2023 Volume 260 Issue 4 Pages 341-346
2023 Volume 260 Issue 4 Pages 341-346
Primary malignant lymphoma confinement to the cauda equina is rare. Only 14 cases of primary malignant lymphoma in the cauda equina have been reported. In these cases, the clinical features were similar to those of lumbar spinal canal stenosis (LSCS). This report describes a case of diffuse large B-cell lymphoma of the cauda equina that was diagnosed after decompression surgery for LSCS. An 80-year-old man presented with gait disturbance due to progressive muscle weakness in the lower extremities over the previous two months. He was diagnosed with LSCS, and decompression surgery was performed. However, the muscle weakness worsened after surgery; therefore, he was referred to our department. Plain magnetic resonance imaging (MRI) revealed swelling of the cauda equina. It demonstrated marked homogenous enhancement by gadolinium-diethylenetriamine pentaacetic acid. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) revealed diffuse accumulation of 18F-FDG in the cauda equina. These imaging findings were consistent with those of cauda equina lymphomas. To confirm the diagnosis, we performed an open biopsy of the cauda equina. Histological examination indicated diffuse large B-cell lymphoma. Considering the patient’s age and activities of daily living, further treatment was not performed. The patient died four months after the first surgery. Rapid progression of muscle weakness, which cannot be prevented by decompression surgery, and swollen cauda equina on MRI may be signs of this disorder. Gadolinium-enhanced MRI, 18F-FDG PET, and histological investigation of the cauda equina should be performed for diagnosing primary malignant lymphoma of the cauda equina.
Primary malignant lymphoma can occur in any part of the central nervous system, including the brain, spinal cord, and cauda equina (Grommes and DeAngelis 2017). However, this pathology is rarely confined to the cauda equina. To the best of our knowledge, only 14 cases have been histopathologically confirmed as primary malignant lymphoma of the cauda equina (Mauney and Sciotto 1983; Toner et al. 1989; Klein et al. 1990; Knopp et al. 1994; Ooi et al. 1996; Khong et al. 2008; Morita et al. 2009; Nishida et al. 2012; Teo et al. 2012; Broen et al. 2014; Nakashima et al. 2014; Shin et al. 2016; Belcastro et al. 2016; Suzuki et al. 2018).
Patients with cauda equina lymphoma may show clinical features similar to those of lumbar spinal canal stenosis (LSCS). So far, no case reports have described patients who suffered from LSCS along with malignant lymphoma of the cauda equina. This report describes a case of diffuse large B-cell lymphoma of the cauda equina that was diagnosed as LSCS and treated with decompression surgery.
An 80-year-old man experienced gait disturbance due to progressive muscle weakness in the lower extremities, severe numbness in both the legs, and polyuria over the last two months. He was admitted to a nearby orthopedic hospital. Manual muscle testing (MMT) revealed grade 2 muscle weakness in the bilateral iliopsoas muscle and grade 1 to 2 weakness in the bilateral tibialis anterior, extensor hallucis longus, and gastrocnemius muscles. Patellar tendon reflexes (PTR) and Achilles tendon reflexes (ATR) were absent bilaterally. The pinprick test revealed hypoesthesia in both lower extremities.
Magnetic resonance imaging (MRI) revealed central canal stenosis at the L3-4 level (Fig. 1A, B). Myelography and subsequent computed tomography (CT) revealed narrowing of the dural tube at L3-4 (Fig. 2A, B). Cerebrospinal fluid (CSF) examination showed a cell count of 100/μL (reference value 0-5 /μL), a protein level of 100 mg/dL (reference value 10-40 mg/dL), and a glucose level of 45 mg/dL (reference value 50-75 mg/dL). Cytological examination of the CSF indicated no malignancy. Hematological tests for tumor markers, white blood cell count, and C-reactive protein levels were within the normal range. Based on neurological, radiological, and biochemical examinations, the patient was diagnosed with LSCS and underwent L3 laminectomy three months after the onset of his symptoms. However, his symptoms did not improve, and muscle weakness in the tibialis anterior worsened. Additional decompression surgery at L4-5 was performed two weeks after the initial surgery, yet the muscle weakness persisted. Since the cause of progressive muscle weakness could not be identified, he was referred to us five weeks after the first surgery.
On arrival at our hospital, the patient’s leg motor strength on MMT was grade 0 to 2 bilaterally. Plain MRI showed good decompression of the dural tube at L3-4 and L4-5. However, cranial to L2-3, swelling of the cauda equina was seen in the dural tube in transverse planes. Swollen cauda equina showed marked enhancement by gadolinium-diethylene triamine pentaacetic acid (Gd-DTPA). The L2 vertebral body, which partially showed high signal intensity on T2-weighted images and was well enhanced by Gd-DTPA, was suspected to be a hemangioma (Fig. 3). 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) demonstrated diffused accumulation of FDG in the cauda equina, with a maximum standardized uptake value (SUVmax) of 22.2 (Fig. 4). There was no other site of malignant lymphoma including elsewhere in the nervous system.
The patient’s serum soluble interleukin 2 receptor (sIL-2R) level was 558 U/ml, which was within the reference value of 205-587 U/ml. Other tumor markers were also within the normal range. The patient’s symptoms and imaging findings suggested that he had a malignant tumor or inflammatory disease. The differential diagnoses included hematological malignancies, metastatic tumors, chronic inflammatory demyelinating polyradiculoneuropathy, hereditary motor sensory neuropathies, and sarcoidosis (Hayashi et al. 2008).
To confirm the diagnosis, we performed an open biopsy of the cauda equina and flow cytometry of the CSF six weeks after the first surgery. Upon L2 laminectomy and durotomy, the cauda equina appeared markedly swollen, and one of the spinal nerves was sacrificed for biopsy (Fig. 5). The level of sIL-2R in CSF was 16,388 U/ml, which was remarkably higher than its reference value of 10 U/ml. Histological examination revealed nerve infiltration by atypical cells with little cytoplasm and large nuclei (Fig. 6A). Immunocytochemistry revealed positivity for cluster of differentiation 45 (CD45) (Fig. 6B), CD20 (Fig. 6C), CD79a (Fig. 6D), B-cell lymphoma 2 (BCL2) (Fig. 6E), BCL6 (Fig. 6F), and a high proliferative index (Ki-67 80%) (Fig. 6G), indicating diffuse large B-cell lymphoma. Flow cytometry of the CSF also supported this diagnosis. The patient was transferred to the Department of Hematology. Several treatment options were considered, such as radiotherapy and chemotherapy; however, further treatment was not performed because of the patient’s age and activities of daily living. The patient died due to aspiration pneumonia four months after the first surgery.
Written informed consent was obtained from the patient.

T2-weighted magnetic resonance images before decompression surgery.
(A) Sagittal view. Slight lumbar canal stenosis (arrow) is detected at the L3-4 level. (B) Axial view at the L3-4 level. The dural tube shows moderate compression (arrow).

Computed tomography findings after myelography before decompression surgery.
(A) Sagittal view. Filling defect (arrow) of the contrast medium is found at the L3-4 level. (B) Axial view at the L3-4 level. The contrast medium is only detected at the rim of the dura mater (arrowhead).

Magnetic resonance images (MRI) six weeks after the initial surgery.
(A) T2-weighted MRI sagittal view. The dural tube at the L3-4 and L4-5 levels is well decompressed (arrow), however, L2-3 or more cranial, no cerebrospinal fluid is found (arrowhead). (B) T2-weighted MRI axial view at the L2 level. Swelling of the cauda equina is seen in the dural tube (arrow). (C) Gadolinium (Gd)-enhanced MRI sagittal view. The cauda equina is clearly and homogenously enhanced (arrowhead). (D) Gd-enhanced MRI axial view. The intracanal region is well-enhanced (arrow).

Findings of 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG PET).
(A) Axial fused PET/computed tomography (CT) image at L2. Accumulation of FDG in the spinal canal (arrow) is detected, where the maximum standardized uptake value is 22.2. (B) Coronal maximum intensity projection PET image. Abnormal FDG uptake along the spinal canal (arrowhead) is demonstrated.
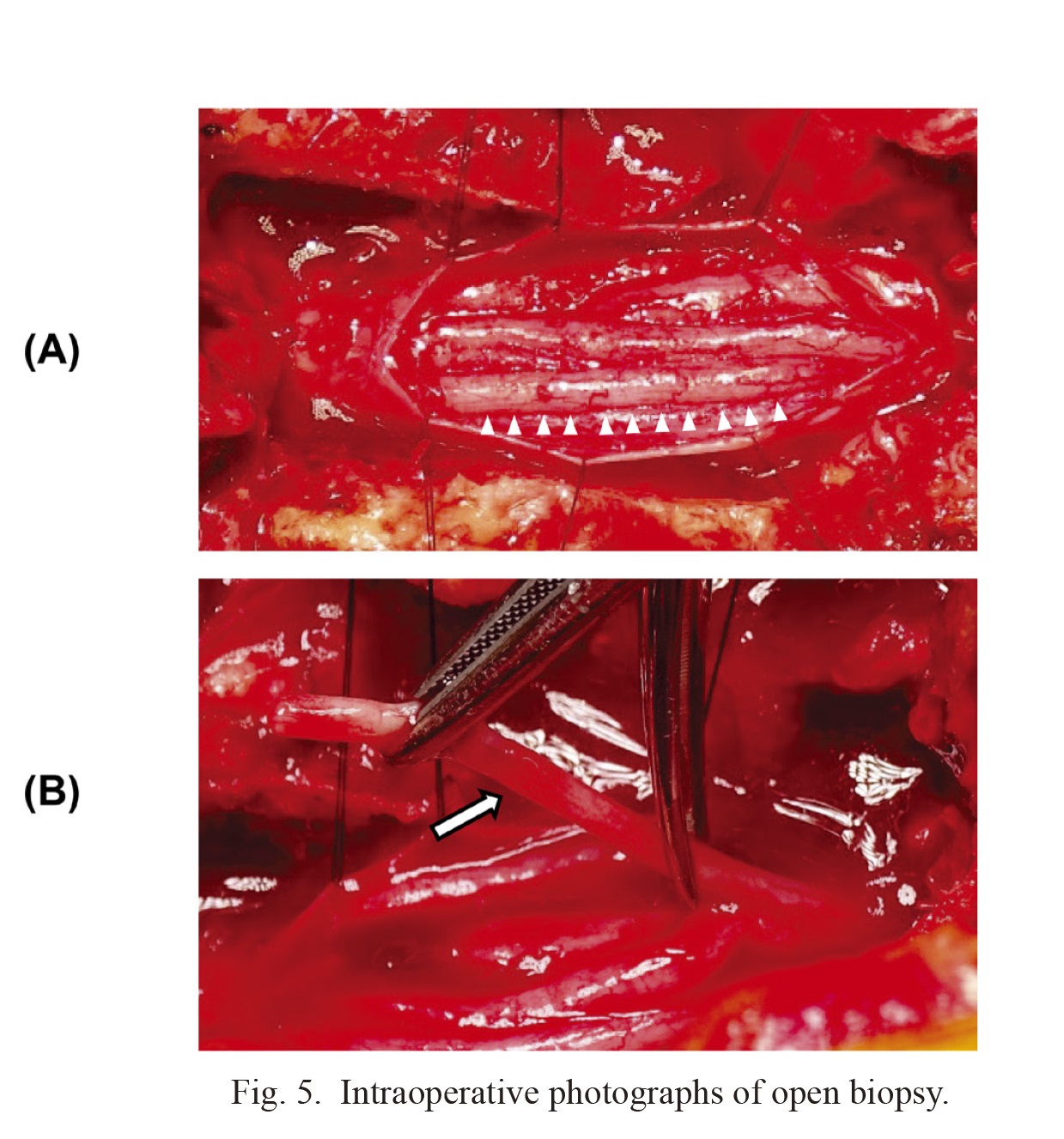
Intraoperative photographs of open biopsy.
(A) After durotomy, swelling of the cauda equina is seen in the intradural space (arrowhead). (B) One of the spinal nerves (arrow) was sacrificed for biopsy.
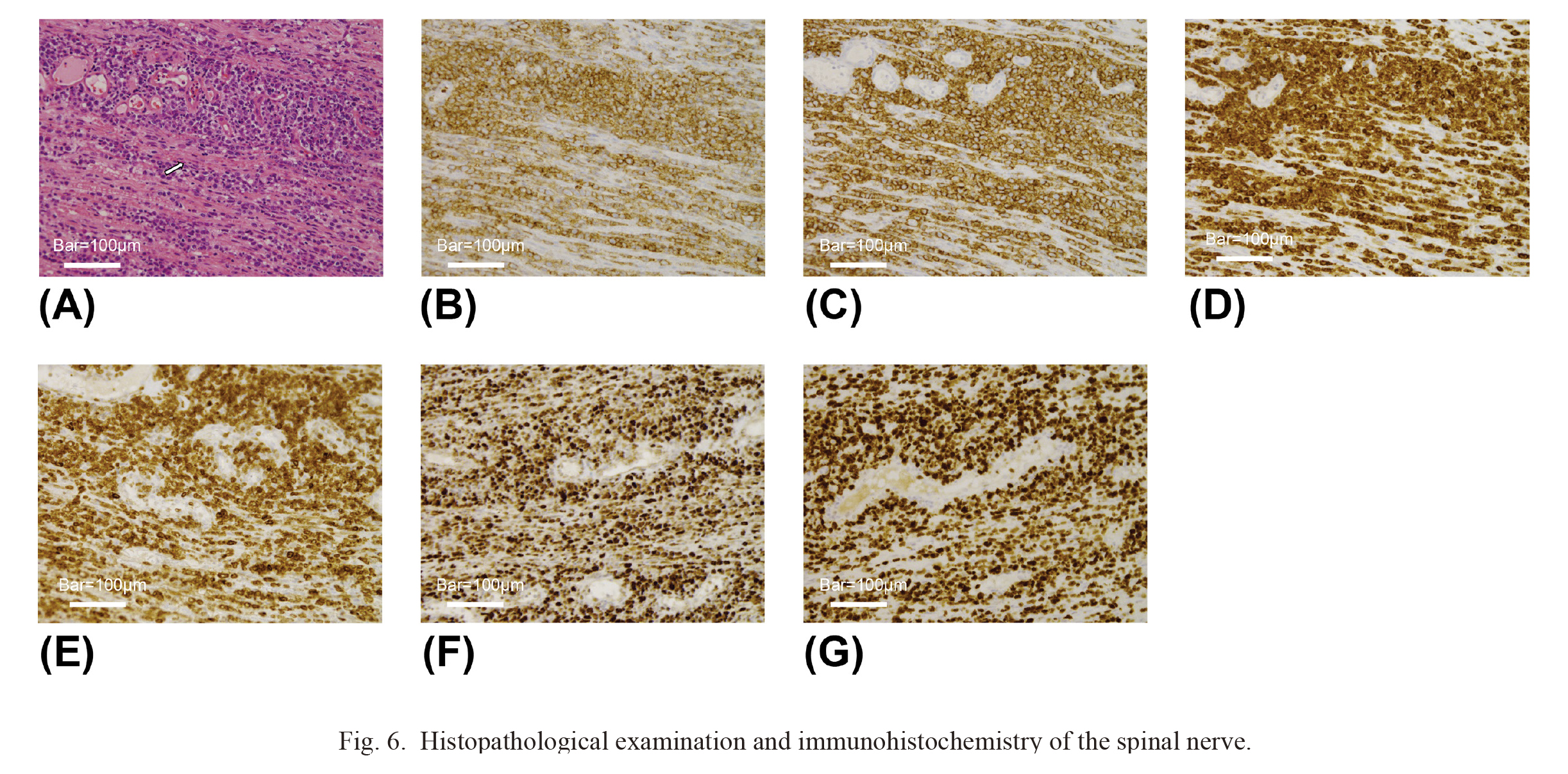
Histopathological examination and immunohistochemistry of the spinal nerve.
(A) Hematoxylin-eosin stain. Atypical cells with a large and bizarre nucleus infiltrate into nerve fibers (arrow) (bar: 100 μm). (B-F) Immunocytochemistry. Atypical lymphoma cells show positive staining by the antibodies of CD45 (B), CD20 (C), CD79a (D), BCL2 (E) and BCL6 (F) (bar: 100 μm). (G) Immunostaining of Ki-67. Atypical lymphoma cells indicate Ki-67 expression. The positive rate of Ki-67 was 80% (bar: 100 μm). CD, cluster of differentiation; BCL2, B-cell lymphoma 2.
Primary malignant lymphoma confined to the cauda equina is extremely rare. To the best of our knowledge, only 14 cases in previous English-language scientific journals have been confirmed by histopathology, and a summary of these patients is shown in Table 1. There were nine men and five women with an average age of 54 years. All patients had bilateral leg pain. The PTR and ATR were recorded in 12 patients and were diminished or absent in all of them. Ten patients showed bilateral anesthesia or hypoesthesia. Muscle weakness was detected in multiple nerve root distributions in 11 patients and progressed within 12 weeks in 10 patients (Mauney and Sciotto 1983; Toner et al. 1989; Klein et al. 1990; Knopp et al. 1994; Ooi et al. 1996; Khong et al. 2008; Morita et al. 2009; Nishida et al. 2012; Teo et al. 2012; Shin et al. 2016). Although patients with acute bilateral drop foot caused by LSCS have been reported (Demetriades et al. 2021), rapid progression of muscle weakness deriving from multiple nerve root distributions is not typically caused by ordinary LSCS (Watters et al. 2008). The patient in this case also developed severe paralysis in multiple nerve roots within 12 weeks. With the benefit of hindsight, it is clear to us that the patient’s progression of symptoms and neurological findings were not due to ordinary LSCS.
Plain MRI was performed in 11 of the reported cases, with nine of these revealing swelling of the cauda equina (Klein et al. 1990; Knopp et al. 1994; Khong et al. 2008; Morita et al. 2009; Nishida et al. 2012; Teo et al. 2012; Broen et al. 2014; Nakashima et al. 2014; Shin et al. 2016; Suzuki et al. 2018). Gd-enhanced MRI was performed in 10 cases, and marked enhancement of the cauda equina was observed in all these cases (Knopp et al. 1994; Ooi et al. 1996; Khong et al. 2008; Morita et al. 2009; Nishida et al. 2012; Broen et al. 2014; Nakashima et al. 2014; Belcastro et al. 2016; Shin et al. 2016; Suzuki et al. 2018). Furthermore, 18F-FDG PET was performed in five cases, which showed abnormal accumulation of FDG in the cauda equina (Broen et al. 2014; Nakashima et al. 2014; Belcastro et al. 2016; Suzuki et al. 2018). Among them, only one study reported an SUVmax of 4.9 (Suzuki et al. 2018); however, our case showed a high SUVmax of 22, which suggested malignancy (Grisariu et al. 2010).
Open biopsy is essential for definitive diagnosis. Except for one case diagnosed by flow cytometry, biopsy of the cauda equina was performed in all cases (Mauney and Sciotto 1983; Toner et al. 1989; Klein et al. 1990; Knopp et al. 1994; Ooi et al. 1996; Khong et al. 2008; Morita et al. 2009; Teo et al. 2012; Broen et al. 2014; Nakashima et al. 2014; Shin et al. 2016; Belcastro et al. 2016; Suzuki et al. 2018), which provided detailed information to ensure optimal therapy (Ito et al. 2020).
Definitive diagnosis at first presentation may have been difficult in the present case, as the patient presented with cauda equina syndrome. The cauda equina may have been judged as swollen; however, it was difficult to determine whether the cauda equina was abnormal because its thickness was affected by the presence of LSCS, and the standard value for cauda equina thickness has not been established (Matsushima et al. 2021). The elevated cell count and protein level in the CSF, which suggested inflammatory or malignant disease, were the only findings that suggested an abnormality of the cauda equina in this case. Swelling of the cauda equina became more prominent after the surgery.
The standard treatment strategies for primary central nervous system lymphoma are radiotherapy, chemotherapy, and combined radiotherapy and chemotherapy treatment. High-dose methotrexate with high-dose cytarabine, followed by radiation therapy, has been shown to improve prognosis (Ferreri et al. 2009), whereas the major toxic effects of chemotherapy, including neutropenia, thrombocytopenia, and anemia, have been reported with a frequency of 90% or more (Grommes and DeAngelis 2017).
Elderly patients are more likely to experience the adverse effects of chemotherapy and radiotherapy (Abrey et al. 2000). The present patient was 80 years old and had severe paralysis, which was not expected to improve after treatment. Therefore, we decided not to perform further treatment for this patient.
In conclusion, the case of a patient with malignant lymphoma of the cauda equina diagnosed after decompression surgery for LSCS is presented. Rapid progression of muscle weakness, which cannot be prevented by decompression surgery, and swelling of the cauda equina on MRI may be the signs of primary malignant lymphoma of the cauda equina. Once these findings are detected, Gd-enhanced MRI and 18F-FDG PET should be performed, and if necessary, the cauda equina should be histologically investigated.
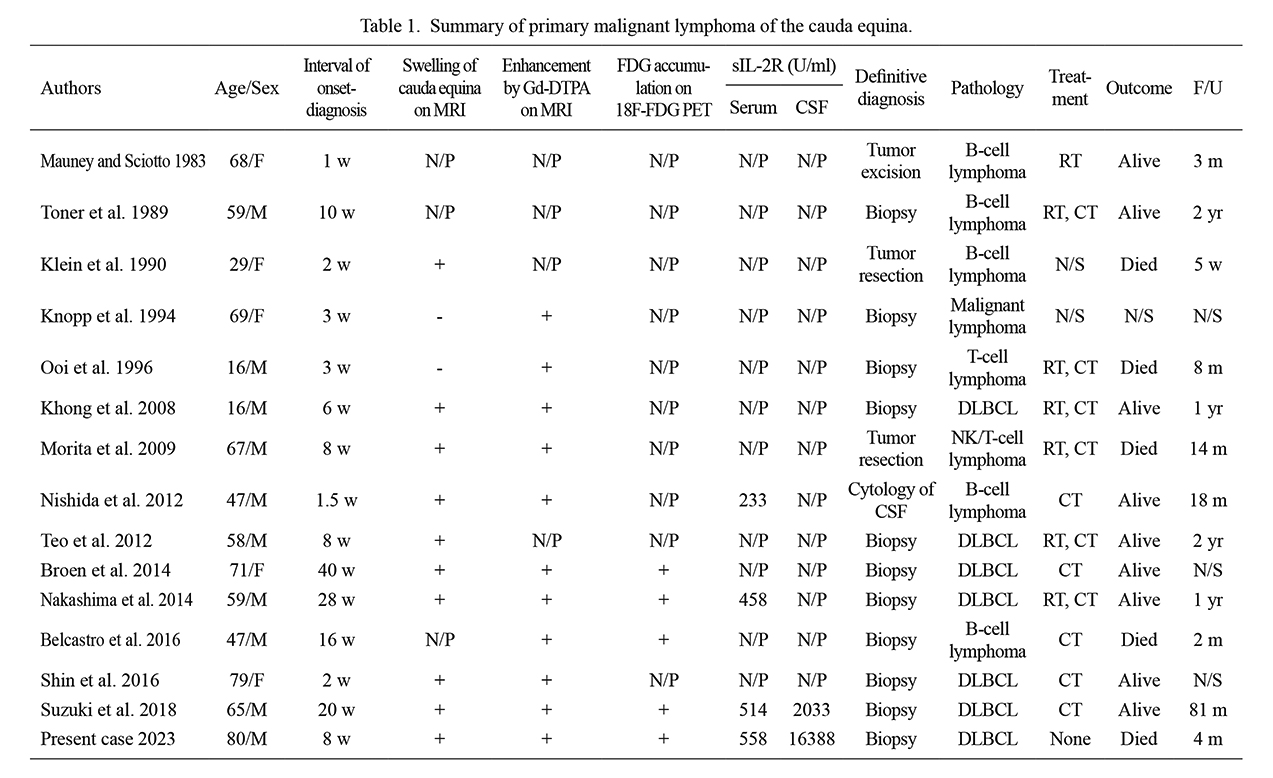
Summary of primary malignant lymphoma of the cauda equina.
MRI, magnetic resonance image; Gd-DTPA, gadolinium-diethylenetriaminepentaacetic acid; FDG, fluorodeoxyglucose; PET, positron emission tomography; sIL-2R, serum soluble interleukin 2 receptor; CSF, cerebrospinal fluid; F/U, duration of follow-up after onset; N/P, not performed; N/S, not stated; DLBCL, diffuse large B cell lymphoma; NK, natural killer; RT, radiation therapy; CT, chemotherapy; M, male; F, female; w, week(s); m, month(s); yr, year(s).
The authors declare no conflict of interest.