-
Hiroyuki Kurata, Takahisa Fujimoto, Sang Kim, Yasukazu Hirao, Kouzou M ...
Session ID: A01
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Bithiophene derivatives bridged with a bis(spirodienone) unit were synthesized and characterized. Lithiation of the thiophene rings of an unsubstituted derivative proceeded without decomposition of the bis(spirodienone) skeleton. Palladium-catalyzed cross-coupling reactions (Suzuki-Miyaura, Sonogashira) with bromides afforded a variety of pai-extended derivatives. Bond breaking and formation under redox conditions were observed by cyclic voltammetry. Conformational change between the s-
cis and s-
trans forms of bithiophene under redox conditions will be a key feature of control of the structures and properties of the compounds.
View full abstract
-
Yoshihiro MATANO, Tooru MIYAJIMA, Tatsuya FUKUSHIMA, Hironori KAJI, Hi ...
Session ID: A02
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Three types of bithiophene-fused benzo[
c]phospholes were synthesized via TiII-mediated intramolecular cyclization of dialkynylated bithiophene derivatives. The X-ray structures, theoretical calculations, UV-vis absorption and fluorescence spectra, and electrochemical measurements have revealed that the structural, optical, and electrochemical properties of the bithiophene-fused benzo[
c]phospholes vary considerably depending on the pi-conjugation modes at the bithiophene subunits. The appropriately ring-annulated sigma3-P derivatives were found to emit fluorescence in the orange-to-red region, and the P-oxo derivatives proved to undergo reversible one-electron reduction in cyclic voltammetry
View full abstract
-
Toshiyuki Kowada, Yoshio Matsuyama, Kouichi Ohe
Session ID: A03
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Oligothiophenes and 9,9'-spirobifluorene derivatives have received considerable attention in the field of organic electronics because of high environmental stability, luminescence, and charge transport properties. On the other hand, there are few reports on spiro compounds containing a heteroarene, such as thiophene or furan. In this study, we synthesized the spiro compounds containing a heteroarene and they showed high luminescence. For example, the photoluminescence efficiency of
2c is 0.89. Furthermore,
2c exhibits a high decomposition temperature (T
d = 438 °C) and glass transition temperature (T
g = 182 °C).
View full abstract
-
Eigo Miyazaki, Ayaka Kaku, Masahito Iwatani, Yuuki Mori, Yuuki Suzuki, ...
Session ID: A04
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Thiophene-fused porphyrazines are potentially interesting compounds as organic electronic materials due to their isoelectronic structure with phthalocyanine. However, their structures and physical properties were not studied because of their poor solubility. In order to elucidate the properties of the thiophene-fused porphyrazine, we have synthesized thiophene-fused porphyrazine derivatives with long alkyl chains at -positions of the thiophene moieties. In the present contribution, their synthesis, physical properties, and crystal structures will be described in detail.
View full abstract
-
Toshihiko Uto, Nobuhiro Yamamoto, Nobukazu Negishi, Yutaka Ie, Yoshio ...
Session ID: A05
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
-
Shoji Tanaka
Session ID: A06
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have been developing step-wise synthetic protocols for mono-molecular integration technology, which integrates the quantum dots, tunnel junctions, and the required passive elements of quantum device on a single macromolecule. In this line, we devised a library of versatile building blocks. The functionality of these blocks is based on the chemical reactivity of 3,4-diaminothiophene component, which can be easily modified to tune the structural and electronic properties of the main p-conjugated chain. It is facile to access to various series of p-conjugated macromolecules using Pd-catalyzed cross-coupling or Ni-induced biaryl coupling reactions of these building blocks. Some typical examples of our step-wise bottom-up architecture will be demonstrated in the presentation.
View full abstract
-
Hiroki Muraoka, Satoshi Ogawa
Session ID: A07
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We attempt to design of new type of multi-steps reversible redox systems containing organic and organometallic redox active flagments by using ferrocene-thiophene hybrid molecules. We have synthesized 1,1'-bis{2-(5-arylthienyl)}-, 1,1'-bis{2-(5'-ferrocenylthienyl)}-ferrocene by palladium catalyzed cross-coupling reactions of 1,1'-bis(bromothienyl)ferrocene with p-substituted phenyl bronic acid or ferrocenylzinc chloride. The structure of 1,1'-bis{2-(5-
p-methoxyphenylthienyl)}ferrocene has been determined by X-ray crystallographic analysis. The crystal structure showed intramoleculer pi-pi stacking between p-methoxyphenyl groups bridged by ferrocene fragment. The electrochemical properties of all compounds were examined by cyclic voltammetry tequnique.
View full abstract
-
TOSHIYUKI HAMURA, TETSU ARISAWA, KEISUKE SUZUKI
Session ID: A08
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Described herein is a synthesis of pi-extended polycyclobutabenzenes, a class of molecules of structural and theoretical interest. The preparation is based on the repeated 2+2 cycloadditions of benzyne and ketene silyl acetal (KSAs), followed by Peterson olefination of the resulting poly-ketone derivatives. By these steps, we were able to prepare various polymethylenecyclobutabenzenes. Structural study on these compounds showed properties related to the strained four-membered ring.
View full abstract
-
Yasuhiro Morisaki, Toshiyuki Sawamura, Takuya Murakami, Yoshiki Chujo
Session ID: A09
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In this study, we developed a simple and novel process for the construction of aromatic ring-layered polymers using a xanthene skeleton as a scaffold. Synthesis, characterization, and optical properties of this class of polymers were investigated in detail. For example, the reaction of diethynyl[2.2]paracyclophane 1, diiodoxanthene 2, and ethynylarenes 3-5 as an end-capping reagent proceeded smoothly to obtain the titled polymers P3-P5 in good yields. The face-to-face structure is attributed to a restricted rotary motion of two aromatic rings attached to 4,5-positions of a xanthene skeleton due to steric hindrance. The optical properties were examined, and an energy transfer from the layered [2.2]paracyclophane to the terminal units via the through-space interaction was observed.
View full abstract
-
Kiyoshi Sato, Shin Tsukishima, Kazunari Takahashi, Motowo Yamaguchi, S ...
Session ID: A10
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
A new approach towards the synthesis of the elusive dicationic heterocoronene, diazoniacoronene, is presented. The fundamental synthetic strategy is based on the intramolecular photo-Diels-Alder type cycloaddition of an azaazoniathia[6]helicene. The improved procedure completely suppress subsequent reaction to form pyridone type byproduct.
Electrochemical study shows the diazoniacoronene has four reversible reduction processes, converted to the monocation radical, the neural, the monoanion radical, and the dianion.
View full abstract
-
Toru Amaya, Takuto Nakata, Michiaki Okada, Maiko Hifumi, Toshikazu Hir ...
Session ID: A11
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
The synthesis of various pi-bowls, including higher strained tribenzannulated sumanene (C42H18), was achieved based on the molecular transformation of sumanene (C21H12).
View full abstract
-
Tomoharu Tanikawa, Masaichi Saito, Jing-Dong Guo, Shigeru Nagase
Session ID: A12
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
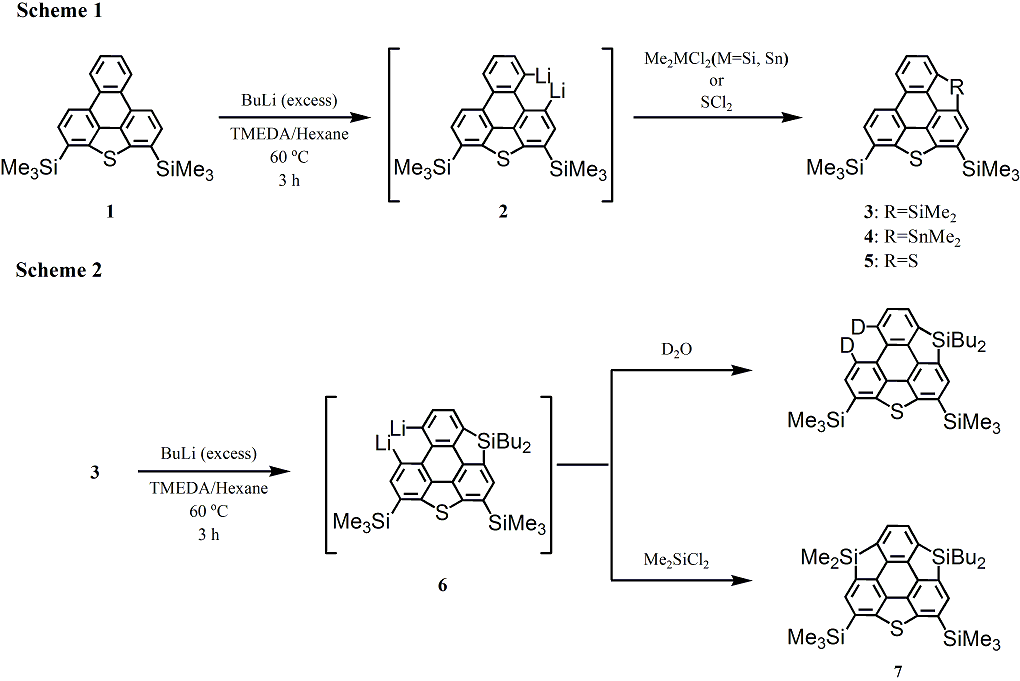
After treatment of triphenylenothiophene
1 with excess butyllithium in hexane-TMEDA, the resulting dilithio derivative reacted with dichlorodimethylsilane, dichlorodimethylstannane and sulfur dichloride to give silolo-
3, stannolo-triphenylenothiophene
4 and triphenylenodithiophene
5, respectively. We finally succeeded in the synthesis of heterasumanene
7 by the lithiation of
3 followed by the addition of dichlorodimethylsilane to the resulting dilithio compound
6. UV-vis spectra of compounds,
1,
3 and
4, were measured and each of absorption maxima was assigned by the aid of TD-DFT calculations.
View full abstract
-
Shinji Toyota, Hiroyuki Onishi, Kazuaki Miyamoto, Testuo Iwanaga, Kazu ...
Session ID: A13
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Some 1,8-anthrylene dimers with acetylene and diacetylene linkers were synthesized by coupling reactions. X-ray analyses revealed that the dimer with two acetylene linkners took a nearly planar structure, whereas its monoanthraquinone analogue suffered from out-of-plane deformations due to the steric hindrance of the inner carbonyl group. The latter compound undergoes self-association in solution, and formed self-assembled monolayer on a graphite surface as revealed by STM. Other cyclic dimers with intraannular alkyl groups were synthesized, and their stereoisomers were isolated. The structures and barrier to isomerization will be reported.
View full abstract
-
NIKAWA Hidefumi, Takashi Kikuchi, Tomoya Yamada, Hidenori Kuga, Tsuyos ...
Session ID: A14
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Endohedral metallofullerenes have attracted special interest since they could lead to new spherical molecules with unique structure and properties that are unexpected for empty fullerenes. In 1991, Smalley and co-workers reported that La@C
60, La@C
74, and La@C
82 were produced especially abundantly in the soot, but only La@C
82 was extracted with toluene. Since then, the chemistry of soluble endohedral metallofullerenes has been started by centering on that of La@C
82, and up to now many soluble endohedral metallofullerenes have been separated and characterized. However, insoluble endohedral metallofullerenes such as La@C
60, La@C
72, and La@C
74, have not yet been isolated although they are regularly observed in the raw soot by mass spectrometry.
We herein report the isolation of La@C
72, La@C
74, and La@C
80 as an endohedral metallofullerene derivative, La@C
72(C
6H
3Cl
2), La@C
74(C
6H
3Cl
2), and La@C
80(C
6H
3Cl
2), respectively. The structural determination has been performed by spectroscopic and finally X-ray crystallographic analysis, and these properties are discussed on the basis of the theoretical study.
View full abstract
-
Atsuya Muranaka, Shuji Yasuike, Ching-Yuan Liu, Joji Kurita, Nagao Kob ...
Session ID: A15
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
The electronic structures of a homologous series of indole and benzofuran derivatives, in which the nitrogen or oxygen atom is replaced by the Group 15 and 16 heavier heteroatoms, have been investigated by means of various spectroscopic techniques coupled with density functional calculations. It was found that the excitation energies of the Group 16 benzoheteroles systematically shift to the red in the order of benzofuran, benzothiophene, benzoselenophene, and benzotellurophene. In contrast, the electronic absorption spectra of the Group 15 benzoheteroles; 1-phenyl derivatives of indole, phosphindole, arsindole, stibindole, and bismuindole, did not exhibit this type of spectral shift. Using the observed spectroscopic properties and TDDFT calculations, the electronic absorption spectra of the present heterocycles were assigned. A molecular orbital analysis was performed to rationalize the effect of replacement of the heteroatom on the electronic structures.
View full abstract
-
Kentaro Yamane, Shinji Nishizuka, Satoko Hayashi, Waro Nakanishi, Taka ...
Session ID: A16
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Extended hypervalent bonds [
m center-
n electron bonds (
mc-
ne:
m >= 4)] higher than 3c-4e are of current interest. Our strategy to construct the extended hypervalent bonds is to employ the interactions caused by direct orbital overlaps between nonbonded atoms. We tried to construct a new building block by the ethynyl group incorporated in the Z-Z bond of Z4 4c-6e (Z = Se and S) at the naphthalene 1,8-positions. 1-(8-
p-YC
6H
4Z)C
10H
6Z[(CC)
mZC
10H
6Z]
n-(CC)
mZC
10H
6(ZC
6H
4Y-
p-8')-1' [
1 (
m = 1;
n = 0; Y = Me; Z = Se) and
2 (
m =
n = 1; Y = Me; Z = Se)] are prepared. The structure of
1 in crystals is determined by the X-ray crystallographic analysis. The six Se---Se-CC-Se---Se atoms align linearly. The linear alignment of six C
2Se
4 atoms is analyzed by the 6c-10e model. The linear alignment of ten C
4Se
6 atoms in
2 is also confirmed in solutions by means of NMR. The linear bonds are analyzed based on the quantum chemical calculations. Investigations on similar compounds containing Z = S and Te in place of Z = Se are in progress.
View full abstract
-
Hiroshi Yamada, Aiko Fukazawa, Shigehiro Yamaguchi
Session ID: A17
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We now report the synthesis of phophonium- and borate-bridged stilbene via intramolecular double cyclization from boryl- and phosphanyl-substituted diphenylacetylene derivatives. This cyclization is applicable to the synthesis of distyrylbenzene derivatives and thiophene-fused derivative. The obtained zwitterionic ladder compounds show totally different photophysical and electrochemical properties from those of the known ladder oligo(p-phenylenevinylene)s.
View full abstract
-
Takahiro Kojima, Jun-ichi Nishida, Shizuo Tokito, Yoshiro Yamashita
Session ID: A18
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, OFETs and OLEDs based on organic semiconductors have attracted much attention. Although organic semiconductors including boron atoms have been reported, the number is limited. Since tricordinated boron has a vacant p orbital in the vertical direction, we tried to increase intermolecular interactions by using this vacant p orbital, and to construct new OFETs based on them.
On the other hand, boronic acids are very famous reagents for the Suzuki-Miyaura coupling reaction. In this work, new compounds containing boron atoms were synthesized by using new synthetic methods. Their physical properties, X-ray single crystal analysis and FET characteristics suggest that these compounds can be applied for organic semiconductor devices.
View full abstract
-
Chitoshi Kitamura, Hideki Tsukuda, Takuya Ohara, Takao Naito, Chika Ma ...
Session ID: A19
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We have prepared anthracenes, tetracenes, and pentacenes, which had various substituents, and investigated optical properties in the solid state. In the case that the substituents are alkyl groups, confomational polymorphs and accompanying molecular arrangement in the solid uderwent a lot of changes. We measured fluorescence in the solid state. Recently we found that we can get Kubelka-Munk spectra, which were calculated from the diffuse reflectance spectra. We will show the Kubelka-Munk spectra of various oligoacenes.
View full abstract
-
SHIGERU SASAKI, KAZUNOBU OGAWA, NOBORU MORITA
Session ID: A20
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Absorption and emission spectra of crowded triarylphosphines were studied and relation with structure and properties was discussed. Triarylphosphines showed absorption in longer wavelength as the structure around the phosphorus becomes more crowded, bond angles around the phosphorus become larger, and oxidation potentials become lower. The crowded triarylphosphines exhibited emission in longer wavelength with smaller Stokes shift as compared with triphenylphosphine reflecting smaller difference of the structures between ground and excited states. A crowded triarylphosphine carrying 4-borylaryl group was synthesized to compare with well-known (4-borylaryl)amine fluorescent dye. The (4-borylaryl)phosphine exhibited absorption and difference betwween the oxidation and reduction potential similar to the corresponding (4-borylaryl)amine. However, emission was observed in ca. 200 nm longer wavelength reflecting introduction of the crowded triarylphosphine moiety.
View full abstract
-
Soichiro Kyushin, Takaya Matsuura, Hideyuki Matsumoto, Hiroaki Horiuch ...
Session ID: A21
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Silyl groups affect photochemical properties of aromatic compounds. When aromatic compounds are substituted by silyl groups, the UV absorption bands show a bathochromic shift and the extinction coefficients of the absorption maxima increase. Theoretical calculations show that these electronic effects of silyl groups are explained by the cooperation of sigma-pi conjugation in the HOMO and sigma*-pi* conjugation of the LUMO.
View full abstract
-
Naoto Hayashi, Akifumi Kanda, Hiroyuki Higuchi
Session ID: A22
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
X-ray structures of quinone dimers, 1a, 2a, 3a, and 4a have been investigated. The distorted quinone moiety into the boat form was observed not only for 1a but also for 2a, which is explainable in terms of the packing demand, but a certain interaction between oxygen atoms may be responsible in part. The torsional potential of 3b and 4b obtained by MO calculations were considerably different from those of MAM and MAAM due to Coulombic interactions.
View full abstract
-
Eigo Isomura, Hideo Enozawa, Tohru Nishinaga, Masahiko Iyoda
Session ID: A23
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, new compounds bearing both magnetism and electrical conductivities are an attractive target. To construct molecules having strong intramolecular π-d interaction, we designed a diad system composed of covalently linked TTF and copper-pyridine moiety. We report here the synthesis and interesting electronic properties of title compounds. Although we did not observed a remarkable magnetic property in the oxidized species derived from 1a and 1b, an interesting intramolecular charge transfer interaction between TTF and Cu(II) has been characterized and will be reported in detail. On the other hand, various supramolecular architectures of 1b and its metal complexes have been constructed toward application into devices in nanotechnology. Thus, long alkylthio derivatives of title compounds form fibrous nanostructures. We first examined the formation of supramolecular structure of the parent compound 1b. In DCM/MeOH (1:10, v/v), 1b self-assembled into purple tape-like structure and this tape showed a conductivity of up to 2.6 x 10
-4 S/cm upon I
2 doping. Copper complexes 2b and 4b also formed flexible and rigid tape-like structure respectively. Furthermore, cation-radical-based intrinsic conducting nanowires were obtained and they showed semiconducting behavior.
View full abstract
-
Taku Hasobe, Atula Sandanayaka, Takehiko Wada, Yasuyuki Araki
Session ID: A24
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Construction of functional molecular assemblies with well-defined shapes and structures are of great interest because of a variety of applications such as optoelectronics. In particular, unidirectionally bar-shaped nanostructures of porphyrins also have potentials for fabrication of nanoscale materials, electronics and photonics because of the characteristic anisotropic structures.
We report a new type of molecular composites: fullerenes-encapsulated porphyrin hexagonal nanorods composed of zinc meso-tetra (4-pyridyl) porphyrin [ZnP(Py)4] and C60 [C60-ZnP(Py)4 nanorod], which are prepared by aiding surfactant: cetyltrimethylammonium bromide (CTAB) in a DMF/acetonitrile mixed solvent. The highly organized C60-ZnP(Py)4 nanorods demonstrate that not only a broad absorption property derived from the supramolecular aggregates, but also significant enhancement of solar energy conversion property based on photoinduced charge separation yielding radical ion pair.
View full abstract
-
Shinichirou Kato, Take-aki Koizumi, Takakazu Yamamoto
Session ID: A25
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
1,8-Naphthyridine has a two pyridine ring-fused structure, and is expected to perform as a strong electron accepting unit. However, there have been few reports about -conjugated polymers containing 1,8-naphthyridine with donor - acceptor architectures so far. In this study, We will report synthesis and optical properties of -conjugated copolymers containing 1,8-naphthyridine in the main chain.
Monomer-1 was prepared by the reaction of 5-bromo-3-aminoquinaldehyde with 4-bromoacetophenone in the presence of KOH as shown in Scheme 1, and characterized by 1H NMR and elemental analysis. Poly-1 and Poly-2 were synthesized by using a Pd-catalyzed Suzuki cross coupling reaction. Poly-1 and Poly-2 gave 5% weight loss temperature at 430 deg and 340 as estimated by thermogravimetric analysis. UV-vis absorption peak of Poly-1 and Poly-2 appeared at 395 nm and 388 nm, respectively. The photo luminescence spectra of Poly-1 and Poly-2 showed emission maxima at 440 and 471 nm in o-dichlorobenzene with quantum yields of 87% and 66 %, respectively.
View full abstract
-
Shigeki Kawabata
Session ID: A26
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Porphyrins and phthalocyanines are very useful for a variety of photochemical and photophysical applications. In order to utilize these chromophores, it is necessary to control their properties favorably. For this purpose, a lot of researchers have synthesized and investigated various porphyrin analogues, such as meso-aza-substituted porphyrins. However, there are few studies on synthesis and characterization of 5,15-diazaporphyrins and their aryl-substituted derivatives. Now, we have succeeded in synthesizing a variety of meso- and beta-aryl-5,15-diazaporphyrins, and characterized their properties.
Hexaaryl substituted 5,15-diazaporphyrins were obtained in good yield in spite of large steric hindrance because of their high solubility. In the absorption spectra, hexaaryl substituted 5,15-diazaporphyrins show large bathochromic shifts in both Soret and Q bands (about 27-35nm bathochromic shift relative to all-alkyl type 5,15-diazaporphyrin).
We report the synthesis of a series of meso- and/or beta-aryl-5,15-diazaporphyrins, and discuss the controlling their photochemical properties.
View full abstract
-
Yusuke Hirata, Torahiko Yamaguchi, Shiro Matsukawa, Yohsuke Yamamoto
Session ID: A27
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, we obtained novel oxidized 16pi octaethyltetraphynylporphyrin(OETPP) and octaisobutyltetraphenylporphyrin(OiBTPP) by the reaction of OETPPLi2 or OiBTPPLi2 with SOCl2. These were the first characterized 16pi porphyrin, although in 2006, Vaid and coworkers also prepared [Li(TPP)][BF4] bearing 16pi porphyrin skeleton by the reaction of Li2(TPP) with thianthrenium tetrafluoroborate (Thn+BF4-). However 16pi porphyrin metal complex was not reported except for [Li(TPP)][BF4]. We report here synthesis and structure of the novel OiBTPPZnCl(ZnCl3) bearing 16pi porphyrin skeleton. The X-ray structure of OiBTPPZnCl(ZnCl3) shows very clear bond alternation and is much more distoreted from the mean plane of porphyrin core than that of OiBTPPZn(H2O).
View full abstract
-
Taro Koide, Gengo Kashiwazaki, Masaaki Suzuki, Ko Furukawa, Atsuhiro O ...
Session ID: A28
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
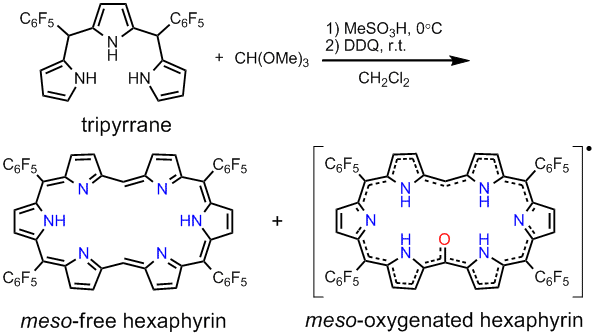
In recent years, increasing attention has been focused on the expanded porphyrins because of their attractive chemical, optical, and coordination properties. However, there have been few reports on expanded porphyrins which have no subsituents on their
meso positions.
We have synthesized
meso-free hexaphyrin as the first example of
meso- and
β-unsubstituted [26]hexaphyrin(1.1.1.1.1.1) by the condensation of 5,10-bis(pentafluorophenyl)tripyrrane with trimethyl orthoformate. Its planar and highly symmetric structure with spectacles-like conformation was confirmed by X-ray crystallographic analysis, and its strong aromaticity was also revealed by
1H-NMR and UV/vis spectrum.
meso-Oxygenated hexaphyrin was identified as a monoradical by ESR and SQUID measurements. It has been confirmed that the
meso-oxygenated hexaphyrin is a stable neutral radical even under air and water. In aerated solution,
meso-free hexaphyrin was slowly changed to
meso-oxygenated hexaphyrin. In the meantime, we found that when dissolved in trifluoroacetic acid-
d (TFA-
d) it exhibited a sharp
1H-NMR spectrum which indicated nonsymmetric and nonaromatic structure. These results demonstrate that large structural and electronic changes in [26]hexaphyrin can be caused by simple removal of two
meso-aryl substituents, hence indicating further promising potentials of expanded porphyrins. These hexaphyrins formed binuclear and mononuclear metal complexes, thus, they can be used as multi-metal coordinating ligands.
View full abstract
-
Yasushi Imada, Takahiro Kitagawa, Hiroki Iida, Takeshi Naota
Session ID: B01
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
In this session we will describe "Aerobic Hydrogenation of Olefins Catalyzed by Noncovalently Dendronized Flavins".
View full abstract
-
Makoto Yasuda, Hideto Nakajima, Sachiko Yoshioka, Akio Baba
Session ID: B02
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Group 13 metal compounds are one of the most important classes of Lewis acids owing to their vacant p-orbitals. Especially boron compounds are known to be strong Lewis acids because of their strong affinity for a heteroatom. However, they have disadvantage as catalysts because they are not likely to release the products. We synthesized sterically strained C3 symmetric cage-shaped borate complexes. The framework changes the metal surroundings which are less sterically hindered. They achieved a high Lewis acidity and catalytic turn-overs. In fact, they effectively catalyzed a hetero Diels-Alder reaction while triphenyl borate showed no catalytic activity. The ab initio caluculations revealed highly accessible vacant MO and its lowered energy level caused by the cage-shaped framework. This means that cage-shaped borate complexes possess higher Lewis acidity than planar-shaped borate complexes. Furthermore, we succeeded in tuning the Lewis acidity of the complexes by changing the tether atom of the complexes. This result indicates that controlling the space around metal center achieves tuning the catalytic ability of the complexes.
View full abstract
-
Teruyuki Matsumoto, Takahiro Sasamori, Norihiro Tokitoh
Session ID: B03
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
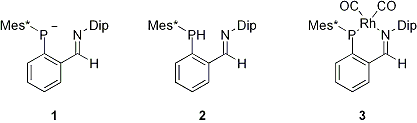
A beta-diketiminato ligand, a monovalent and bidentate ligand, has attracted much attention in several field. On the other hand, a ligand bearing (a) low-coordinated phosphorus atom(s) has also been noticed as a unique ligand from the viewpoint of its electronic feature. Although a monovalent and bidentate ligand bearing a low-coordinated phosphorus atom is expected to show interesting features, only few reports on such a unique ligand has appeared so far. In this paper, we have designed a novel iminophosphido ligand 1 to elucidate a new class of ligands and achieved the synthesis of a compound 2, which should be a good precursor of 1. Precursor 2 was treated with rhodium chloride under the basic conditions to afford an expected rhodium complex 3, which is the first iminophosphido complex. We will also report a deprotonation reaction of precursor 2 with a base and the complexation reactions of precursor 2 with several types of metals.
View full abstract
-
Takahiro Kanei, Hiroshi Ikeda, Kazuhiko Mizuno
Session ID: B04
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Previously, we have proposed efficient orbital interaction between the radical and cation parts of the 1,4-diarylcyclohexane-1,4-diyl radical cation, which is a key intermediate in the photoinduced electron-transfer (PET) Cope rearrangement of 2,5-diaryl-1,5-hexadienes. In this work, we studied 1,4-diarylbutane-1,4-diyl radical cations, generated by PET reaction of 1,2-diarylcyclobutanes, with nanosecond absorption spectroscopy on laser flash photolysis. We also investigated molecular geometry and orbital interaction between the radical and cation parts of the radical cations with time-dependent density functional theory.
View full abstract
-
Ryoichi Akaba, Kazusada Motegi, Koji Kawase, Tsuyoshi Sumiya, Kazufumi ...
Session ID: B05
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Photroinduced electron transfer reactions have been carried out for several diaryethanals to study the reactivity of their radical catons with 2,4,6-triphenylpyrilium salts as a sensitizer in dichloromethane. Laser flash photolyses have shown that the radical cations are actually formed and they may undergo C-C bond cleavage and/or deprotonation finally giving benzophenone derivatives in the presence of oxygen. The reactivity will be analyzed and discussed on the basis of the results of molecular orbital calculations with PM3 and DFT/6-31G(d) levels of theory.
View full abstract
-
Tomoko Sakai, Yoshihisa Fujiwara, Manabu Abe
Session ID: B06
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
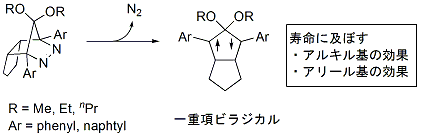
Combined experimental and computational studies were performed to obtain the structural and substituent effects on the reactivity of localized singlet 1,3-diradicals. When the n-propoxy group was introduced at the C2 position of the 1,3-diyls, the dramatic increase of the lifetime, = 1931 ns, was observed, compared to that of 2,2-dimethoxy-substituted 1,3-diyl ( = 320 ns), to give quantitatively the ring-closing product. When the 2-naphthyl group was introduced at the C1 and C3 positions of the 1,3-diyl, the long-lived singlet diradical with a lifetime of 1098 ns was observed. The dramatic increase of the lifetime suggest that a steric interaction between the aryl group and alkoxy group would be one of the important factors to determine the rate constant of the ring-closingg step.
View full abstract
-
Takashi Hirano, Hiroshi Ohba, Yuto Takahashi, Shojiro Maki, Haruki Niw ...
Session ID: B07
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Bioluminescence of the ostracod Cypridina (Vargula) and the jellyfish Aequorea gives a high quantum yield of light production. This indicates that the chemiexcitation process in the bioluminescence reaction produces an excited molecule with a high efficiency. These bioluminescent systems use imidazopyrazinone (imidazo[1,2-a]pyrazin-3(7H)-one) compounds as the bioluminescence substrates, Cypridina luciferin and coelenterazine, respectively. To establish the chemiexcitation mechanism of the bioluminescence, we investigated the chemiluminescence of a series of 6-arylimidazopyrazinones as a bioluminescence model system. In addition, we investigated the fluorescent properties of the light-emitter compounds (amidopyrazines) and carried out DFT calculations of the molecules related to the chemiexcitation process. From these results, we propose the mechanism for the highly efficient chemiexcitation in the Cypridina and Aequorea bioluminescence: thermal decomposition of a neutral dioxetanone intermediate having an electron-donating substituent gives the singlet-excited state (S1) of a light-emitter molecule with a strong intramolecular charge transfer (ICT) character via an ICT transition state (TS) [ICT TS->S1 route in the charge transfer induced luminescence (CTIL) mechanism].
View full abstract
-
Yuta Takano, Akinori Yomogida, Hidefumi Nikawa, Takatugu Wakahara, Tak ...
Session ID: B08
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
La@C
82 is one of the endohedral metallofullerenes which have attracted special attention because they engender new spherical molecules with unique electronic properties. We have found that four isomers of benzyl mono-adducts, La@C
82(CH
2C
6H
5), are obtained by the radical coupling reaction in a toluene solution of La@C
82. Thermolysis of 3-triphenylmethyloxazolidin-5-one (
1) may generate benzyl radical by hydrogen abstraction from toluene, which affords the radical coupling product with La@C
82. The same mono-adducts are also obtained by photoirradiation of La@C
82 in toluene in the absence of 1. These reactions are applicable to paramagnetic metallofullerenes, such as La@C
82 and Ce@C
82. Photoirradiation of La@C
82 in 1,2-dichlorobenzene in the presence of tetrachlorotoluene also affords the mono-adducts(
3a-
3d). Single-crystal X-ray structure analysis for
3d reveals its unique structure. Theoretical calculation confirms that the cage carbons having high spin densities are selectively attacked by radical species to form the singly bonded mono-adducts linked by a carbon-carbon single bond.
View full abstract
-
Masaaki Suzuki, Atsuhiro Osuka, Tyuji Hoshino, Saburo Neya
Session ID: B09
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
meso-Aryl substituted [26]hexaphyrins(1.1.1.1.1.1) (
26ArH) are higher homologues of porphyrin, bearing six pyrrole units in their macrocycles.
26ArH shows characteristic photochemical, electrochemical, and structural properties unlike those of porphyrins. When sterically unhindered aryl or alkynyl groups were introduced at the meso positions of
26ArH, conformational interconversion between Type-I and Type-II could be seen during the molecular thermodynamic processes. Such a conformational change allowed the reaction-active unsturated bonds to contact in a close proximity, resulting in formation of a vinylene- or an indene- intramolecular bridging structure.
View full abstract
-
Kei Kurotobi, Michihisa Murata, Yasujiro Murata
Session ID: B10
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, studies on functionalization of fullerenes have attracted much attention. Especially, that of open-cage fullerene derivatives by transformation of fullerene skeleton is very interesting for the synthesis of endohedral fullerenes and heterofullerenes. Herein, we report the synthesis of novel open-cage fullerene derivatives by functionalization at the rim of orifice.
View full abstract
-
Md.M. Rahman Badal, Masaaki Mishima
Session ID: B11
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
To obtain information on the transition structures for a two-step mechanism of reactions of ketenes with amine, involving a nucleophilic attack of amine to the C=O bond of ketene proceeding via an amide enol intermediate (or zwitterion), the substituent effects for respective processes of amination of phenylketene have been studied. The rate constants were measured using laser flash photolysis with time-resolved infrared detection of transients (LFP-TRIR).
The substituent effects for the second order rate constants of both processes were analyzed using the Yukawa-Tsuno equation. A value of 0.49 with an r value of 1.25 and a of 0.54 with an r of 1.61 were obtained for a nucleophilic attack of amine to the ketene and for the following reaction of the amide enol intermediate, respectively. The small values and remarkably large r values may be due to the duality of electronic interactions between substituents and reaction sites at the transition state.
View full abstract
-
Takao Okazaki, Toshikazu Kitagawa, Kenneth K. Laali
Session ID: B12
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Persistent carbocations derived from polycyclic aromatic hydrocarbons in superacid solutions were analyzed by NMR measurements. Their signals were assigned by 2D NMR techniques and positive charge delocalizations were estimated on the base of relative 13C chemical shift values to those of parent hydrocarbons. Electronic structures were also studied by the DFT method.
View full abstract
-
Kazunori Miyamoto, Takuji Okubo, Masahito Ochiai
Session ID: B13
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Report here for the first time is generation of labile cyclopentenyl cation from 1-cyclopentenylbromanes(III) 3 by thermal solvolysis, which relies on the hyperleaving group ability of hypervalent arylbromanyl(III) groups. Thermal solvolysis of 1-cyclopentenylbromanes(III) 3a,b at 20-50 C affords cyclopentanone, 1-chlorocyclopentene, (1-cyclopentenyl)halobenzenes, and 1-cyclopentenylhalogans(III) depending on the conditions, being the trapping products of 1-cyclopentenyl cation by the solvents and by the leaving group bromoarenes. Both deuterium labeling experiments and reaction kinetics strongly suggest an SN1 mechanism involving generation of cyclopentenyl cation.
View full abstract
-
Masashi Hojo, Tadaharu Ueda, Toshiyuki Nakano
Session ID: B14
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
We report the effects of metal and non-metallic salts on the solvolysis reaction rates of 1-adamantyl halides (S
N1) and hexyl halides (S
N2) in
N,
N-dimethylacetamide (DMA)-H
2O mixed solvents. The exponential increase in solvolysis rates of typical S
N1 substrates in the presence of alkali metal and alkaline earth metal perchlorates have been explained by the direct chemical interaction between the metal cations and the halide ions from the substrates at the activated states. Contrastingly, non-metallic salts (e.g. Et
4NClO
4) caused decreases in the S
N1 solvolysis rates in a good accordance with the distortion of water structure. In the presence of halide and tosylate salts, however, the solvolysis of an S
N2 substrate occurred after or at the same time of the anion exchange between the leaving group of a substrate and the anion of an added salt.
View full abstract
-
Nobuyoshi Yoshimura, Makoto Sato, Hiroshi Yamataka
Session ID: B15
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Anomalous relation between rates and equilibrium for the proton-transfer reactions of nitroalkanes is known as nitroalkane anomaly. In a typical example, an anomaly was illustrated by larger-than-unity Br?nsted a coefficients of 1.3-1.5 for the reactions of XC6H4CH2NO2 with various bases. Recent experiments have revealed that aci-nitro species is involved in deprotonation-protonation reactions of nitroalkanes. In the present study, detailed analyses of various phases of the reactions of XC6H4CH2NO2 were carried out computationally, which showed that the reaction is more complex than normally thought.
View full abstract
-
Daisuke Kaneno, Yasumitsu Suzuki, Shuji Tomoda
Session ID: B16
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
LiAlH
4 reduction of acyclic carbonyl compounds possessing a heteroatom at the a-position (R = Me) give
R*,
S*-diastereoisomers predominantly via
Re attack. These diastereoselectivities are interpreted in terms of the chelation model, where Li interacts with both oxygen atoms of the substrate ketones. However, stereochemical reversal is observed if a large substituent, such as aryloxy group, is introduced at the a-position (R = Aryl). Theoretical calculations at the B3LYP/6-31+G(d,p) level on the reduction of a-aryloxy ketones with LiAlH
4 assuming the chelated transition state could not reproduce the reversal of stereoselectivity. Therefore calculations were performed using the non-chelated transition state models with a few solvent molecules (dimethyl ether). It was found that two solvent molecules coordinate with lithium ion throughout the reaction and that they sterically prevent the formation of the chelated transition structure. Optimized transition structures with two dimethyl ether molecules at the Li nicely reproduced the observed data.
View full abstract
-
Koichi Ohno, Satoshi Maeda
Session ID: B17
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently developed Scaled Hypersphere Search Method was applied to finding the lowest transition state leading to selection of the chirality's of the hydrogenation product of CH3COCH2COOCH3 by (R)-RuHCl-BINAP. Lower transition structures (TS) were systematically searched on the quantum chemical potential energy hyper-surface with a huge dimension of 285. Among 38 TS of R-Type and 10 TS of S-type, the lowest one is an R-type which was found to be considerably lower than those of S-type, which indicates the excellent ability of RuHCl-BINAP in the asymmetric synthesis.
View full abstract
-
Zheng XU, Zhifang LI, Guoqiao LAI,, Jianxiong JIANG, Huayu QIU, Mitsuo ...
Session ID: B18
Published: 2008
Released on J-STAGE: May 18, 2009
CONFERENCE PROCEEDINGS
FREE ACCESS
A systematic DFT study of the substituent effects on the transition structures and energies of the insertions of a silylene into Si-X bonds of YZ
2Si-X (X = H, Cl) via three-membered cyclic transition states has disclosed important electronic and steric factors to determine the rates and selectivity. The migrating atom X affects not only n(Si)-sigma*(Si-X), ppai(Si)-sigma(Si-X), and ppai(Si)-n(X) interactions but also the geometry of the three-membered ring TSs, and in turn, the steric effects between the silylene and the secondary substituents Z. Electronegative Y located in the same plane with the three-membered ring accelerates the insertions through the n(Si)-sigma*(Si-Y) interaction, while the extent depends on X and Z.
View full abstract