COVID-19 administered antibacterial agents for empirical therapy or due to the overdiagnosis of bacterial co-infection. In addition, whether antibiotic therapy is associated with post-COVID-19 conditions requires further investigation.
This study has several limitations. First, the obtained findings may not be applicable to the current situation of the COVID-19 pandemic, as a variety of SARS-CoV-2 variant strains have emerged and most people have been vaccinated against the virus after the study period. Second, the measured SpO2 values could be less reliable than the measures of oxygen saturation in arterial blood for the correct diagnosis of hypoxia. However, the medical staffs at the isolation facility repeatedly measured the SpO2 values and checked the correctness of the way of measuring the SpO2 in cases with low value. Third, not all patients with pneumonia could have been identified as many participants were not studied radiologically. Consequently, the impact of having pneumonia at admission to the facility on hypoxia development or prolonged isolation period remains uncertain. Finally, the clinical severity level at onset could have influenced the prescription of antibiotics before COVID-19 diagnosis. However, we checked and confirmed that the prevalence of the evaluated symptoms at onset did not significantly differ between those with and without antibiotics.
In conclusion, higher ages and male sex were identified as significant predictors for later developing hypoxia or having prolonged isolation period with persistent acute symptoms among mild-to-moderate COVID-19 patients with lower risk stratification scores. History of atopic diseases, prescription of antibiotics for related symptoms, and the presence of cough symptoms at onset were further identified as predictors for prolonged isolation period. The used risk stratification scoring model before determining the admission to the facility was suggested to be useful in stratifying the risk for developing hypoxia, whereas it was not useful in predicting a prolonged isolation period with persistent acute symptoms.
Acknowledgments
We thank the staff of the Miyagi Prefectural Government for their cooperation in collecting the records and the healthcare workers who cared for the patients and diligently recorded patient information in the isolation facility. We also thank the staff of the Kanagawa Prefectural Government for allowing us to cite and introduce the Kanagawa Model to estimate severe COVID-19 in this study. This study was supported by JSPS KAKENHI Grant Number JP21K10367.
Author Contributions
Y.T., T.A., and T.I. conceived and designed the study. Y.T. and T.I. obtained the data. Y.T. and T.A. analyzed the data and performed the statistical analyses. Y.T. and T.A. drafted the manuscript, and T.I., S.S., R.O., N.A., R.A., T.K., J.T., A.K., M.O., S.T., M.A., and K.O. contributed to the final manuscript. All authors approved the final manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Akaishi,
T.,
Onodera,
T.,
Takahashi,
T.,
Harigae,
H. &
Ishii,
T.
(2022) Reports of acute adverse events in mRNA COVID-19 vaccine recipients after the first and second doses in Japan. Sci. Rep., 12, 15510.
-
Arons,
M.M.,
Hatfield,
K.M.,
Reddy,
S.C.,
Kimball,
A.,
James,
A.,
Jacobs,
J.R.,
Taylor,
J.,
Spicer,
K.,
Bardossy,
A.C.,
Oakley,
L.P.,
Tanwar,
S.,
Dyal,
J.W.,
Harney,
J.,
Chisty,
Z.,
Bell,
J.M.,
et al.
(2020) Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med., 382, 2081-2090.
-
Centers for Disease Control and Prevention (CDC)
(2022) COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. Atlanta, GA: U.S. Department of Health and Human Services. https://www.cdc.gov/drugresistance/covid19.html [Accessed: October 22, 2022].
-
Chan,
E.D.,
Chan,
M.M. &
Chan,
M.M.
(2013) Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir. Med., 107, 789-799.
-
Dumas,
A.,
Bernard,
L.,
Poquet,
Y.,
Lugo-Villarino,
G. &
Neyrolles,
O.
(2018) The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol., 20, e12966.
-
Fan,
G.,
Tu,
C.,
Zhou,
F.,
Liu,
Z.,
Wang,
Y.,
Song,
B.,
Gu,
X.,
Wang,
Y.,
Wei,
Y.,
Li,
H.,
Wu,
X.,
Xu,
J.,
Tu,
S.,
Zhang,
Y.,
Wu,
W.,
et al.
(2020) Comparison of severity scores for COVID-19 patients with pneumonia: a retrospective study. Eur. Respir. J., 56, 2002113.
-
Furusawa,
Y.,
Obata,
Y.,
Fukuda,
S.,
Endo,
T.A.,
Nakato,
G.,
Takahashi,
D.,
Nakanishi,
Y.,
Uetake,
C.,
Kato,
K.,
Kato,
T.,
Takahashi,
M.,
Fukuda,
N.N.,
Murakami,
S.,
Miyauchi,
E.,
Hino,
S.,
et al.
(2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 504, 446-450.
-
Gottlieb,
M.,
Sansom,
S.,
Frankenberger,
C.,
Ward,
E. &
Hota,
B.
(2020) Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad. Emerg. Med., 27, 963-973.
-
Guan,
W.J.,
Liang,
W.H.,
Zhao,
Y.,
Liang,
H.R.,
Chen,
Z.S.,
Li,
Y.M.,
Liu,
X.Q.,
Chen,
R.C.,
Tang,
C.L.,
Wang,
T.,
Ou,
C.Q.,
Li,
L.,
Chen,
P.Y.,
Sang,
L.,
Wang,
W.,
et al.
(2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J., 55, 2000547.
-
Hashimoto,
T.,
Perlot,
T.,
Rehman,
A.,
Trichereau,
J.,
Ishiguro,
H.,
Paolino,
M.,
Sigl,
V.,
Hanada,
T.,
Hanada,
R.,
Lipinski,
S.,
Wild,
B.,
Camargo,
S.M.,
Singer,
D.,
Richter,
A.,
Kuba,
K.,
et al.
(2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature, 487, 477-481.
-
He,
Y.,
Wang,
J.,
Li,
F. &
Shi,
Y.
(2020) Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections. Front. Microbiol., 11, 1302.
-
Ioannou,
G.N.,
Locke,
E.,
Green,
P.,
Berry,
K.,
O’Hare,
A.M.,
Shah,
J.A.,
Crothers,
K.,
Eastment,
M.C.,
Dominitz,
J.A. &
Fan,
V.S.
(2020) Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw. Open, 3, e2022310.
-
Ishii,
T.,
Kushimoto,
S.,
Katori,
Y.,
Kure,
S.,
Igarashi,
K.,
Fujita,
M.,
Takayama,
S.,
Abe,
M.,
Tanaka,
J.,
Kikuchi,
A.,
Abe,
Y.,
Imai,
H.,
Inaba,
Y.,
Iwamatsu-Kobayashi,
Y.,
Nishioka,
T.,
et al.
(2021) Predictors of SARS-CoV-2 positivity based on RT-PCR swab tests at a drive-through outpatient clinic for COVID-19 screening in Japan. Tohoku J. Exp. Med., 253, 101-108.
-
Kanagawa Prefectural Government, Japan
(2020) Kanagawa Model for the risk stratification of local COVID-19 patients. https://www.pref.kanagawa.jp/docs/ga4/covid19/facilities/model.html [Accessed: September 11, 2022] (in Japanese).
-
Kanda,
Y.
(2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant., 48, 452-458.
-
Karami,
Z.,
Knoop,
B.T.,
Dofferhoff,
A.S.M.,
Blaauw,
M.J.T.,
Janssen,
N.A.,
van Apeldoorn,
M.,
Kerckhoffs,
A.P.M.,
van de Maat,
J.S.,
Hoogerwerf,
J.J. &
Ten Oever,
J.
(2021) Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect. Dis. (Lond.), 53, 102-110.
-
Kato,
Y.
(2021) Case management of COVID-19 (secondary version). JMA J., 4, 191-197.
-
Kikuchi,
A.,
Arita,
R.,
Ono,
R.,
Tadano,
Y.,
Saito,
N.,
Akaishi,
T.,
Kanno,
T.,
Osawa,
M.,
Takayama,
S.,
Abe,
M.,
Onodera,
K. &
Ishii,
T.
(2022) Response to glucocorticoid therapy in patients with mild to moderate coronavirus disease 2019 at a Japanese care facility. Tohoku J. Exp. Med., 257, 97-106.
-
Killerby,
M.E.,
Link-Gelles,
R.,
Haight,
S.C.,
Schrodt,
C.A.,
England,
L.,
Gomes,
D.J.,
Shamout,
M.,
Pettrone,
K.,
O’Laughlin,
K.,
Kimball,
A.,
Blau,
E.F.,
Burnett,
E.,
Ladva,
C.N.,
Szablewski,
C.M.,
Tobin-D’Angelo,
M.,
et al.
(2020) Characteristics associated with hospitalization among patients with COVID-19 - Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb. Mortal. Wkly. Rep., 69, 790-794.
-
Knight,
S.R.,
Ho,
A.,
Pius,
R.,
Buchan,
I.,
Carson,
G.,
Drake,
T.M.,
Dunning,
J.,
Fairfield,
C.J.,
Gamble,
C.,
Green,
C.A.,
Gupta,
R.,
Halpin,
S.,
Hardwick,
H.E.,
Holden,
K.A.,
Horby,
P.W.,
et al.
(2020) Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ, 370, m3339.
-
Liang,
W.,
Liang,
H.,
Ou,
L.,
Chen,
B.,
Chen,
A.,
Li,
C.,
Li,
Y.,
Guan,
W.,
Sang,
L.,
Lu,
J.,
Xu,
Y.,
Chen,
G.,
Guo,
H.,
Guo,
J.,
Chen,
Z.,
et al.
(2020) Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern. Med., 180, 1081-1089.
-
Malik,
P.,
Patel,
U.,
Mehta,
D.,
Patel,
N.,
Kelkar,
R.,
Akrmah,
M.,
Gabrilove,
J.L. &
Sacks,
H.
(2021) Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid. Based Med., 26, 107-108.
-
Moeti,
M.,
Gao,
G.F. &
Herrman,
H.
(2022) Global pandemic perspectives: public health, mental health, and lessons for the future. Lancet, 400, e3-e7.
-
National Center for Immunization and Respiratory Disease, Division of Viral Diseases
(2020) Science Brief: Evidence Used to Update the List of Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19. In CDC COVID-19 Science Briefs, Centers for Disease Control and Prevention (US), Atlanta, GA, USA.
-
National Institute of Health
(2022) Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/ [Accessed: July 16, 2022].
-
Petrilli,
C.M.,
Jones,
S.A.,
Yang,
J.,
Rajagopalan,
H.,
O’Donnell,
L.,
Chernyak,
Y.,
Tobin,
K.A.,
Cerfolio,
R.J.,
Francois,
F. &
Horwitz,
L.I.
(2020) Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ, 369, m1966.
-
Round,
J.L.,
Lee,
S.M.,
Li,
J.,
Tran,
G.,
Jabri,
B.,
Chatila,
T.A. &
Mazmanian,
S.K.
(2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science, 332, 974-977.
-
Sakurai,
A.,
Sasaki,
T.,
Kato,
S.,
Hayashi,
M.,
Tsuzuki,
S.I.,
Ishihara,
T.,
Iwata,
M.,
Morise,
Z. &
Doi,
Y.
(2020) Natural history of asymptomatic SARS-CoV-2 infection. N. Engl. J. Med., 383, 885-886.
-
Shi,
H.,
Han,
X.,
Jiang,
N.,
Cao,
Y.,
Alwalid,
O.,
Gu,
J.,
Fan,
Y. &
Zheng,
C.
(2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis., 20, 425-434.
-
Stokes,
E.K.,
Zambrano,
L.D.,
Anderson,
K.N.,
Marder,
E.P.,
Raz,
K.M.,
El Burai Felix,
S.,
Tie,
Y. &
Fullerton,
K.E.
(2020) Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb. Mortal. Wkly. Rep., 69, 759-765.
-
Takayama,
S.,
Namiki,
T.,
Ito,
T.,
Arita,
R.,
Nakae,
H.,
Kobayashi,
S.,
Yoshino,
T.,
Ishigami,
T.,
Tanaka,
K.,
Kainuma,
M.,
Nochioka,
K.,
Takagi,
A.,
Mimura,
M.,
Yamaguchi,
T. &
Ishii,
T.
(2020) A multi-center, randomized controlled trial by the Integrative Management in Japan for Epidemic Disease (IMJEDI study-RCT) on the use of Kampo medicine, kakkonto with shosaikotokakikyosekko, in mild-to-moderate COVID-19 patients for symptomatic relief and prevention of severe stage: a structured summary of a study protocol for a randomized controlled trial. Trials, 21, 827.
-
Takayama,
S.,
Ono,
R.,
Arita,
R.,
Saito,
N.,
Suzuki,
S.,
Tadano,
Y.,
Akaishi,
T.,
Tanaka,
J.,
Kanno,
T.,
Kikuchi,
A.,
Ohsawa,
M.,
Onodera,
K.,
Abe,
M.,
Ido,
K.,
Nakamura,
N.,
et al.
(2021) Usefulness of portable chest radiography and blood sampling for prompt medical response in COVID-19 isolation facilities : two cases of moderate stage I COVID-19. J. Hosp. Gen. Med., 3, 92-96.
-
Terada,
M.,
Ohtsu,
H.,
Saito,
S.,
Hayakawa,
K.,
Tsuzuki,
S.,
Asai,
Y.,
Matsunaga,
N.,
Kutsuna,
S.,
Sugiura,
W. &
Ohmagari,
N.
(2021) Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID-19 in Japan. BMJ Open, 11, e047007.
-
The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team
(2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) - China, 2020. China CDC Wkly., 2, 113-122.
-
Vaughn,
V.M.,
Gandhi,
T.N.,
Petty,
L.A.,
Patel,
P.K.,
Prescott,
H.C.,
Malani,
A.N.,
Ratz,
D.,
McLaughlin,
E.,
Chopra,
V. &
Flanders,
S.A.
(2021) Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin. Infect. Dis., 72, e533-e541.
-
World Health Organization (WHO)
(2022a) COVID-19 Weekly Epidemiological Update. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-july-2022 [Accessed: August 15, 2022].
-
World Health Organization (WHO)
(2022b) Origin of SARS-CoV-2. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/origins-of-the-virus [Accessed: October 22, 2022].
-
Wu,
Z. &
McGoogan,
J.M.
(2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA, 323, 1239-1242.
-
Yamada,
G.,
Hayakawa,
K.,
Matsunaga,
N.,
Terada,
M.,
Suzuki,
S.,
Asai,
Y.,
Ohtsu,
H.,
Toyoda,
A.,
Kitajima,
K.,
Tsuzuki,
S.,
Saito,
S. &
Ohmagari,
N.
(2021) Predicting respiratory failure for COVID-19 patients in Japan: a simple clinical score for evaluating the need for hospitalisation. Epidemiol. Infect., 149, e175.
-
Yeoh,
Y.K.,
Zuo,
T.,
Lui,
G.C.,
Zhang,
F.,
Liu,
Q.,
Li,
A.Y.,
Chung,
A.C.,
Cheung,
C.P.,
Tso,
E.Y.,
Fung,
K.S.,
Chan,
V.,
Ling,
L.,
Joynt,
G.,
Hui,
D.S.,
Chow,
K.M.,
et al.
(2021) Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut, 70, 698-706.
-
Zhao,
Y.,
Chen,
F.,
Wu,
W.,
Sun,
M.,
Bilotta,
A.J.,
Yao,
S.,
Xiao,
Y.,
Huang,
X.,
Eaves-Pyles,
T.D.,
Golovko,
G.,
Fofanov,
Y.,
D’Souza,
W.,
Zhao,
Q.,
Liu,
Z. &
Cong,
Y.
(2018) GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol., 11, 752-762.
-
Zheng,
Z.,
Peng,
F.,
Xu,
B.,
Zhao,
J.,
Liu,
H.,
Peng,
J.,
Li,
Q.,
Jiang,
C.,
Zhou,
Y.,
Liu,
S.,
Ye,
C.,
Zhang,
P.,
Xing,
Y.,
Guo,
H. &
Tang,
W.
(2020) Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect., 81, e16-e25.










