2024 Volume 262 Issue 4 Pages 263-268
2024 Volume 262 Issue 4 Pages 263-268
Anamorelin (ANAM) is a novel ghrelin receptor agonist for the treatment of cancer cachexia. In clinical trials of ANAM, glucose metabolism disorders as adverse effects were relatively frequent, however, when and how they occur remains unclear. Moreover, the safety in patients with pancreatic cancer and/or diabetes has not been clarified because most previous studies focused on patients with non-small cell lung cancer and had excluded patients with poorly controlled diabetes. Herein, a 66-year-old man with advanced pancreatic cancer and diabetes was administered ANAM, and acute hyperglycemia was developed and could be monitored by the self-monitoring of blood glucose (SMBG). Increasing the insulin dose failed to control hyperglycemia adequately, but the hyperglycemia ameliorated quickly after ANAM discontinuation. The continuous glucose monitoring (CGM) revealed that the sensor glucose levels had remained in the high range throughout the day during ANAM administration despite using 1.5 times more insulin. Our report is one of the few that describe the details of ANAM-induced hyperglycemia and provides important information for the safe and effective use of ANAM.
Cancer cachexia is defined as a multifactional syndrome characterized by an ongoing loss of skeletal mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support in a consensus report of European Palliative Care Research Collaborative, and leads to progressive functional impairment (Fearon et al. 2011). Cancer cachexia can be associated with poor quality of life (QOL), tolerance and response to anticancer therapy, and survival (Zhang and Garcia 2015; Nishikawa et al. 2021). However, there are few pharmacological therapies with efficacy as the standard of care due to the balance between efficacy and adverse events. Corticosteroids increase appetite, but their effects are short-lived, and progestins increase appetite and body weight but have the risk of serious adverse effects (Arends et al. 2021).
Ghrelin is a peptide endogenously secreted mainly from the stomach, and is an endogenous ligand of the growth hormone secretagogue receptor (GHS-R) (Kojima et al. 1999). The ghrelin signal regulates energy metabolism through a variety of actions, including promoting growth hormone (GH)/insulin-like growth factor (IGF)-I secretion, increasing appetite, and promoting lipogenesis and gastric emptying (Müller et al. 2015). Ghrelin administration improved food consumption in both animal models and human studies (DeBoer et al. 2007; Neary et al. 2004; Hiura et al. 2012). In relation to cancer cachexia, elevated ghrelin levels are often observed in patients with cancer cachexia, presumably as a physiological response to the decrease in body weight and food intake (Argilés and Stemmler 2013; Shimizu et al. 2003). In addition, decreasing endogenous ghrelin levels in cancer cachexia predict both lean and fatty tissue loss (Fouladiun et al. 2005). From these findings, ghrelin has been considered for application in the treatment of cancer cachexia. Ghrelin also has anti-inflammatory effects and was found to ameliorate endotoxin-induced anorexia in a murine model of cancer cachexia (Chen et al. 2015). Because inflammatory cytokines such as tumor necrosis factor-alpha, interleukin-1-beta, and interleukin-6 are involved in the pathogenesis of cancer cachexia by modulating gene-expression profiles in adipose tissue and muscle cells and by inducing tissue catabolism (Akamizu and Kangawa 2010; Fearon et al. 2012; Baazim et al. 2022), ghrelin may be expected to improve the immunological pathophysiology of cancer cachexia. However, the clinical use of ghrelin itself is problematic because of its short half-life and the need for intravenous administration (Zhang and Garcia 2015).
Anamorelin (ANAM) is an orally active, selective ghrelin receptor agonist that is expected to improve cancer cachexia by increasing appetite, body weight, and lean body mass (Garcia et al. 2013; Pietra et al. 2014; Zhang and Garcia 2015). Clinical trials have shown that ANAM improved anorexia symptoms and increased the lean body mass in cancer cachexia patients with non-small cell lung cancer (Takayama et al. 2016; Temel et al. 2016; Currow et al. 2017; Katakami et al. 2018) or advanced gastrointestinal (gastric, pancreatic, and colorectal) cancer (Hamauchi et al. 2019; Naito et al. 2022). In these clinical studies of ANAM, the incidence of glucose metabolism disorders was about 10% at most, and that of grade 3 or higher was very low (Takayama et al. 2016; Temel et al. 2016; Currow et al. 2017; Katakami et al. 2018; Hamauchi et al. 2019). On the other hand, when and how the glucose metabolism disorders occurred has not yet been discussed. In addition, the efficacy and safety of ANAM in patients with pancreatic cancer and/or diabetes have not been clarified because many of these trials had been conducted in patients with non-small cell lung cancer and had excluded patients with poorly controlled diabetes.
We herein report a case of a patient with advanced pancreatic cancer and diabetes who developed hyperglycemia immediately after initiation of ANAM, and the clinical course was monitored by the self-monitoring of blood glucose (SMBG) and the continuous glucose monitoring (CGM). The acute hyperglycemia improved rapidly after discontinuation of ANAM, suggesting that it was induced by the pharmacological action of ANAM.
A 66-year-old man experienced fatigue and body weight loss of about 9 kg in 6 months. He was on medical treatment for hypertension and insomnia by his family physician, and he had been identified as having impaired glucose tolerance for several years, but had not been diagnosed with diabetes. Abdominal ultrasonography revealed a pancreatic mass, and he was referred to our hospital for close examination. Since he had developed obstructive jaundice, he was admitted to our hospital, and a bile duct stent was implanted. His HbA1c was 7.7% at the admission. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a pancreatic head mass in contact with the superior mesenteric artery (SMA) and pancreatic duct dilation. Adenocarcinoma was detected by brush cytology from the pancreatic duct, and he was diagnosed with unresectable pancreatic head cancer with SMA invasion. His oncologist planned chemotherapy with gemcitabine and nab-paclitaxel (GnP), and the chemotherapy was initiated after discharge.
He was referred to our department for diabetes care after the initial dose of the first cycle of GnP therapy. At the first visit to our department, his body mass index was 20.1 kg/m2 (weight 51.4 kg, height 160 cm), his fasting plasma glucose was 215 mg/dL, his HbA1c was 9.3%, and his endogenous insulin secretion was preserved (fasting serum CPR 3.50 ng/mL, negative for glutamic acid decarboxylase antibody) (Table 1). He was diagnosed with diabetes and was started multiple daily injection using insulin aspart and insulin glargine. After receiving 6.6 mg of dexamethasone on the days of chemotherapy, his blood glucose level temporarily elevated, so he was instructed to increase insulin aspart for 2-3 days after dexamethasone administration. Fig. 1 shows the time course of the SMBG for nine weeks from the final dose of the first cycle to the end of the third cycle of GnP therapy. After the final dose of the first cycle, the chemotherapy-induced hyperglycemia could be corrected by insulin dose adjustment.
Although he did not have symptoms of chemotherapy-induced nausea or anorexia, the start of ANAM was planned because of significant weight loss prior to the start of chemotherapy. ANAM was initiated after the start of the second cycle, and his blood glucose levels elevated acutely (Fig. 1). At the next visit after the initiation of ANAM, he disclosed that he felt hungrier and had an increased appetite but did not consume excessive amounts of snacks or soft drinks. His insulin dose was increased weekly, and the total daily dose of insulin (TDD) reached more than doubled from before ANAM started, but his hyperglycemia was not fully controlled. During the period when he stopped taking ANAM for a few days at his own decision, his blood glucose levels tended to decrease (Fig. 1). Although the effect of ANAM on weight gain was obtained, ANAM was discontinued at the end of the second cycle at the patient’s request due to excessive hunger and persistent hyperglycemia. After the discontinuation of ANAM, his blood glucose levels quickly dropped and his insulin dose had to be decreased. Finally, his blood glucose levels were maintained within an acceptable range without hypoglycemia by about half the TDD during ANAM administration (Fig. 1). Between the first course to the third course, there were no differences in other conditions that could affect blood glucose levels, such as changes in chemotherapy regimen or development of infections. Fig. 2 shows his blood glucose variability through CGM for six days before and after the discontinuation of ANAM, respectively (the period marked “CGM” in Fig. 1). Other conditions were the same, including dexamethasone administration, but his sensor glucose levels while taking ANAM had remained in the high range throughout the day, including at night, even though he had used approximately 1.5 times more insulin aspart and 1.5 times more insulin glargine.
The authors have obtained written informed consent from the patient for this manuscript.

Laboratory data at the first visit to our department.
WBC, count of white blood cells; RBC, count of red blood cells; Hb, hemoglobin; PLT, count of platelets; T-Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; γ-GTP, gamma glutamyl transferase; BUN, blood ureanitrogen; Cre, crea tinine; UA, uric acid; TP, total protein; Alb, albumin; TG, triglyceride; HDL-C,high density lipopr otein cholesterol; LDL-C, low density lipoprotein cholesterol; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; CRP, C-reactive protein; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; GA, glycoalbumin;CPR, C-peptide immunoreactivity; GADA, glutamic acid decarboxylase antibody.
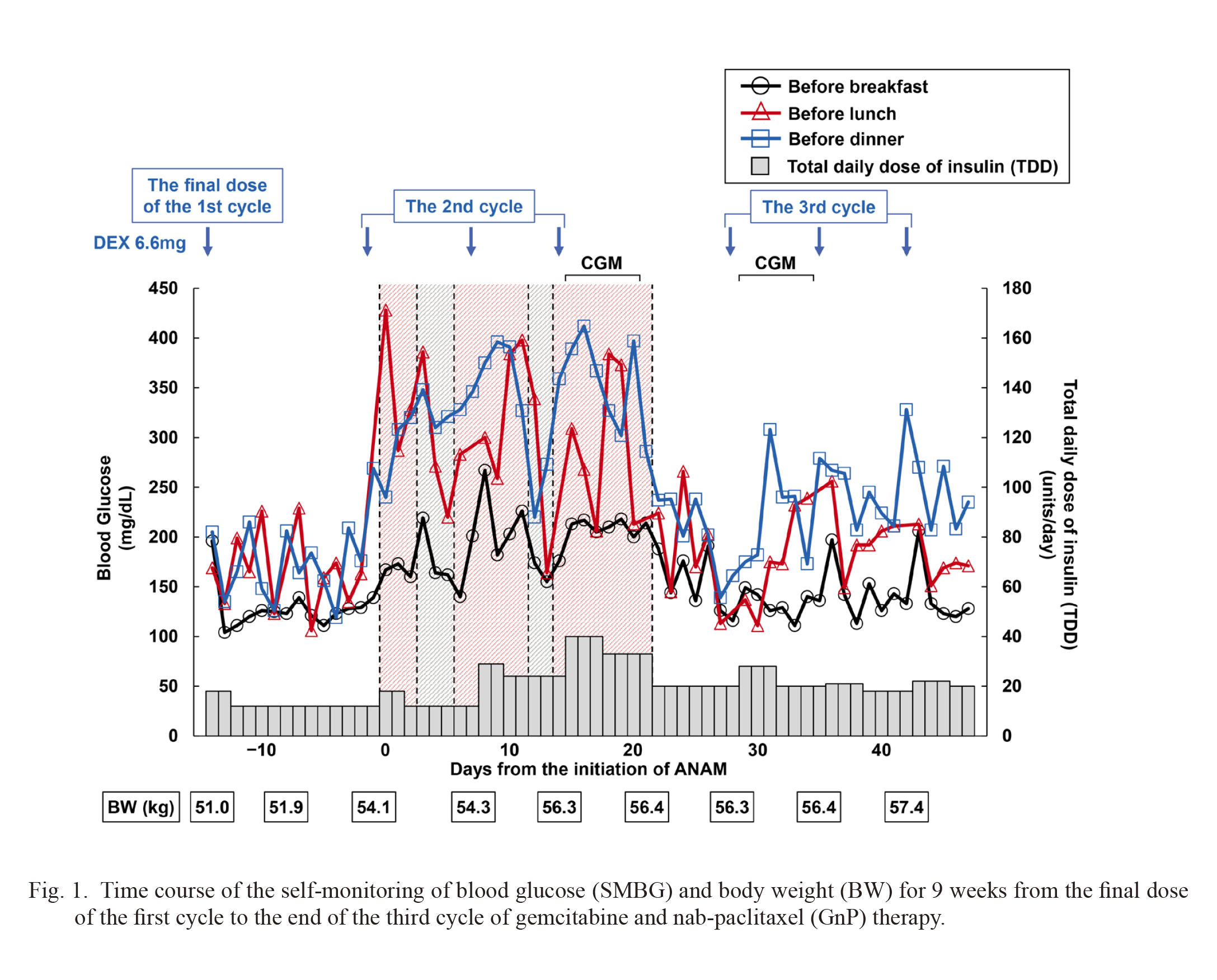
Time course of the self-monitoring of blood glucose (SMBG) and body weight (BW) for 9 weeks from the final dose of the first cycle to the end of the third cycle of gemcitabine and nab-paclitaxel (GnP) therapy.
Black circles, red triangles, and blue squares show blood glucose levels before breakfast, lunch, and dinner, respectively. The bar graphs show the total daily dose of insulin (TDD). Blue arrows indicate 6.6 mg of dexamethasone (DEX) administration. Red shaded areas indicate the duration of anamorelin (ANAM) administration. Gray-shaded areas indicate the duration of stopping ANAM by himself. “CGM” indicates the period of continuous glucose monitoring (CGM).

Continuous glucose monitoring (CGM) data for 6 days on anamorelin (ANAM) (red) or not on ANAM (blue), and mean insulin dose during that period.
Solid lines indicate the median of sensor glucose levels and dotted lines show its 10 and 90 percentiles.
In the present case, intensive insulin therapy was started after he was diagnosed with diabetes, and the increase in blood glucose levels caused by dexamethasone was improved once by adjusting the insulin dose. However, after starting ANAM administration, the same dose of insulin did not control the hyperglycemia at all, not even with approximately more than twice the amount of insulin. His hyperglycemia was severe, and he could have developed ketosis or ketoacidosis if insulin had not been used. Increased food intake might have contributed to the increase in blood glucose levels because of his weight gain after starting ANAM, however, the time course of his blood glucose levels, which elevated rapidly after initiation of ANAM and improved quickly after the discontinuation of ANAM, strongly suggested that the pharmacological effects of ANAM were the primary cause of the hyperglycemia.
Three crucial features were observed in our case. First, hyperglycemia occurred shortly after the initiation of ANAM. In pancreatic β cells, stimulation of GHS-R suppresses insulin secretion through the decreased cAMP production, activation of voltage-dependent potassium channels, attenuation of cell membrane excitability, and inhibition of Ca2+ influx (Yada et al. 2014). In addition, ghrelin directly enhances glucagon secretion from pancreatic α-cells via GHS-R (Chuang et al. 2011; Heppner and Tong 2014). The effects of ghrelin signaling on insulin sensitivity are complicated, but acute ghrelin effects induce insulin resistance in peripheral tissues (Vestergaard et al. 2008; Heppner and Tong 2014). Therefore, ANAM, a GHS-R agonist, may cause acute hyperglycemia by suppressing insulin secretion, enhancing glucagon secretion, and inducing peripheral insulin resistance. Second, our patient had advanced pancreatic cancer. Many previous studies of ANAM had included patients with non-small cell lung cancer (Takayama et al. 2016; Temel et al. 2016; Currow et al. 2017; Katakami et al. 2018). Two studies included patients with gastrointestinal cancer (Hamauchi et al. 2019; Naito et al. 2022), but only a few patients with pancreatic cancer. In a recent single-center retrospective study of only patients with advanced pancreatic cancer, the frequency of hyperglycemia leading to discontinuation of treatment with ANAM was higher than in previous studies (Takeda et al. 2023), and this is the only study of exclusively pancreatic cancer patients. At this time, the evidence for the efficacy and safety of ANAM in patients with pancreatic cancer is insufficient. Since patients with advanced pancreatic cancer have a higher prevalence of diabetes than those with other types of cancer (Aggarwal et al. 2013; Hart et al. 2016), they may be more prone to hyperglycemia caused by ANAM. Finally, our case had developed diabetes prior to the initiation of ANAM. Previous studies excluded uncontrolled diabetes (Takayama et al. 2016; Katakami et al. 2018; Hamauchi et al. 2019), and there have been no studies of ANAM exclusively in patients with diabetes. Therefore, the safety of ANAM in patients with diabetes is entirely unknown. Because patients with diabetes have relative or absolute insulin deficiency, they may need to be aware of the possibility of hyperglycemia due to ANAM, regardless of the type of cancer.
Since insulin is an anabolic hormone (Dimitriadis et al. 2011), in patients with pancreatic cancer and/or diabetes who have a background of impaired β cell function, poor insulin action via the GHS-R may not only induce an elevation of the blood glucose levels but may also counteract the anabolic effect expected with ANAM. In our case, he had lost body weight probably due to hypercatabolism and insulin insufficiency caused by the pancreatic cancer, and had gained body weight with the initiation of treatment for diabetes, prior to ANAM administration. After starting ANAM administration, his body weight increased although his blood glucose levels were not adequately controlled, which might have resulted from the effects that the insulin deficiency due to the decreased insulin secretion via the GHS-R was resolved to some extent by increasing exogenous insulin, and that the ghrelin-like actions of ANAM promoted protein anabolism, lipogenesis, and increasing appetite. After the discontinuation of ANAM, his appetite decreased to the similar level as before ANAM administration but he did not lose weight again, presumably due to the improvement of cachexia by short-term ANAM and the continuation of treatment for diabetes. When initiating ANAM in patients with pancreatic cancer and/or diabetes, it may be necessary to consider increasing the insulin dosage or adding insulin secretagogues to prevent hyperglycemia and to achieve the sufficient benefit of ANAM.
In our case, we considered that insulin resistance and β-cell dysfunction derived from advanced pancreatic cancer, in addition to his original relative insulin deficiency, led to overt diabetes. Furthermore, ANAM suppressed insulin secretion, enhanced glucagon secretion, and induced insulin resistance in peripheral tissues, resulting in markedly acute hyperglycemia. However, we did not evaluate changes in insulin, glucagon, GH, or IGF-I secretion, and we could not obtain evidence on how these hormone secretions fluctuated. If frequent hormone measurements or hormone secretion tests had been performed during the ANAM administration, it might have been possible to discuss the effects of ANAM on the secretion of these hormones.
ANAM is expected to be a breakthrough treatment for cancer cachexia via the activation of ghrelin signaling. However, it is not yet clear which patients should be aware of hyperglycemia and how to manage it when initiating ANAM because of limited real-world clinical findings. Very recently, it was reported that pancreatic cancer and a history of diabetes were associated with adverse effects of ANAM on glucose metabolism, with a median onset of adverse effects being 17 days after ANAM treatment (Ohta et al. 2023). On the other hand, the course of our case indicates that hyperglycemia can develop very quickly. Further research is needed for the safe and effective use of ANAM, and that will help patients suffering from cancer cachexia.
We would like to thank Mr. Brent K. Bell (Tohoku University, Japan) for English editing of the manuscript. We also thank all the medical staff who cared for the patients.
J.Y. and M.U. contributed to relevant discussions and wrote, reviewed and edited the manuscript. H.O., Y.K., and Y.K. contributed to the overall relevant discussions and reviewed the manuscript.
The authors declare no conflict of interest.