-
Siddiki S. M. A. Hakim, Takashi Sugimura
Session ID: 1P063
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Metathesis of two allyoxy groups tethered by optically active 2,4-pentanediol with a Grubbs catalyst resulted in a cyclic dimer in a high yield. Change of the allyoxy group to the higher analogues did not result in intramolecular metathesis, but resulted in cyclic dimers of 18-26 membered ring. The size enlargement of the tether part was then studied. The tether structure was strictly related to the efficiency of the intramolecular reaction and the stereoselectivity. The optimized yield of the cyclic product is more than 95%, and Z-selectivity is in a range of 75->95%. Diastereoselectivity with related substrates will be presented.
View full abstract
-
Kazushi Miyata, Koki Miura, Morifumi Fujita, Takashi Sugimura
Session ID: 1P064
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have recently reported that the reaction of 4-acyloxybut-1-enes with iodosylbenzene gives an O-cyclization product via acyloxy rearrangement, and carried out the asymmetric variant using lactic acid-derived hypervalent iodine reagent. In this paper, we will discuss enantioselectivity in dioxytosylation of styrenes using the optically active iodine(III) reagents.
View full abstract
-
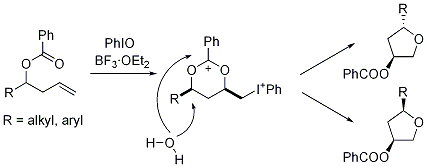
Yuya Okubo, Hiroshi Suzawa, Morifumi Fujita, Takashi Sugimura
Session ID: 1P065
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have recently reported that the reaction of 4-acyloxybut-1-enes with iodosylbenzene gives a tetrahydrofurane product via a 1,3-dioxan-2-yl cation intermediate. Stereochemistry of the tetrahydrofuranylation is highly controlled by alkyl substituents to give a diastereoselective product. The diastereoselectivity is rationalized by electronic and steric effect of the substituents of the 1,3-dioxan-2-yl cation intermediate.
View full abstract
-
Kota Saito, Haruyasu Asahara, Takumi Oshima
Session ID: 1P066
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We investigated the acid-catalyzed skeletal rearrangement reaction of variously
m-,
p-substituted diaryl homoquinone epoxides. The epoxide-opening reactions was accompanied by the transannular cyclization of
endo-aryl group. Both
ortho and
ipso transannular products were obtained. We also examined the kinetic substituent effects on these epoxide ring-opening reactions. The results indicated that the ring cleavage of epoxide involves the remote π-aryl participation.
View full abstract
-
Md.M. Rahman Badal, Masaaki Mishima, Annette D. Allen, Thomas T. Tidwe ...
Session ID: 1P067
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
LFP of diazoacetylpyrazine and diazoacetylpyrimidine in CH3CN with 266 nm light gave the corresponding ketenes and stable azacyclobutenone ylides. Pyrazylketene in the presence of n-BuNH2 decayed with 5.6 x108 M-1s-1, and the absorption at 1674 cm-1 grew with the same rate constant as the decay of the ketene. This intermediate decayed with a rate constant of 1x102 s-1, which is independent of concentration of n-BuNH2. On the other hand, in the reaction of the pyrazylketene with Et2NH the intermediate (1684 cm-1) decayed much faster with a rate constant of 8.3 x107 M-1s-1, and gave the amide. Similar results were obtained for the pyrimidylketene generated from photolysis of diazoacetylpyrimidine. It was found that there is a significant difference in property of the transient between n-BuNH2 and Et2NH.
View full abstract
-
Makoto Sato, Hiroshi Yamataka
Session ID: 1P068
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
The Beckmann rearrangement (BR) takes place when ketoxime is treated with acid, but when the migrating atom group is stable as the cation, a fragmentation reaction (BF) also occurs. Theoretical calculations with 3-phenyl-2-propanone oxime have suggested that both rearrangement and fragmentation reactions proceeded via a common transition state (TS). Ab initio molecular dynamics (MD) calculations performed from each transition state of the substituted derivatives showed that the reaction pathway for some derivatives was bifurcated after passing the corresponding TS region. The potential energy surface (PES) search indicated that a ridge on PES, lying between BR region and BF region, divided MD trajectories into two reaction channels. Therefore, we conclude that BR and BF proceed via a common TS, and reaction products (pathway) are affected by dynamics effect.
View full abstract
-
Tetsuji Katori, Hiroshi Yamataka
Session ID: 1P069
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
For the Schmidt reaction, it is expected that not only rearrangement but also fragmentation reaction takes place depending on substituent as in the Beckmann rearrangement. In this study, we analyzed pathways of the Schmidt reaction of substituted benzyl methyl ketones by MO and MD calculations to clarify how a change of a reaction pathway occurred. It was found that as substituent became electron donating, reaction pathway changed from rearrangement to fragmentation through single TS as in the Beckman rearrangement by the MO calculations. The MD calculations revealed that the reaction of the borderline of rearrangement and fragmentation gave the two different kinds of products from common TS. In addition, the ratio of rearrangement and fragmentation changed with the electronic nature of substituent. The results of the MO and MD calculations are compared, and the mechanism of this reaction is analyzed.
View full abstract
-
shuhei Itoh, hiroshi yamataka
Session ID: 1P070
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Unimolecular dissociation of 3-choloro-2-cyano-2-phenylpropanol anion was investigated using molecular orbital calculations and molecular dynamics simulations.
According to molecular orbital calculations, the reaction mechanism gradually varies from E1cB to E2 by changing a substituent of phenyl group electron withdrawing -groups.
On the other hand, molecular dynamics simulations were indicative of non-IRC pathway. Thus, the C-Cl bond dissociation was delay from than the C-C bond dissociation in compassion to IRC pathway. In the 4-nitrophenyl substituent compound, two type trajectories were obtained: E2 and E1cB pathway.
These results indicated that E1cb-E2 borderline mechanism is complex behavior.
View full abstract
-
Kazuki Mori, Shu Hasimoto, Kazuhisa Sakakibara
Session ID: 1P071
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Stable conformations in the bulk-phase, dynamic behavior and related physical properties of proton-conducting ionic liquids such as Diethylmethylammmonium Trifluoromethanesulfonate [DEMA][TfOH] and Diethylmethylammmonium bis(trifluoromethylsulfonyl)imide [DEMA][HTFSI] have been investigated by Molecular Dynamics(MD) Calculation and
1H and
13C NMR Spectroscopy.
View full abstract
-
Noriaki Ochi, Yoshihide Nakao, Hirofumi Sato, Yoshihiro Matano, Hirosh ...
Session ID: 1P072
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
New palladium(II) phosphorus-containing hybrid calixphyrin complex
1 was synthesized, recently. Although the palladium(II) complex was believed to be unfavorable for the oxidative addition,
1 efficiently catalyzes the Heck reaction which takes place through oxidative addition. Herein, we wish to theoretically discuss the reaction course of the oxidative addition of phenylbromide to
1 and the role of calixphyrins in the reaction. We found that the thiophene and pyrrole rings dissociate from the palladium center to generate vacant coordination sites at the metal center, accompanied by the reduction of the palladium center from +2 to zero oxidation state. In this process, the population of LUMO of calixphyrin considerably decreases, which indicates that the calixphyrin changes from anion to neutral species. This clearly shows that the flexibility of calixphyrin platform plays important roles in this oxidative addition.
View full abstract
-
Atsushi Ishikawa, Yoshihide Nakao, Hirofumi Sato, Shigeyoshi Sakaki
Session ID: 1P073
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Photoinduced epoxidation reaction of olefins, catalyzed by RuII(TMP)(CO) (TMP = tetra(2,4,6-trimethyl)-phenylporphyrine) is theoretically investigated, where model complex, RuII(Por)(CO) is employed (Por = unsubstituted porphyrin). Geometry was optimized for singlet, triplet, and quintet states. The ground state is singlet, and structural difference is quite small between singlet and triplet spin states. For Ru(Por)(CO) and its radical cation, excitation energies are calculated by TD-DFT method. A characteristic absorption at 411 nm, experimentally shown by UV-vis. Spectrum of Ru(TMP)(CO), is assigned to be intra-ligand pi-pi* excitation. Blue shift of this peak in the radical cation is reproduced well by the calculation, and this strongly supports the existence of radical cation species in the reaction process. After the pi-pi* excitation, the intersystem crossing occurs and Ru(Por)(CO) takes the triplet state. The spin density of this state is calculated, and it is concluded that an unpaired electron is delocalized over porphyrin ring. For the epoxidation process of olefins, the optimized transition state of doublet Ru(Por)(CO)O- indicates that the epoxidation undergo the carbon radical compound; in other word, this computational result strongly supports the "carbon radical mechanism" of olefin epoxidation. The transition state is also optimized for another olefins and axial ligand. These results indicate that the nature of transition state is strongly influenced by the axial ligand. One of the important results is that the strong ligand field of CO ligand leads to high selectivity of olefin epoxidation catalyzed by RuII(TMP)(CO).
View full abstract
-
Takashi Hosoya, Yoshihide Nakao, Hirofumi Sato, Shigeyoshi Sakaki
Session ID: 1P074
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Anhydrosugar formation is an important reaction in thermal degradation of cellulose, and the detail mechanism has been still unknown. In this study, reaction pathways from cellulose into 1,6-anhydro-beta-D-glucopyranose (a major anhydrosugar) were calculated at MP4//B3LYP level with using methyl beta-D-glucoside as a model structure of cellulose. As a result, it was indicated that the product is formed through the intramolecular substitution of the O(6) atom on the C(1) atom of the reactant after the gradual conformational changing of the reactant from
4C1 to
1C4. The calculated activation barrier (52.5 kcal/mol) agreed well with the experimental activation energy (~55 kcal/mol) reported for cellulose.
View full abstract
-
Atsuki YANAGISAKO, Kaoru NOBUOKA, Satoshi KITAKOKA, Yuichi ISHIKAWA
Session ID: 1P075
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In the Diels-Alder reaction using imidazolium ionic liquids with charge delocalized anions, high stereoselectivity is shown. These ionic liquids show weaker anion- cation interaction. This situation allows hydrogen bonding between the C
2 hydrogen of the imidazolium and the carbonyl oxygen of the dienophile and act like a Lewis acid catalyst. In this study, we report that the effect of ionic liquid with charge delocalized anion as a Diels-Alder reation media in various conditions and Lewis acid catalyst in various reactions.
View full abstract
-
TAKAO FUJITA, SATOSHI KITAOKA, KAORU NOBUOKA, KAZUHIKO MURATA, KEIICHI ...
Session ID: 1P076
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In recent years, ionic liquids have received a considerable upsurge of interest as electrolytes for various electrochemical devices, such as Li-ion batteries, dye-sensitized solar cells. This is mainly attributable to their superior properties, such as non-flammability, non-volatility and intrinsic ion conductivity. High ionic conductivity and fast ion mobility (including the doped ions such as Li
+ for Li batteries) are the requirements for ionic liquids as supporting electrolytes in high-energy devices. This means that the viscosity of ionic liquids must be as low as possible. Therefore, we synthesized multiple low-viscosity ionic liquids. We report the viscosity, ion conductivity and the melting point of these ionic liquids.
View full abstract
-
Koichi ISHIBASHI, Hirohito TSUE, Kazuhiro MATSUI, Hiroki TAKAHASHI, Sa ...
Session ID: 1P077
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
To investigate the solid-state complexation of nitrogen-bridged calixarene analogues, azacalix[7]arene heptamethyl ether
2 has been prepared by applying a 5+2-fragment coupling approach using Buchwald-Hartwig aryl amination reaction. X-ray crystallographic analysis revealed that azacalix[7]arene
2 adopted a highly distorted 1,2-alternate conformation in the solid state as a result of intramolecular NH/O hydrogen bonding interactions. In the crystal, molecules of
2 are mutually interacted by intermolecular NH/O- and CH/pi interactions to establish one-dimensional (1D) hexane-filled nanochannel crystal architecture. Similarly to our recently reported azacalix[6]arene
1, the desolvated crystalline powder material of
2 was capable of selectively and rapidly adsorbing CO
2 among the four main components of the atmosphere. The adsorption capacity of
2 for CO
2 nearly doubled as compared to that of
1 because of the formation of the 1D nanochannel with almost twice the volume of the latter.
View full abstract
-
Sekai IWAMA, Masahiro HORIGUCHI, Hiroki TAKAHASHI, Hirohito TSUE, Rui ...
Session ID: 1P078
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have investigated whether preferential
enrichment, an enantiomeric resolution phenomenon that is
regarded as an event of complexity, can occur regarding
amino acids classified into a racmic compound crystal.
Here we report that the amino acids leucine and alanine show a quite
similar phenomenon to that of preferential enrichment whenever slightly D- or L-enriched leucine or alanine of 5 % ee are
recrystallized from the mixed solvent of water and ethanol
at room temperature.
View full abstract
-
Atsushi Sato, Koichi Tosaki, Naruyoshi Komiya, Takeshi Naota
Session ID: 1P079
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In this session we will describe "Enantioselective rotational isomerization of palladium cyclic dinuclear complex bearing bis-salicylaldimine ligand".
View full abstract
-
Kazuki Ogata, Akio Tannna, Shigeaki Kamuro, Masayuki Hieda, Hikaru Tak ...
Session ID: 1P080
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In this session, we will describe "Synthesis, Structure and C-C Bond Forming Properties of N-Bound Ruthenium Cyanocarbanion Complexes
."
View full abstract
-
Eiji Takahashi, Hikaru Takaya, Takeshi Naota
Session ID: 1P081
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In this session we will describe "Development and Color Change Mechanism of Vapochromic Crystals of Aromatic Diimide Derivatives."
View full abstract
-
Yumiko Tsuruoka, Yosuke Ohyama, Yoichi Habata
Session ID: 1P082
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, we have reported that tetra-armed cyclen bearing two 3,5-difluorobenzyl groups at positions 1- and 7-, and two 4-pyridylphenylmethyl groups at positions 4- and 11- of the cyclen ring forms a trefoil-typed Ag
+ complex (ligand : Ag
+=3:5). From the X-ray crystal structure, we found that the trefoil has a big cavity in the structure. In this paper, we report X-ray structure and TG-DTA of the trefoil-benzene complex. We have used several aromatic compounds, such as benzene, toluene, and naphthalene, as guest molecules of the trefoil. X-ray structures indicated that toluene and naphthalene might be in the cavity, but these molecules are seriously disordered. Only benzene complex was solved. The benzene molecule is incorporated in the center of the trefoil. In order to confirm inclusion of benzene molecule, TG-DTA was measured. The weight loss corresponding to one molecular of benzene was observed at decomposition temperature of the complex. The structures of the complexes with the other aromatic compounds are now in progress.
View full abstract
-
Mari Ikeda, Kinuko Ogura, Aya Matsu-ura, Yoichi Habata
Session ID: 1P083
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
New pyridine-containing chiral ligands having alpha-naphtylethyl ((
R)-
2), and beta-naphytylethyl ((
R)-
3) groups as a chiral moiety have been prepared. When an equimolar amount of Hg(OTf)
2 was added to these ligands, significant CD spectral changes were observed. On the other hand, no spectral changes were observed, when Cd(NO
3)
2, Zn(OTf)
2, and HgCl
2 were added. To investigate stoichiometry between Hg
2+ and (
R)-
2,
1H-NMR titration was carried out. A proton signal at 8-position of alpha-naphtyl group shifted to down field about 0.7 ppm with broadening when an equimolar amount of Hg(OTf)
2 was added. These results suggested that the stoichiometry between Hg
2+ and (
R)-
2, is 1:1. Cold ESI-MS of a 1:1 mixture of Hg(OTf)
2 and (
R)-
2 showed ion peaks at m/z = 703, 1555, and 3259 arising from [2(
R)-
2+2Hg
2++2OTf
-]
2+, [(
R)-
2+2Hg
2++3OTf
-]
+, and [4(
R)-
2+4Hg
2++7OTf
-]
+, respectively.
View full abstract
-
Yasuhiro Ueda, Kennosuke Itoh, Yoshio Ito, Toshio Kawato
Session ID: 1P084
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Pd(II) Complexes of pyridine ligands containing poly ether groups extracted alkali metal ions efficiently. In order to apply this system to the recognition and extraction of anions, we synthesized 3-substituted and 3,5-disubstituted pyridines having amide functional groups. To evaluated the ability of the Pd(II) complexes of these ligands for recognition of several anions, we examined
1H NMR titrations in chloroform-
d. As the result, interactions of Pd(II) complexes with HSO
4-, NO
3- Cl
- and H
2PO
4- ions were recognized. Among them HSO
4- and H
2PO
4- ion showed the largest value association constant with the Pd(II) complexes. In order to improve the association constant, we replaced the amide group by thioamide group to increase the NH acidity. As the result, the ion selectivity was intact and the association constant for HSO
4- ion was increased successfully.
View full abstract
-
Tomoka MAEKAWA, Yuji TANAKA, Susumu TSUDA, Jun TERAO, Nobuaki KAMBE
Session ID: 1P085
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Insulated conjugated molecules have a high potential application to organic electronics for reducing their aggregations and enhancing their fluorescence efficiency, chemical stability, and solubility. Herein, we describe a synthetic methodology of a highly insulated conjugated molecule via double self-inclusion of a guest molecule bearing two permethylated cyclodexitrins and fixation of the pseudorotaxane with two [1]rotaxanes as stopper units by Sonogashira coupling reaction in 50% aqueous MeOH. The structural conformation of the insulated conjugated molecule that four macrocycles are fixed to an axle molecule in a [5]rotaxane system was investigated by general NMR techniques. The corresponding reference compounds that the axle molecule is insulated with two macrocycles or fully naked were also synthesized in
iPr
2NH-THF systems. The shielding effects of macrocycles for an axle fluorophore of the rotaxane and the reference compounds were estimated by Stern-Volmer titration experiment.
View full abstract
-
Yuji Tanaka, Susumu Tsuda, Jun Terao, Nobuaki Kambe
Session ID: 1P086
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Insulated conjugated molecular wires have attracted much attention because of their interesting optical and electronic behavior attributed to blocking the interchain interactions of conjugated molecular wires. Herein, we describe the novel synthetic method for a polyrotaxane as a highly insulated conjugated molecular wire using a pseudorotaxane as a monomer. The pseudorotaxane was prepared via double self-inclusion of symmetric oligo(phenyleneethynylene) derivatives attached with two cyclodextrins in aqueous MeOH. Formation of this pseudorotaxane was confirmed by 2D ROESY NMR spectroscopy in D2O:CD3OD = 1:1. Then formed pseudorotaxane was polymerized by Hay coupling reaction in aqueous MeOH. According to size-exclusion chromatography (SEC) analysis of the obtained polyrotaxane, the average molecular weight and the average repeating units were estimated as 119,000 and 43, respectively using polystylene as a calibration standard. The polyrotaxane was readily soluble in common organic solvents such as chloroform, ethylacetate and THF. Atomic force microscopy (AFM) images of the polyrotaxane on mica substrate indicated that the shape of a polyrotaxane was highly rigid linear and the chain length was above 200nm.
View full abstract
-
Makoto Uchimura, Genichi Konishi, Jyunji Watanabe
Session ID: 1P087
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We describe synthesis of a novel liquid crystal based on 1,4-bis(biphenyl-4-yl)buta-1,3-
dyine (BDPDA-OCx). This molecule exhibited a thermotropic liquid crystal phase. In particular, BDPDA-OC6 showed a sybotactic nematic phase. Little is known about this phase in the low molecular weight liquid crystal compound. We consider this characteristic phase transition behavior is derived from the effect of long mesogen moiety.
View full abstract
-
Sakiko Suzuki, Kazuko Nakazono, Toshikazu Takata
Session ID: 1P088
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
tert-Ammonium salt in crown ether type rotaxane enable to the reversible site conversion of the wheel on axle by neutralization. This pH responsible molecule is expected as a molecular device. We recently investigated an effective synthesis of
tert-ammonium salt type rotaxane by reductive N-methylation of the corresponding
sec-ammonium salt type rotaxane. This time
sec-ammonium salt type rotaxane was treated with an excess of sodium triacetoxyborohydride, and N-ethylated
tert-amine-type rotaxane was obtained in quantitative. We also examined the other reductive N-alkylation with variable sodium triacyloxyborohydride. We will describe the experimental detail and the structures of each
tert-amine and ammonium salt-type rotaxane in solution state.
View full abstract
-
Fumitaka Ishiwari, Kei-ichiro Fukasawa, Takashi Sato, Yasuhito Koyama, ...
Session ID: 1P089
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
A pseudorotaxane consisting of a sec-ammonium salt having a terminal hydroxyl group and axially asymmetric crown ether was end-capped by the esterification with a benzoic acid having an ethynyl group to the corresponding rotaxane was prepared as the monomer. It was polymerized by Rh cat. and gave corresponding polyacetylene having axially asymmetric rotaxane structure in side chain. This polymer was treated with acetic chloride and gave N-acetylated polymer, which showed a Cotton effect around polyacetylene's main chain chromophore 480 nm.
View full abstract
-
Hirofumi Nobukuni, Fumito Tani, Yuichi Shimazaki, Yoshinori Naruta, Ke ...
Session ID: 1P090
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, there has been great interest in the design and synthesis of nanotubular assemblies composed of porphyrin derivatives. They are expected to offer tailored one-dimensional spaces and to be employed in technological applications. The author has projected to construct a new nanotube of cyclic porphyrin dimers which can self-assemble through complexation of the pyridyl groups with hydrogen-bonding donors or metal ions. Interestingly, X-ray crystallography reveals a self-assembled nanotube of cyclic porphyrin dimers without an additional hydrogen-bonding donor or metal ion. The cyclic molecules stack through nonclassical C-H...N hydrogen bonds and pi-pi interactions of pyridyl groups to form the tubular structure. A similar tubular assembly is also observed in the crystal packing of its inclusion complex with C60. The C60 molecules are linearly arranged to form a supramolecular peapod. To the best our knowledge, this is the first example of an X-ray crystal structure determination for a supramolecular peapod. Its structure and photoelectric properties will be discussed.
View full abstract
-
Shinzi Tafuku, hajime Iwamoto, Takeharu Haino
Session ID: 1P091
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Previously, we have reported the synthesis of [2]- and [3]catenanes via the olefin metathesis reaction of the pseudorotaxane formed from a crown ether and a secondary ammonium salt. The selectivity of [2]- and [3]catenanes was dependent on the concentration of the pseudorotaxane. We applied this synthetic strategy for the synthesis of higher order [n]catenanes. In this paper, we will present the details of the synthesis of a [5]catenane.
View full abstract
-
Hiroshi Saito, Masahiro Tanaka, Takeharu Haino
Session ID: 1P092
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have reported that tris(phenylisoxazolyl)benzene derivatives act as a good gelator of various organic solvents, and their helical columnar assemblies formed in solution. In this session, we will present the synthesis of asymmetrically substituted tris(phenylisoxazolyl) benzene derivatives, attached polar functional moieties as the side chain, and their self-assembly to form the gels of simple alcohols.
View full abstract
-
Eri Hirai, Takeharu Haino
Session ID: 1P093
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have reported the noncovalent synthesis of fullerene-containing supramolecular polymeric networks formed by the iterative host–guest interaction of [60]fullerene and calix[5]arene. This supramolecular interaction can be utilized for the supramolecular cross-linking of fullerene-containing polymers. This should force the fullerene-containing polymers to come together to bring their aggregates. In this session, we will present a new method to regulate the size of the fullerene-containing polymers by the supramolecular interaction.
View full abstract
-
Takeharu Haino, Katsushi Sakamoto, Hajime Iwamoto
Session ID: 1P094
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have reported the supramolecular polymeric nano networks formed by the molecular-recognition-directed self–assembly between a calix[5]arene and C
60. Covalently-linked double-calix[5]arenes take up C
60 into their cavities. This complementary interaction creates a strong non-covalent bonding; thus, the iterative self-assembly between dumbbell fullerene
1 and ditopic host
2 can produce the supramolecular polymer networks in the solid state. In this presentation, we will present the detail of the formation of the supramolecular fullerene-containing aggregates in solution.
View full abstract
-
Hajime Iwamoto, Saori Nishi, Takeharu Haino
Session ID: 1P095
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have developed a molecular container based on a calix[4]arene and a metallo-porphyrin. The container encapsulated
N-containing small aromatic molecules within the confined inner cavity due to van der Waals and coordination interactions. Pyridine can be accommodated within the cavity, but not 4-methylpyridine can't. The strict size selectivity for the guest encapsulation was observed. In this presentation, we will present the detail of the guest encapsulation by the molecular container.
View full abstract
-
Fumihiro Kayamori, Daisuke Murayama, Hajime Abe, Masahiko Inouye
Session ID: 1P096
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Meta-ethynylpyridine polymers and oligomers, consisted of pyridine rings linked by acetylene bonds at 2,6-positions, associate with saccharides by multipoint hydrogen bonding to form helical structures. The helical sense of the complexes is biased by chirality of the guest saccharides to show characteristic induced circular dichroisms (CDs) at the adsorptive region of the ehynylpyridines. However, one must use a large excess of saccharide to efficiently construct stable helices. Therefore, we designed a new class of ethynylpyridine oligomers which are covalently linked with saccharide templates through appropriate spacers. Compared to the intermolecular association, this intramolecular one is expected to reduce an entropic loss and stabilize a higher-order helical structure. Alkylene and phenylene spacers were used for this purpose, and glucose, galactose, and mannose were chosen as a saccharide template. The oligomers display strong induced CDs, indicating the effective formation of the intramolecular helical complexes. The phenylene spacer makes the complex more rigid, so that induced CDs were observed even in a competitive medium such as CH2Cl2/ MeOH(3/1).
View full abstract
-
Shunsuke Takashima, Hajime Abe, Masahiko Inouye
Session ID: 1P097
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have studied artificial polymers consisted of pyridine rings linked at 2,6-positions with acetylene bonds. The artificial polymers can recognize various kinds of saccharides and form helical higher-order structures. In the present study, we newly developed such class of artificial polymers bearing azacrown side chains. The higher-order structure of the polymers was expected to be regulated by cation recognition at the azacrown moieties.
The synthesis of the new polymer is as follows. Citrazinic acid was converted to methyl 2,6-dibromoisonicotinate, and it was reduced and brominated to yield 2,6-dibromo-4-(bromomethyl)pyridine. Then, the bromine atom of the side chain was replaced with 1-aza-24-crown-8. The target polymer bearing azacrown side chains was obtained by repeating Sonogashira reaction on the monomer. Addition of a guest saccharide into a solution of the polymer induced a CD band around 340 nm, which indicates that host-guest association occurred between the polymer and the guest to form a chiral higher-order structure. Furthermore, when a polyammonium adduct was added to the mixture of the polymer and the saccharide, the induced CD band was remarkably enhanced, suggesting the presence of heterotropic positive allosterism.
View full abstract
-
Satoshi OGAWA, Sayuri INOUE, Kazuaki SHIMADA, Hiroki MURAOKA
Session ID: 1P098
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We synthesized tetrathiafulvalene derivatives containing slkoxysiliy terminal with donor ability (EDO-TTFCnSi (n=3,8)) and estimated electrochemichal properties by cyclic voltammetry. We prepared self-assembled monolayers (SAMs) of the synthesized organic molecules on a silicon oxide substrate. The structural property of the SAMs was characterized by X-ray photoelelectron spectroscopy (XPS) and water contact angle (WCA).
View full abstract
-
Mayumi Kudo, Kaori Sato, Takayuki Hanashima, Kosuke Katagiri, Isao Azu ...
Session ID: 1P099
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Aromatic secondary urea such as
N,N'-diphenylurea exists in a
trans-amide form both in the crystal and in solution, whereas
N,N'-dimethylated urea exists in
cis form in the crystal, and predominantly in a
cis form in solution. The
cis-urea bonds can be applied to construct intramolecular aromatic multi-layered structures, and in the case of
meta-linked compounds, the crystal structure showed the helical conformation with all-
R or all-
S of the axis chirality. However, the two enantiomeric structures of the helix could not be distinguished in solution, possibly due to the rapid equilibrium between the enantiomers.
In this study, we investigated the dynamic behavior of aromatic
cis-ureas bearing a chiral substituents on the urea bonds by UV/CD spectra and theoretical calculations, and concluded that aromatic multi-layered urea bearing chiral TEG groups with
S configuration would exist in all-
R helical structure in solution. Further, we tried to construct self-assembled monolayers of aromatic layered ureas on Au(111) substrate in order to analyze the electrochemical properties of aromatic multi-layers.
View full abstract
-
Mio Matsumura, Atsuya Muranaka, Masanobu Uchiyama, Hyuma Masu, Isao Az ...
Session ID: 1P100
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Aromatic secondary amides such as benzanilide exist in
trans-amide form, whereas
N-methylated benzanilides exists in
cis form in the crystal and predominantly in
cis form in solution. The
cis conformational preference is general as observed in various
N,
N'-dimethylated ureas and guanidines, and can be applied to construct the aromatic molecules with unique conformational properties. In this study, we designed functional porphyrin derivatives bearing
trans (-NH-CO-R) or
cis (-NMe-CO-R) amide/urea bond, including porphyrin dimmers or porphyrin-benzene-hybrid type compounds. 2,3,7,8,12,13,17,18-Octaethylporphyrin (OEP) was nitrated to afford 5-nitro-OHP in 64 %, which was reduced to 5-amino derivative. Although simple acylation of the amino group was unsuccessful, the conditions using
n-BuLi as a base and benzoyl chloride afforded 5-benzamido-OEP.
N-Methylated derivative was obtained by direct
N-methylation of the amide by the conditions using NaH and MeI. 5- Benzamido-OEP showed
trans conformation in the crystal and exists predominantly in
trans form in solution deduced from
1H NMR, while
N-methylated derivative exists mainly in
cis form in solution. Further derivatization and their conformational and functional analyses are now in progress.
View full abstract
-
Naoki Akamatsu, Chitoshi Kitamura, Takeshi Kawase, Takashi Kobayashi, ...
Session ID: 2P001
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In the solid-state physical properties, molecular arrangement or packing is one of the most important factors. In the field of organic semiconductor, pi-overlap between pi-systems is essential to get increased mobilities. The attainment of pi-overlap may be a matter of luck. Recently, we found that the anthracene having weak polarization along the long molecular axis can adopt antiparallel stacking. To examine the generality, we prepared several oligoacenes possessing polarization along the long molecular axis. We are going to report X-ray crystal structural analysis and optical properties in the solid state of their acenes.
View full abstract
-
Hideki Tsukuda, Chitoshi Kitamura, Takeshi Kawase, Takashi Kobayashi, ...
Session ID: 2P002
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We prepared tetracenes which have alkyl groups at the 1,4,7,10-positions and investigated the effect of alkyl chain length into molecular structure, molecular arrangement in the solid, and solid-state optical properties. Recently we have synthesized new tetracenes which have alkyl groups at 1,7- or 1,10-positions. We will report their crystal structure and optical properties in the solid state.
View full abstract
-
MIKI Kaori, MATSUMOTO Kouzou, NEHIRA Tatsuo, Gennaro Pescitelli, HIRAO ...
Session ID: 2P003
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have synthesized phenyl-(2-pyridyl)-(3-pyridyl)-(4-pyridyl)methane
1 and the corresponding tris(pyridine
N-oxide)
2 as prototypal chiral molecules based on tetraarylmethane framework. Both
1 and
2 are resolved by a chiral HPLC, which is confirmed by the measurement of CD spectra of the separated two fractions. We also determine their absolute configuration by the comparison of the experimental CD spectrum with the calculated CD curve based on time-dependent density functional theory (TDDFT). We assign the relationship of the absolute configuration of
1 and
2 by measurement of CD spectrum of
2 derived from oxidation of the optically active
1. The result is accordance with that obtained by the calculated CD curve based on TDDFT. Furthermore, We have also synthesized the related chiral molecules
3 and
4, in which the phenyl group in
1 is replaced by pyradinyl or 2-pyrimidinyl group, respectively. These chiral molecules are expected to exhibit metal-chelating properties, which would be detected by CD spectroscopy. We already resolved these compounds by a chiral HPLC. We will also report the chiroptical properties, absolute configuration determination, and metal-binding properties of
3 and
4.
View full abstract
-
Yoko Daifuku, Yasukazu Hirao, Kouzou Matsumoto, Hiroyuki Kurata, Takes ...
Session ID: 2P004
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have revealed the characteristic properties of the singlet biradical componds involing two phenalenyl units. By X-ray structure analysis, these compounds form one-dimensional (1D) chains with a slipped stacking arrengement and an average &pi-&pi distance is substantially shorter than the van der Waals contact of carbon atoms. That is, according to a contribution of singlet biradical, these compounds have strong intermolecular interaction in the crystal-state and two unpair electrons could delocalize by the resonance between intra- and inter molecular interaction over one-dimensional (1D) chains. In this context, we have designed and synthesized 1,2-bis(phenalenyl -1-ylidene)- butatriene (BPLB), that is, acethylene-bridged bisphenalenyls. The biradical form may be responsible for the strong and sharp absorption band &lambdamax = 670 nm in solution. We will report the synthesis and some properties of BPLB.
View full abstract
-
Akihito Konishi, Takeshi Kawase, Yasukazu Hirao, Kouzou Matsumoto, Hir ...
Session ID: 2P005
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Dibenzopentalenes as a 4n-pai electron system would exhibit highly amphoteric redox properties; therefore, they have potential utilities for functional dyes. Recently, we found a nickel mediated reaction forming dibenzopentalenes from 2-bromoethynylbenzenes. Besides, the arylnickel(II) complex as an intermediate of the reaction was isolated, and the structure can be confirmed by an X-ray crystallographic analysis. Here, we discuss the proposed reaction mechanism and the properties of newly synthesized dibezopentalene derivatives.
View full abstract
-
Tatsuya Masuko, Jun-ichi Nishida, Yoshiro Yamashita
Session ID: 2P006
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Much attention has been focused on organic molecular-based opto-electronics materials. Especially, compounds containing both electron donor and acceptor units are highly polarized systems which undergo intramolecular charge transfer. Therefore, those compounds are expected to have small HOMO-LUMO gaps which lead to properties such as absorptions in the near-infrared region, nonlinear optical properties, and single component conductivity. These properties are interesting in terms of applications to semiconductors, nonlinear optical materials and dye-sensitized solar cells.
Previously, we reported donor-acceptor compounds prepared by cycloaddition reaction of tetracyanoethylene (TCNE) with acetylenes. In this study, instead of TCNE, methyl 2,3,3-tricyanoacrylate or dimethyl 2,3-dicyanofumarate were used to control the LUMO levels of donor-acceptor compounds. We will report their synthesis and physical properties.
View full abstract
-
Yujiro FUJIWARA, Jun-ichi NISHIDA, Yoshiro YAMASHITA
Session ID: 2P007
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Low-cost, low temperature process, large area, and flexible electronic device can be made by using the organic materials. pi-Conjugated molecules composed of acene units and aromatic rings can take orthogonal conformation. This effect is expect to weaken intermolecular interactions and give high fluorescence quantum yield. On the other hand, acene units and aromatic rings can form pi-staking to afford two dimensional charge transportation. Here, we will report their synthesis and physical properties. It aimed at p-type organic semiconductor with highly effective luminescence characteristic and high mibility.
View full abstract
-
Masashi Mamada, Jun-ichi Nishida, Shizuo Tokito, Yoshiro Yamashita
Session ID: 2P008
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, organic field-effect transistors (OFETs) have attracted much attention as an alternative to amorphous Si technology because of their potential applications to lightweight, low cost and flexible devices. For achievement of high performance of OFETs, the development of new organic material is very important as well as the improvement of device fabrication techniques. We have reported organic semiconductors with a thiazolothiazole ring as core structure, whose devices exhibited high performance p- and n-type OFET characteristics. As an extension of this work, we have now focused on a benzo[1,2-
d:4,5-
d']bisthiazole (BBT) ring which is a pi-extended system of thiazolothiazole. BBT derivatives were applied for OFET as n-channel semiconductors and their devices showed high performance OFET characteristics.
View full abstract
-
YASUMASA KUGA, KENGO HASEGAWA, KENITIRO WATANABE, JUNICHI NISHIDA, SHI ...
Session ID: 2P009
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, complexes with heavy metals such as iridium and platinum have attracted much attention as organic EL materials that show phosphorescence and highly effective ELs. Especially, complexes with a 2-phenylpyridine structure are known to be highly effective and are researched widely. On the other hand, there are development of complexes with new conjugate systems is not reported still important to enhance the EL performance.
In this research, the relationship between substituent effects and properties was studied by changing substituents 2-vinylpyridines in the Pt complexes.
View full abstract
-
Takeshi Nakagaki, Shin-ichiro Kato, Teruo Shinmyozu
Session ID: 2P010
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have studied the synthesis and the inclusion behavior of the macrocyclic host molecule which has the intercavity sandwiched by two pyromellitic diimide moieties and the size of the cavity is supposed to be suitable for including pi-donating benzene derivatives. The association constants between the macrocycle and polymethoxybenzenes were defferent reflecting the number and the position of the methoxy groups and this difference was suggested to be occured only in the cavity of the macrocycle. This results indicate that small substituents like a methoxy group show significant influence on the inclusion behavior in macrocylic cavity.
View full abstract
-
Motonori Watanabe, Masahiko Shibahara, Kenta Goto, Teruo Shinmyozu
Session ID: 2P011
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
The novel two- and three- layered[3.3]paracyclophanes that had the acceptor were synthesized. In the UV/Vis and Fluorescence spactra, the Red shifted was observed with accumulating of benzene ring.
In the cyclic voltammogram, the donor character has increased with accumulating of benzene ring. It is scheduled to synthesize CP that builds in the donor molecules other than benzene, and to compare and to examine it in the future.
View full abstract
-
Md.Ershad Haim, Yogesh Sangvikar, Tetsuo Iwanaga, Teruo Shinmyozu
Session ID: 2P012
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have planned the synthetic strategy of molecular tubes with long and rigid cavities composed of 1,4-dibromopyromellitic diimide-based cyclophanes to use them as host molecules to accommodate guest molecules by charge transfer interactions. For this purpose, we want to design and synthesize [2+2] and [3+3] cyclophanes from 1,4-bis(aminomethyl)benzene derivatives and 1,4-dibromopyromellitic dianhydride for the construction of molecular tubes.Then introduction of ethynyl group to the bromo-substituted macrocycle by Sonogashira reaction and successive Glaser coupling may lead to the construction of tubular molecules. We want to report here these results.
View full abstract