2023 Volume 261 Issue 4 Pages 283-289
2023 Volume 261 Issue 4 Pages 283-289
Diabetic foot ulcers are caused by nerve abnormalities and vascular lesions in the distal lower limbs of diabetic patients. However, the causes of diabetic foot ulcers are diverse and the treatment process is complex. Therefore, understanding the pathogenesis of diabetic foot ulcers through lncRNA and formulating effective means are the key to the cure of patients. Tissues were collected from 76 diabetic foot ulcer patients and 50 non-diabetic patients undergoing traumatic amputation. Human dermal fibroblasts (HDFs) were induced by high glucose to obtain diabetic foot ulcer cell model. The lncRNA SNHG16 (SNHG16) and miR-31-5p expression in tissues and cells was detected by real-time quantitative reverse transcription PCR (RT-qPCR). Cell Counting Kit-8 (CCK-8) and Transwell assays were used to evaluate the biological behavior of the cells, and the association between SNHG16 and miR-31-5p was explored by luciferase reporting assay. SNHG16 was distinctly expressed in diabetic foot ulcer tissue samples, while miR-31-5p was decreased. In vitro cell function assays confirmed that the proliferation level was inhibited in the constructed diabetic foot ulcer cell model (HG group), as was the migration and invasion ability. After transfection with silencing SNHG16, the biological behavior of the cells was promoted. Mechanistically, SNHG16 sponge miR-31-5p regulated disease progression. Recovery experiments revealed that miR-31-5p inhibitor counteracted the effect of silencing SNHG16 on cell viability. SNHG16 knockdown may regulate the biological function of cells by targeting miR-31-5p to promote wound healing and ameliorate the condition of diabetic foot ulcer patients.
Diabetic foot ulcer (DFU) is a chronic and complex foot complication caused by sensory and motor neuropathy in diabetic patients (Li et al. 2023). The worldwide incidence of DFU is about 6.3%, the mortality rate is 11%, and the lifetime probability of developing the disease can be as high as 34% (Kifelew et al. 2020; Hernandez-Guedes et al. 2023). Statistics indicate that DFU is the leading cause of non-traumatic lower limb amputation, in which more than half of DFU patients develop infections, and 20% of infected patients will require amputation, with a mortality rate of about 22% (Jiang et al. 2022; Kim et al. 2022). In particular, a variety of factors including age, smoking or vascular lesions, may induce the occurrence of DFU, but the pathogenesis has not been specifically explained, and the patients are prone to recurrent wound infection and poor treatment outcomes (Sawaya et al. 2020). Therefore, understanding the pathogenesis of DFU and finding reasonable treatment strategies are very momentous for both social medicine and individual patients.
Fibroblasts are adaptable, highly divided and easily cultured in vitro, which are involved in tissue repair and wound healing (Esteban-Vives et al. 2019). It has been proposed that fibroblasts accumulate in large numbers at the site of wound healing in patients, where they not only proliferate but also promote the formation of granulation tissue and blood vessels. In addition, growth factors produced by fibroblasts have the potential to accelerate and coordinate wound healing (Lu et al. 2021).
As a molecule of great concern, long non-coding RNAs (lncRNAs) have revealed key functions in the study of different diseases. Critically, a large number of effective lncRNAs have been reported in the discussion of DFU. SNHG16 was initially identified as an oncogene with a length of 2,435 nucleotides and located on chromosome 17q25.1 (Liu et al. 2021a; Xia et al. 2022). In the past literature evidence, SNHG16 was confirmed to be associated with diabetes-related diseases and may be a therapeutic target. For example, SNHG16 aggravated renal injury by regulating the ELF4A3/TLR4 axis and mediated the treatment of diabetic nephropathy in the latest study (Liu et al. 2023). More notably, lncRNAs participate in biological processes through sponge microRNAs (miRNAs) (Guo et al. 2022). SNHG16 targeted miR-124-3p to affect cell reproduction and promote the deterioration of colorectal cancer via mediating the miR-124-3p/MCP-1 axis (Chen et al. 2022). Zhang et al. (2023) also revealed that silencing SNHG16 sponge miR-31-5p mediates SFN to slow the progression of nasopharyngeal carcinoma. MiR-31-5p was found to have an active function in diabetic wound healing (Yan et al. 2022), which regulated the development of fibroblasts in the study of the mechanism of hypertrophic scar formation (Wang et al. 2017).
Therefore, by verifying the expression quantity of SNHG16 and miR-31-5p in DFU tissues, we conducted in vitro cell assays to focus on the effect of abnormal expression of SNHG16 on high glucose-induced fibroblasts. In addition, exploring the relationship between SNHG16 and miR-31-5p to realize the molecular mechanism of DFU, providing a new understanding for the treatment of DFU wound healing.
Ulcer margin tissues were provided by 76 DFU patients who were treated at The First Affiliated Hospital of Fujian Medical University, and 50 non-diabetic patients who underwent traumatic amputation surgery during the same period provided corresponding healthy tissues. The collected tissue specimens were simply washed and stored in cold storage. The diagnostic criteria of DFU patients were referred to the management guidelines of the International Working Group on the Diabetic Foot. The study was conducted in accordance with the requirements of the ethics committee of The First Affiliated Hospital of Fujian Medical University, and the consent of the patients and their guardians was obtained.
Cell processing and high glucose inductionHuman dermal fibroblasts (HDFs) were selected as control cells and purchased from Gibco (Grand Island, USA). The cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA; control group) or DMEM medium containing 5.5 mM D-glucose (HG group), and 10% fetal bovine serum (FBS) was also added to the above medium. During the culture at 37°C and 5% CO2, cells were passaged ten times before subsequent manipulation (Manivong et al. 2022).
Cell transfectionThe cells induced by high glucose were subjected to transfection experiments using the lipofectamine 3000 kit (Invitrogen, Carlsbad, CA, USA) (Fan et al. 2023). GenePharma (Shanghai, China) designed and provided silencing negative control (si-NC), silencing SNHG16 (si-SNHG16), miR-31-5p inhibitor NC and si-SNHG16-miR-31-5p inhibitor.
RT-qPCR assayTRIZOL reagent (Invitrogen)extracted the total RNA from tissues and cells. After measuring the RNA concentration and quality with a spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA), a reverse transcription kit manufactured by Takara (Kusatsu, Japan) was used for cDNA synthesis, a step which should ensure that no degradation of the RNA has occurred. The RT-qPCR reaction system included the cDNA template and the relevant reagents in SYBR Green PCR Master Mix Kit (Thermofisher Scientific, Waltham, MA, USA). The reaction was performed on an AB 7900 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA)with GAPDH and U6 as the reference genes (Zhang et al. 2021). RT-qPCR assays were performed for 3 min at 95°C, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. The relative quantity of SNHG16 and miR-31-5p were calculated according to the obtained Ct values. The primer sequence is shown as follows: SNHG16 forward: 5’-CAGTCAGCCT CAGTTTCCAA-3’, reverse: 5’-AGGCAGGGCTGTGCTGAT-3’; miR-31-5p forward: 5’-AGCTGGGAGGCAAGATGCT-3’, reverse:5’-TGGTGTCGTGGAGTCG-3’.
Cell biological behavior assaysAfter cells were transfected into 96-well plates (5 × 103 cells/well) and incubated for 24 h, then CCK-8 reagent was added to the cells at specific time points (0 h, 24 h, 48 h), and the absorbance value (450 nm) was determined for each well after continued culture for 4 h. Finally, the cell proliferation was evaluated according to the obtained data.
For the migration assay, the cells were first seeded in serum-free culture medium and placed in Transwell upper chambers. Next, the cells were incubated in Transwell chamber (3 × 105 cells/well) for 24 h at an incubator with 37℃ and 5% CO2. Finally, the cells that migrated into the complete medium of the lower chamber were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet, and 5 sites were randomly selected for microscopic counting. The same procedure was performed to measure cell invasion when Matrigel (Corning, Corning, NY, USA) was applied to the Transwell chamber.
Luciferase report assayThe wild-type or mut-type binding sites of SNHG16 and miR-31-5p were cloned into the pmirGLO vector to obtain WT-SNHG16 or MUT-SNHG16. Mimic NC or miR-31-5p mimic were co-transfected into cells with the participation of lipofectamine 3000 kit. Then the change of activity was measured by luciferase reporter gene assay system after 48 h transfection.
Statistical analysisData processing and statistical analysis were performed using GraphPad Prism 7 software and presented in the form of standard mean ± deviation (SD). The difference between two groups of data was compared by the Student’s t-test, and the difference between multiple groups was compared by one-way analysis of variance. Each trial set up three parallel groups and was performed in triplicate or more. In addition, the difference was identified as statistically significant when P < 0.05.
SNHG16 and miR-31-5p expression were evaluated after RNA isolation and extraction of the collected 76 DFU tissues and 50 healthy control tissues. Compared with the control group, SNHG16 was relatively increased in DFU (Fig. 1A), while miR-31-5p was relatively down-regulated in DFU (Fig. 1B).

Levels of SNHG16 and miR-31-5p in sample tissues.
(A) SNHG16 was up-regulated in diabetic foot ulcer (DFU) tissues. (B) MiR-31-5p was low expressed in DFU tissues. ***P < 0.001, in comparison with the control group.
Based on the efficacy of fibroblasts in the treatment of diabetes and wound healing, HDFs were used as the in vitro study object and as the control group, and then DFU cell model was obtained by high glucose induction, which was named HG group. Silencing SNHG16 (si-SNHG16) was transfected into DFU cell model to construct HG-si-SNHG16. Fig. 2A illustrated the transfection results, in which SNHG16 level was enhanced in cells of HG group, while it was obviously decreased in HG-si-SNHG16 group cells. Based on CCK-8 assay, we found that the OD value (450 nm) of HG group cells decreased, but cell proliferation was more active after transfection with si-SNHG16 in Fig. 2B. Transwell assay confirmed that the behavior of cells in HG group was inhibited compared with the control HDFs group, and knockdown of SNHG16 promoted cell migration (Fig. 2C) and invasion ability (Fig. 2D). According to the above, we predicted that silencing SNHG16 might facilitate the biological behavior of DFU cells and wound healing.

The regulation of SNHG16 knockdown on cell biological behavior.
(A) Transfection efficiency of silencing SNHG16. (B) The level of change in cell reproduction. (C, D) Knockdown of SNHG16 promoted cell migration and invasion ability. Data are shown as mean ± SD. *P < 0.05, ***P < 0.001, in comparison with the control group; #P < 0.05, ##P < 0.01, in comparison with the high glucose (HG) group.
In Fig. 3A, the predicted binding sites of SNHG16 and miR-31-5p were found. And in Fig. 3B, the luciferase activity was significantly down-regulated after co-transfection of WT-SNHG16 and miR-31-5p mimic, while the activity was unchanged after transfection of MUT-SNHG16. Further analysis by RT-qPCR demonstrated that miR-31-5p level was decreased in HG group, and transfection of si-SNHG16 caused an increase in miR-31-5p level (Fig. 3C). That is, SNHG16 directly sponges and negatively regulates miR-31-5p.

MiR-31-5p is the downstream target of SNHG16.
(A) SNHG16 and miR-31-5p had linking sites. (B) Results of luciferase activity assay. (C) miR-31-5p was elevated after transfection with si-SNHG16. Data are shown as mean ± SD. *P < 0.05, ***P < 0.001, in comparison with the control group; #P < 0.05, in comparison with the HG group.
To clarify the molecular mechanism of SNHG16 targeting miR-31-5p in DFU, we transfected si-SNHG16-miR-31-5p inhibitor into DFU cell models, and HG-si-SNHG16-miR-31-5p inhibitor group was constructed. Fig. 4A described the transfection results of the cells, where miR-31-5p was markedly decreased after transfection with si-SNHG16-miR-31-5p inhibitor, compared with HG-si-SNHG16 group cells. According to Fig. 4B, SNHG16 knockdown improved the growth level of DFU cell model, while transfection with si-SNHG16-miR-31-5p inhibitor restored the inhibition of fibroblasts induced by high sugar. Similarly in Fig. 4C, D, si-SNHG16-miR-31-5p inhibitor reversed the promotion effect of si-SNHG16 on DFU cell migration and invasion.
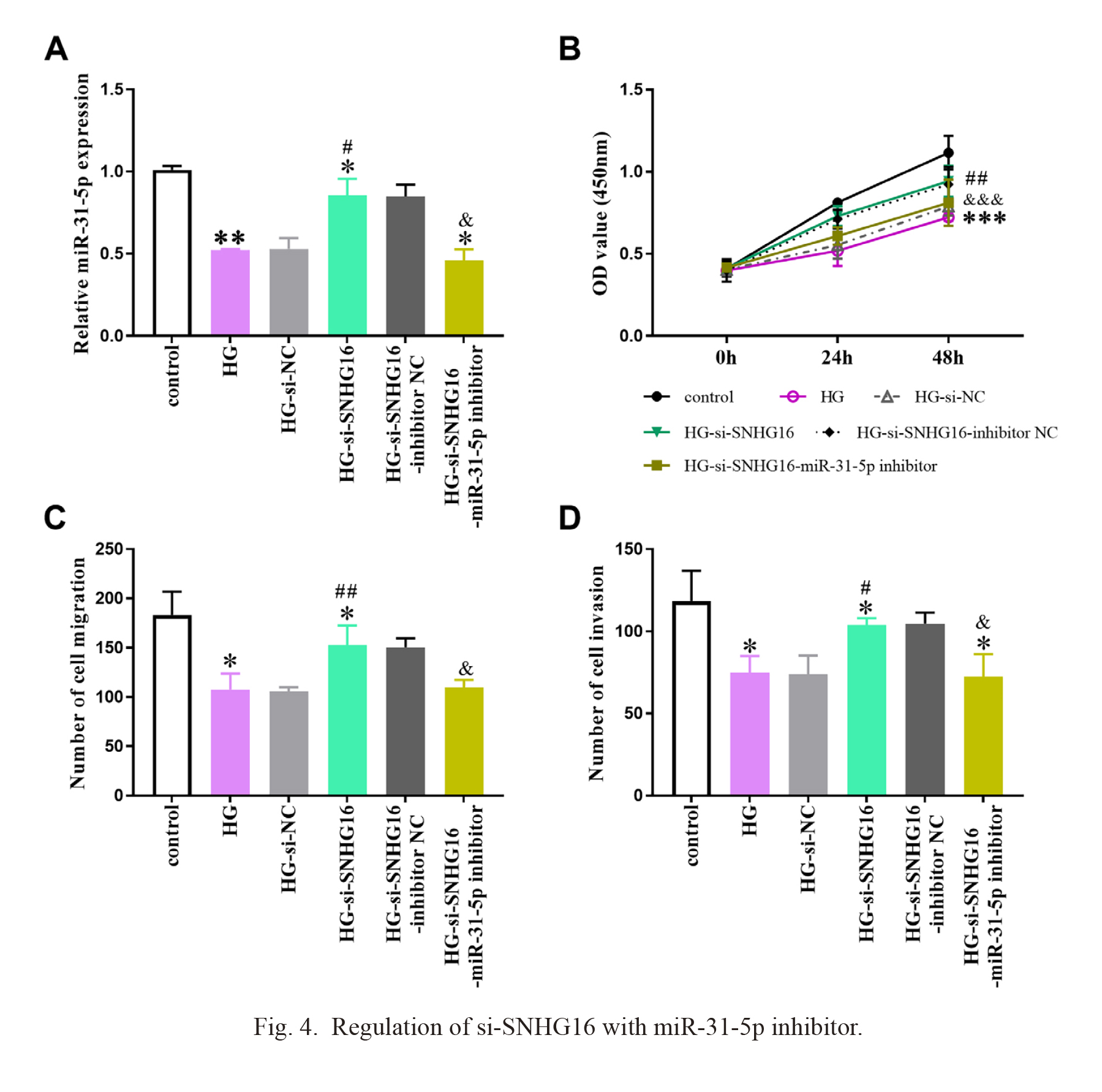
Regulation of si-SNHG16 with miR-31-5p inhibitor.
(A) Expression of miR-31-5p after cell transfection. (B-D) After transfection with si-SNHG16-miR-31-5p inhibitor, the promoting effect of si-SNHG16 on cell activity was restored. HG, high glucose. Data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, in comparison with the control group; #P < 0.05, ##P < 0.01, in comparison with the HG group; &P < 0.05, &&&P < 0.001, in comparison with the HG-si-SNHG16 group.
Diabetes can seriously threaten the health and life of patients, causing vascular and neurological lesions, and lead to a variety of complications (Tran et al. 2020). At the same time, the high glucose environment in the patient’s body can make it difficult to heal infections or wounds, eventually leading to amputation or even death. The immunity of DFU patients is poor, easy to be affected by pathogenic factors, blood supply ability is impaired, and mild wounds are prone to ulcer formation (Lawrence et al. 2022). Currently, the treatment of DFU is based on the severity of the ulcer, but the results are always unsatisfactory, and many patients are still at risk of amputation. Therefore, the exploitation of new prevention and treatment methods is still urgent.
In our study, SNHG16 was positively expressed in DFU tissue specimens, while miR-31-5p level was down-regulated. In previous studies, SNHG16 as a promising tumor-related RNA, was confirmed to function in different cancers (Chen et al. 2020; Gong et al. 2020; Ni et al. 2020). Furthermore, SNHG16 was highly expressed in human retinal microvascular endothelial cells (HRMECs) treated with high glucose and provided a therapeutic possibility for diabetic retinopathy by promoting cell dysfunction (Cai et al. 2021). Serum SNHG16 was up-regulated in patients with diabetic nephropathy, and its sponged miR-106a/KLF9 to aggravate podocyte injury (He and Zeng 2020). These all imply that SNHG16 is highly expressed in diabetes-related diseases. We also recognize that high glucose conditions and various biological factors affect the rate of wound healing in diabetic patients (Liu et al. 2021b). Based on the value of fibroblasts in wound healing, HDFs was selected as the study object and cells were induced by high glucose medium to obtain DFU cell models. In vitro assays showed that, unlike normal fibroblasts, the biological behaviors and activities of the cells were obviously suppressed by high glucose, while recovered after transfection with si-SNHG16. It is speculated that low level of SNHG16 may contribute to the reproduction of fibroblasts and have the significance of cell repair. Similarly, aberrant expression of H19 (Li et al. 2020) and CASC2 (He et al. 2022) was demonstrated to repair DFU wounds in mice when regulating the proliferation of fibroblasts and inhibiting their apoptosis.
In the molecular mechanism of DFU, miR-31-5p was identified as a target of SNHG16 by luciferase reporter assay, and silencing SNHG16 up-regulated the content. MiR-31-5p was decreased in the serum of diabetic female patients prone to fracture (Heilmeier et al. 2022) and in the wounds of diabetic mice (Yan et al. 2022), which was consistent with our detection results. In addition, miR-31-5p is not only involved in the pathogenesis of systemic lupus erythematosus (Li et al. 2022) and prostate cancer (Zhao et al. 2020), but more relevantly, miR-31-5p has also been described as the target of Hsa_circ_0037128 to further cause podocyte injury in diabetic nephropathy, which was proposed by Fang et al. (2022). To understand the regulatory mechanism between SNHG16 and miR-31-5p, we co-transfected si-SNHG16-miR-31-5p inhibitor into DFU cell models. Down-regulation of miR-31-5p reversed the enhanced effects of SNHG16 knockdown on cell proliferation, migration and invasion. Therefore, we realized that SNHG16 in DFU mediates the progression of DFU by directly targeting miR-31-5p, and SNHG16 may be a potential target for prevention and treatment of DFU. Unfortunately, our study did not provide the reference mouse assays, which will be the focus of our future research. Meanwhile, the study of SNHG16 as a competing RNA regulating miR-31-5p target gene may also help us to better understand the regulatory mechanism of SNHG16 in DFU.
In summary, in order to develop more effective therapeutic approaches for DFU and relieve the pressure on patients and medical stress, the present study confirmed for the first time that SNHG16 regulated the DFU progression through mediating miR-31-5p. SNHG16 was up-regulated and miR-31-5p level was decreased in DFU. Silencing SNHG16 may negatively regulate miR-31-5p and improve the biological behavior of DFU cell model, thereby suppressing the process of DFU and promoting wound healing.
This study was funded by Startup Fund for scientific research, Fujian Medical University (Grant number: 2020QH1045).
The authors declare no conflict of interest.