2025 Volume 266 Issue 1 Pages 81-85
2025 Volume 266 Issue 1 Pages 81-85
This study aimed to investigate the correlation between plasma brain natriuretic peptide levels and left ventricular shortening fraction in Fukuyama congenital muscular dystrophy. The correlation between brain natriuretic peptide levels and left ventricular shortening fraction in patients with genetically confirmed Fukuyama congenital muscular dystrophy was retrospectively investigated. The patients at Tokyo Women’s Medical University were followed-up from October 2015 to October 2020. The brain natriuretic peptide level was measured using a specific immunoradiometric assay kit. Left ventricular shortening fraction was defined as a degree of left ventricular systolic function within 3 months before and after brain natriuretic peptide measurements. In addition to left ventricular shortening fraction, the ratio of early filling to early diastolic and the maximum velocity of tricuspid regurgitation, which are measures of diastolic function, were investigated. In total, 27 sets of brain natriuretic peptide levels and echocardiography results were obtained from 16 patients with Fukuyama congenital muscular dystrophy. Excellent correlations were found between the brain natriuretic peptide levels and left ventricular shortening fraction (R2 = 0.8755). Patients with a left ventricular shortening fraction of > 20% had low brain natriuretic peptide levels. However, the brain natriuretic peptide values of patients with a left ventricular shortening fraction of < 20% were > 40 pg/mL. In Fukuyama congenital muscular dystrophy, the brain natriuretic peptide level was exponentially correlated with left ventricular shortening fraction. However, it did not elevate until the advanced stage of left ventricular systolic dysfunction.
Fukuyama congenital muscular dystrophy (FCMD) is the second most common childhood-onset muscular dystrophy in Japan. It is an autosomal recessive disorder caused by FKTN gene mutations. Moreover, it is characterized by cardiomyopathy, skeletal muscle wasting, and brain malformation, resulting in heart failure, motor impairment, mental retardation, and seizures (Fukuyama et al. 1981; Kobayashi et al. 1998; Nakanishi et al. 2006; Yamamoto et al. 2017).
Brain natriuretic peptide (BNP) is a common biomarker of heart failure. It is synthesized as a proBNP, and this process is mainly stimulated by myocardial wall stress (Weber and Hamm 2006). Upon release into the circulation, proBNP is cleaved in equal proportions into the biologically active 32-amino acid C-terminal fragment (i.e., BNP) and the inactive N-terminal fragment (NT-proBNP), which are released into the blood and are likely to increase in patients with reduced renal dysfunction (Weber and Hamm 2006). Vasan et al. (2002) showed that BNP was a useful marker for detecting moderate to severe left ventricular (LV) systolic dysfunction in a healthy population. However, it was not useful for identifying mild LV systolic dysfunction. Similarly, Mori et al. (2002) reported that the BNP levels of patients with Duchenne muscular dystrophy (DMD) who presented with an LV systolic function (LVSF) of > 15% was low. However, the BNP levels of patients with an LVSF of < 15% exponentially increased. Yamamoto et al. (2017) found that the correlation between LV ejection fraction and BNP levels was not significant in FCMD. However, the study did not include patients with a relatively severe LV dysfunction. We have often experienced patients with FCMD manifesting reduced LVSF despite mild BNP elevation. The Japanese Circulation Society and The Japanese Heart Failure Society Joint Working Group stated that BNP > 40 pg/mL as “unlikely to have heart failure, but should be followed-up if possible” in common heart failure (Tsutsui et al. 2019). However, we have often experienced that BNP > 40 pg/mL is manifested enough reduced LVSF in patients with FCMD. The current study aimed to determine the correlation between BNP levels and severe LVSF in patients with FCMD to reminder the above point.
We retrospectively investigated patients with genetically confirmed FCMD who were followed-up at the Tokyo Women’s Medical University from October 2015 to October 2020. Genetic diagnosis was confirmed using polymorphic microsatellite markers (i.e., D9S2105-D9S2170-D9S2171-D9S2107) or the polymerase chain reaction method (Saito et al. 2000).
Immediately after blood sampling, the BNP level was measured using a specific immunoradiometric assay kit (LUMIPULSE Presto® BNP assay kit, FUJIREBIO, Tokyo, Japan). LVSF was defined as a degree of LV systolic function: LVSF = (left ventricular dimension at end-diastolic – left ventricular dimension at end-systolic)/left ventricular dimension at end-diastolic. Within 3 months before and after the BNP measurements, to assess diastolic function, echocardiography (Philips EPIQ 7, Philips Ultrasound, Bothell, WA) was performed to measure the ratio of peak early diastolic to mitral annulus velocity (e’) to peak early diastolic LV inflow velocity (E) and the maximum velocity of tricuspid regurgitation (TR) (Nagueh et al. 2016).
Nonlinear regression analysis was performed to evaluate the correlation between the BNP levels and LVSF. Linear regression analysis was performed to evaluate the correlation between BNP levels or LVSF and age. A p value of < 0.05 indicated statistically significant difference. GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA) was used for statistical analysis.
Ethical approvalThe ethics committee of Tokyo Women’s Medical University approved this study (approval no. 2020-067). The study conducted was in accordance with the ethical standards formulated in the Helsinki Declaration of 1964. A written informed consent was obtained after providing the parents of the patients with a written and verbal information about the objectives, methodology, and expected benefits of the study.
This study included 16 patients with FCMD (11 boys and 5 girls). Ten patients were homozygous for the Japanese founder mutation (homozygotes). Meanwhile, six patients had a compound heterozygous mutation, which was a combination of the Japanese founder mutation and a missense mutation (heterozygotes). The mean age at recruitment was 10 (range: 4-17) years. In terms of motor functions, the patients achieved developmental milestones up to sitting up. In total, 27 sets of BNP values and echocardiography results were obtained from the patients. The median BNP levels of the patients was 5.5 (range: 4.0-68.8) pg/mL. The LVSF ranged from 0.13 to 0.40. The median E/e’ and the maximum velocity of TR were 8.95 (range: 5.5-12.9) and 2.0 (range: 1.8-2.6) m/s, respectively. The median body mass index was 13.4 (range: 9.0-16.5) kg/m2, and the median serum cystatin C level was 0.70 (range: 0.42-0.88) mg/L. None of the patients presented with renal failure or acute illness. Table 1 shows the baseline characteristics of the patients.
The BNP levels of patients with LVSFs of > 20% were < 40 pg/mL. However, the BNP levels of patients with LVSFs of < 20% were > 40 pg/mL. The BNP levels was correlated with the LVSF based on the nonlinear regression model (R2 = 0.8755), but not according to the simple linear regression model (R2 = 0.5778) (Fig. 1). No correlation was observed between the E/e’ and the maximum velocity of TR (Fig. 2). LVSF reduced with age (R2 = 0.5544), but BNP levels showed poor correlation with age (R2 = 0.2176).
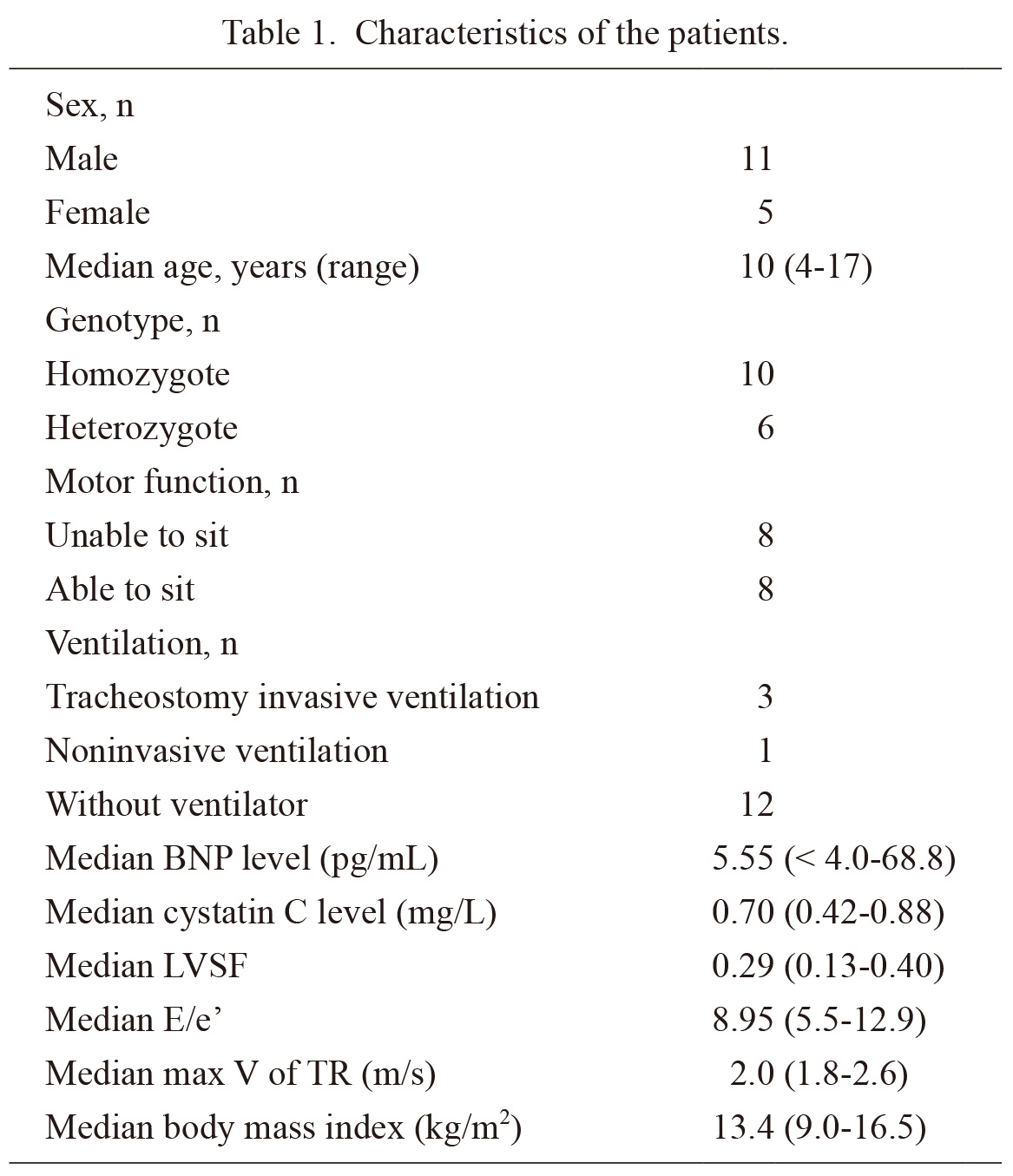
Characteristics of the patients.
BNP, brain natriuretic peptide; LVSF, left ventricular shortening fraction; E, peak early diastolic–left ventricular inflow velocity; e’, peak early diastolic to mitral annulus velocity; Max V of TR, maximum velocity of tricuspid regurgitation.

Correlation between the BNP levels and LVSF.
(A) The BNP level was significantly correlated with LVSF. R2 = 0.8755. Y = (Y0 − plateau) × exp (−K × X) + plateau. Y0 = 2,093, plateau = 4.485 ± 2.558, K = 25.67 ± 8.494. (B) The BNP level was moderately correlated with LVSF. R2 = 0.5778. Y = −237.1X + 81.21. BNP, brain natriuretic peptide; LVSF, left ventricular systolic dysfunction.

Correlation between the BNP level and diastolic function.
BNP was not correlated with the (A) E/e’ and (B) the max V of TR. BNP, brain natriuretic peptide; E, early filling; e’, early diastolic; Max V of TR, maximum velocity of tricuspid regurgitation.
This study investigated the correlation between BNP levels and LVSF in patients with FCMD. Results showed that an excellent correlation was found between the BNP levels and LVSF, which could directly reflect LVSF. The BNP levels of patients with FCMD exponentially elevated with LV dysfunction progression. Patients with an LVSF of > 20% had low s levels. However, the BNP levels of patients with an LVSF of < 20% were > 40 pg/mL. BNP > 40 pg/mL might be a cutoff level in heart failure complicated with FCMD. These findings were similar to those of a previous study on patients with DMD (Mori et al. 2002). However, they were different from those of a previous study on FCMD (Yamamoto et al. 2017). Myocardial wall stress is the main stimulus for increased BNP synthesis and secretion (Weber and Hamm 2006). Patients with DMD can perform minimal activities. However, their myocardial wall might not receive severe stress. The BNP levels are significantly high in the ischemic area of the endocardium in various conditions such as myocardial infarction (Morita et al. 1993). In patients with dystrophinopathies, fibrosis could be found in the epicardial area (Verhaert et al. 2011). Hence, the BNP levels might not elevate substantially in the early stage in cardiomyopathies such as FCMD. This is because it is present in the subepicardial area, not in the endocardium, as in FCMD. Compared with the current cohort who had developmental milestones up to sitting up, the cohort in a previous study might have a milder cardiac function. The BNP levels do not elevate in the terminal stage of heart failure. The BNP cutoff values for detecting heart failure varied. However, two studies have reported that a BNP level of > 100 pg/mL had a good sensitivity and specificity for heart failure (Maisel et al. 2002; Wieczorek et al. 2002). Based on our results, the BNP cutoff value for detecting LV systolic dysfunction in patients with FCMD should be lower than that in patients with other common conditions causing heart failure. Hence, physicians should consider echocardiogram in patients with a BNP level of > 40 pg/mL.
However, there was no correlation between the BNP levels and diastolic function parameters. The American Society of Echocardiography and the European Association of Cardiovascular Imaging recommended the cutoff values of < 14 for E/e’ and > 2.8 m/s for the maximum velocity of TR as diagnostic markers of diastolic dysfunction (Nagueh et al. 2016). E wave indicates early diastolic wave, which can reflect flow from the left atrium to the LV by LV relaxation. e’ is a peak diastolic mitral annulus velocity measured on tissue Doppler echocardiography, which can also reflect LV relaxation. E/e’ can be linearly correlated with left atrium pressure; therefore, E/e’ can be a significant factor in LV diastolic function (Ohte et al. 2022). Patients with diastolic dysfunction could have increased BNP levels (Kitzman et al. 2002). Nevertheless, none of the patients in this study had an abnormal E/e’ or maximum velocity of TR, and the high BNP levels were likely caused by systolic dysfunction. BNP has been reported as a predictive marker of diastolic dysfunction. Among 181 participants, Grewal et al. (2008) reported that those with a BNP level of > 100 pg/mL had a left atrial volume index of > 28 mL/m2. Abhayaratna et al. (2006) reported that NT-pro BNP was a marker of diastolic dysfunction in a population-based sample of 1,229 older adults.
Owing to poor metabolism, patients with renal dysfunction can have high BNP levels. However, patients with obesity can have low BNP levels because of endocytosis in the adipose tissue (Weber and Hamm 2006). In the current cohort, none of the patients had renal dysfunction or obesity. An elevated BNP is associated with infection, age, and being female. Our cohort did not include any patients with infection or acute illness. Additionally, exploring the effect of sex in our cohort is difficult because the five female patients had neither BNP levels < 40 pg/mL nor LVSF < 0.20. Age could be an important factor, influencing the relationship between BNP levels and LVSF. LVSF may be decreased in proportion to age in our cohort, which is consistent with a previous report (Nakanishi et al. 2006). However, BNP levels showed a poor correlation with age and were variable even in older patients. It was highly likely BNP levels represented LVSF independently, although we could not perform multivariate analysis because of limited number of cases.
The current study had several limitations. First, it had a retrospective design, and the number of patients with data on echocardiography and BNP levels was small. Second, diastolic dysfunction might not have been assessed. This is because E/e’ and maximum velocity of TR were only the parameters used and the other recommended parameters (i.e., left atrial volume index) (Nagueh et al. 2016) were not available. To further validate the correlation between the BNP levels and LVSF, future studies should include a larger number of patients, particularly those with LV systolic dysfunction. To validate the findings of this study, we are considering a prospective study focused on progressive stage patients.
In conclusion, the BNP levels was exponentially correlated with LVSF in patients with FCMD, and it elevated in the terminal stage of LV systolic dysfunction.
Conceptualization: Takatoshi Sato; Data curation: Takatoshi Sato, Yuki Kihara, Kumiko Ishiguro, Minobu Shichiji; Formal analysis: Takatoshi Sato; Funding acquisition: Keiko Ishigaki; Investigation: Takatoshi Sato, Yuki Kihara, Kumiko Ishiguro, Minobu Shichiji; Methodology: Takatoshi Sato, Atsuhito Takeda, Keiko Ishigaki; Project administration: Takatoshi Sato, Terumi Murakami, Keiko Ishigaki; Resources: Takatoshi Sato, Eriko Shimada, Kei Inai; Software: Takatoshi Sato; Supervision: Satoru Nagata; Validation: Takatoshi Sato, Keiko Ishigaki; Visualization: Takatoshi Sato; Writing- original draft: Takatoshi Sato; Writing- review & editing: Takatoshi Sato, Keiko Ishigaki.
This research was supported by AMED under grant number 23ek0109611h0002.
The authors declare no conflict of interest.