-
Keisuke KITAJIMA, Kazuki DEMACHI, Takamichi AOKI, Tomoki OGOSHI, Tada- ...
Session ID: A01
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
A series of pillar[5]arene derivatives with alkyl groups of different length were synthesized. The conformational characteristics of the pillar[5]arene derivatives were investigated by dynamic
1H NMR measurements. As the length of alkyl chains become long, the motion of pillar[5]arene derivatives was suppressed. We synthesized novel [2]rotaxane consisting of C0 as a wheel and viologen derivative as a axle. We will also describe conformational characteristics of C0 in the [2]rotaxane.
View full abstract
-
WATARU SETAKA, KENTARO YAMAGUCHI
Session ID: A02
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Much attention has been focused on the chemistry and properties of molecular machines, in which mechanical motions of parts of the molecules are observed. Novel framed molecular gyro-tops having a phenylene rotator encased in three long alkenyl and alkyl-spokes were synthesized as novel molecular machines. Rapid rotation of the phenylene rotator of the molecular gyro-tops was confirmed by solid-state
2H NMR spectroscopy, which showed changes in the optical properties of a single crystal, specifically, the thermodynamic birefringence modulation. These results are the first application of the dynamic state of molecular motion in a crystal causing an optical change.
View full abstract
-
Hirohito Tsue, Kohei Ono, Satoshi Tokita, Koichi Ishibashi, Kazuyuki M ...
Session ID: A03
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Nitrogen-bridged calixarene analogues (azacalixarenes) have recently emerged as a new family of calixarene, and their intriguing complexation behaviors based on the introduction of nitrogen atoms as the bridging units have thus far been disclosed. To shed a light on solid-state complexation by azacalixarenes, we have newly synthesized azacalix[5]arene pentamethyl ether. As an eventual outcome, rapid and selective adsorption of CO
2 among the four main components of the atmosphere was observed on the crystalline powder of the azacalix[5]arene under ambient condition. The synthesis, molecular and crystal structures, and solid-gas adsorption behavior of the cyclic pentamer will be discussed.
View full abstract
-
Masamichi Yamanaka, Hirofumi Adachi, Naoaki Toyoda, Kenji Kobayashi
Session ID: A04
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Multifunctionalized cavitand 1 was synthesized in six steps from a tetrabromo cavitand. A 1:1 mixture of 1 and Pd(dppp)(OTf)2 or Pt(dppp)(OTf)2 in CDCl3 or CD2Cl2 quantitatively self-assemble into a supramolecular hybrid capsule through hydrogen bond and metal-ligand coordination bond. Cavity of the capsule is filled with triflate ion and penetrated alkyl groups of ureides. The capsule encapsulates a neutral guest molecule such as 4,4'-diiodobiphenyl with the assistance of an anion. Encapsulation complex was identified by NMR technique. The kinetics of guest exchange is controllable by the polar solvent. Furthermore, remarkable differences of the guest exchange kinetics were observed according to the phosphine ligand on the metal complex.
View full abstract
-
Saburo Neya, Takashi Matsugae, Masaaki Suzuki, Tyuji Hoshino
Session ID: A05
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
A novel pyridine-containing porphyrin isomer was synthesized by a metal-templated reaction in the presence of Ni
2+ ion and was given a trivial name "pyricorrole". UV-visible absorption spectrum of pyricorrole Ni(II) complex (
PyC-Ni) exhibited Soret-like and Q-like absorption bands, while
1H NMR spectrum of
PyC-Ni was suggesting existence of the aromatic ring current effect by the 18π electronic circuit, judged from the downfiled shifts of the signals due to the peripheral protons. X-ray crystal structure of
PyC-Ni revealed its planar framework with a mean-plane deviation of 0.032 Å and small displacement of Ni atom form the mean plane (0.002 Å). The bond lengths between the Ni and N1-4 atoms were 1.975, 1.875, 1.794, and 1.851 Å, respectively, indicating that the narrow inner cavity of pyricorrole was comparable to that of corrole. Therefore, pyrricole can be an interface molecule between porphyrin and corrole.
View full abstract
-
Ken-ichi Yamashita, Kazuyuki Kataoka, Shouichi Takauchi, Motoko Asano, ...
Session ID: A06
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Whereas an increasing number of the procedures for the
meso-functionalization of the porphyrin have been reported, many of them require an activation of the porphyrin core or an addition of suitable transition metal catalysts. In this presentation, we present a simple procedure for the introduction of N-containing functional groups into the porphyrin core through nucleophilic substitution reaction. For example,
meso-azidoporphyrins are obtained almost quantitatively by simple treatment of
meso-bromoporphyrins with azide anion in aprotic polar solvents without any additives. Ni complexes are moderately stable, and can be isolated, whereas freebase
meso-azidoporphyrins are thermally less stable, and smoothly decomposed into corresponding
meso-aminoporphyrins as a main product. A treatment of an excess of azide anion and sodium ascorbate to
meso-bromoporphyrin affords
meso-aminoporphyrin almost quantitatively.
View full abstract
-
Chihiro Maeda, Naoki Yoshioka, Atsuhiro Osuka
Session ID: A07
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Carbazole, an analogue of pi-expanded-pyrrole, can be regarded as a part of porphyrin, which reminds us doubly-pyrrole-bridged carbazole dimer would be a new porphyrinoid. Here we report the synthesis of pyrrole/thiophene-bridged cyclic carbazole dimer and trimer through the annulation reaction as a new synthetic strategy of porphyrinoids. Glaser coupling reaction of diethynylcarbazole 1 provides butadiyne-bridged carbazole dimer 2, trimer 3, and tetramer 4. Their structures are examined by NMR, absorption, fluorescence spectroscopy, and X-ray diffraction analyses. The X-ray crystal structures of 2 and 3 reveal their unique bent-shape macrocycles. Cu(I)-mediated annulation reaction of 2 and 3 with amines provide pyrrole-bridged cyclic carbazole dimer 5a-5c and trimer 6 which exhibit high fluorescence quantum yields. Annulation reaction of 2 with sodium sulfide provides thiophene-bridged carbazole 7. 7 can be oxidized to 8 which exhibits distinct aromaticity and NIR absorption bands.
View full abstract
-
Yohei Haketa, Hiromitsu Maeda
Session ID: A08
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Boron complexes of dipyrrolyldiketones as pi-conjugated acyclic anion receptors can be considered as essential building units to form supramolecular structures. Covalent linkage of the anion receptors affords the versatile oligomer systems that exhibit the anion-driven dynamic conformation changes and assembling behaviors. Meta-phenylene-bridged oligomers construct various helical structures, such as [1 + 1]-, [1 + 2]-, and [2 + 2]-type complexes, depending on the numbers of receptor units and terminal substituents. On the basis of the [1 + 1]-type single helix, acyclic dimer can be transformed to a macrocyclic structure with an extremely high anion-binding affinity.
View full abstract
-
Yuki Hasegawa, Masaki Yamamura, Shigehisa Akine, Tatsuya Nabeshima
Session ID: A09
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
In this study, we report the synthesis and guest recognition ability of novel platinum(II) cleft hosts containing dipyridoacridine platinum(II) complexes or terpyridine platinum(II) acetylide complexes. The former cleft host showed a higher affinity to electron-rich aromatic guests such as catechol. Guest recognition of the cleft host was evaluated by
1H NMR and UV-Vis titration experiments. The
1H NMR titration indicated that upfield shifts of the dipyridoacridine protons and downfield shifts of the protons inside the spacer were observed upon complexation with catechol. The association constant (
Ka, CD
3CN) at room temperature was estimated to be 2500 ± 600 M
-1. In UV-Vis titration, CT bands appeared owing to interaction between the cleft host and catechol. The acetylide cleft host also showed a high affinity toward planar anionic aromatic guests. For example,
1H NMR titration suggested that 1-pyrenecarboxylic acid interacted with the cleft host in the presence of DBU as a base. The estimated association constant (
Ka, CD
3CN : CDCl
3 = 1 : 3) at room temperature is 540 ± 60 M
-1.
View full abstract
-
Zhiou Li, Norifumi Kishi, Michito Yoshizawa, Munetaka Akita
Session ID: A10
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Fluorescent molecules have widespread applications from fundamental sciences to practical materials and devices. Among them, molecular hosts with fluorescent properties have recently attracted much attention as potential chemosensors, biological probes, and light-emitting diodes. Here we report novel highly fluorescent coordination capsules consisting of anthracene frameworks. Upon simply treatment with M(II) ions (M = Ni, Pd, Pt, and Zn), anthracene-containing bis-monodentate ligands are quantitatively self-assembled into M(II)-linked coordination capsules with ca. 1.5 nm outer diameter. It is noteworthy that the Zn(II)-linked capsule shows strong blue fluorescence by UV-light irradiation, which is in contrast to isostructural Pd(II)-, Pt(II)-, and Ni(II)-linked capsules that show non or weak emissions.
View full abstract
-
Junji IWASA, Qing-Fu SUN, Sota SATO, Makoto FUJITA
Session ID: A11
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Previously, we reported about the construction of M6L12 and M12L24 coordination spheres. Here, we report the self-assembly of giant, M24L48 coordination spheres from 24 metal ions (M) and 48 curved bridging ligands (L). Because of the self-assembled critical structure of this multi-component system is highly sensitive, even a slight change of the mixed-ligand ratio critically switches the final structure between M24L48 and M12L24 coordination spheres.
View full abstract
-
Sekai IWAMA, Rajesh GONNADE, Yuko MORI, Hiroki TAKAHASHI, Hirohito TSU ...
Session ID: A12
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We report that the 1:1 co-crystal of (DL)-phenylalanine and fumaric acid shows the "preferential enrichment" phenomenon upon recrystallization from water. Recrystallization of the DL-co-crystals led to a remarkable alternating enrichment of the two enantiomers up to 85% ee in the mother liquor, together with slight enrichment(< 6% ee) of the opposite enantiomer in the deposited crystals. The mechanism of this "preferential enrichment" phenomenon is rationalized on the basis of i) the comparison of solubility between the DL-co-crystals and the L-ones, ii) the observation of polymorphic transition during crystallization by in situ ATR-FTIR spectroscopy, iii) the characterization of deposited crystals by powder X-ray diffraction and DSC measurements.
View full abstract
-
Kenta Furuyoshi, Yoshimasa Makita, Shinichi Fujiwara, Akiya Ogawa
Session ID: A13
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Molecular capsules have been of much interest to chemists because they provide a reaction field within the space. Hemicryptophane is composed of a catalytic site and suitable binding pocket for a particular substrate in the internal space. In this study, a Verkade's base-included hemicryptophane, which has a phosphorous centre embedded in the cavity, was synthesized. The reaction of hemicryptophane 1 with dimethylaminochlorophosphine furnished phosphonium salt-included hemicryptophane 2 in 62% yield. The structure was confirmed by NMR and ESI-MS. Addition of t-BuOK into the DMSO solution of 2 resulted in Verkade's base-included hemicryptophane 3.
View full abstract
-
Fumihiro Kayamori, Hajime Abe, Masahiko Inouye
Session ID: A14
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Ethynylpyridine oligomers covalently linked with saccharide templates were studied. The oligomers form chiral helical structures by intramolecular hydrogen bonds. To fix the helical structures, alkene side chains were introduced on the pyridine rings of the oligomers. By Grubbs reaction at these alkene side chains, the pitches of the helix could be bridged. Subsequently, the saccharide template was removed by the use of an acidic resin. The change of the retention time on GPC analyses suggested that the molecular size of the substrate decreased after the reaction. In addition, the CD activity was retained for the substrate, indicating that the chirality was retained even after removal of the saccharide template.
View full abstract
-
Kengo Ohkubo, Hiroyuki Hayashi, Satoru Karasawa, Noboru Koga
Session ID: A15
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Supramolecular assemblies exhibiting external-stimuli responsiveness such as heat, pH, light, pressure, etc. have been collecting many interests as a new type of functional material. We reported that the C3 symmetrical framework of benzene-1,3,5-trisurea derivatives (EgmCnU) having oligo(ethylene glycol) (Egm) and alkyl chains (Cn) showed a lower critical solution temperature (LCST), which is a temperature becoming from clear solution to turbid by decreasing the solubility on warming. Interestingly, we also found that the difference of only one methylene unit in the alkyl chains changed to the formations of nanoparticles above the temperature of LCST, drastically. Furthermore, EgmCnU derivatives with tertiary amine (EgmNCnU) showed LCST behaviors highly sensitive pH dependence. This time, analogous compounds having the side chains inserted the amino-TEMPO and alkylamine units between alkyl and ethylene glycol chains were prepared as a precursor for the functional assembly. In this presentation, we will report the preparation of functional small-sized molecules, the formation of pH responsive nanoparticles with LCST, and their magnetic behaviors.
View full abstract
-
Jun Nakamura, Tetsuo Okujima, Noboru Ono, Kiyomasa Hosokawa, Kengo Suz ...
Session ID: A16
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
The fluorescence spectra of the free-base acenaphthoporphyrins(AceP) in CHCl
3 showed two emission bands at 660 and 720 nm, whose intensity drastically changed depending on the excitation wavelength. This fact indicates that two electronic structures exist in the free-base AcePs and their fluorescence is emitted from one structure in higher quantum yield (λ
em 660 nm) and from the other in lower yield (λ
em720 nm), respectively.
View full abstract
-
Tomohiro Higashino, Takahiro Miura, Shohei Saito, Atsuhiro Osuka
Session ID: A17
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Recently, 4npi Möbius aromaticity of singly twisted pi-conjugated compounds as Möbius strip has attracted much attention. However, (4n+2)pi Möbius antiaromaticity remains unexplored. Here, we report hexaphyrin phosphorus complexes that are characterized as the Möbius antiaromatic compounds from NMR investigation and X-ray diffraction analysis.
View full abstract
-
Atsuro TAKAI, Claude P. GROS, Jean-Michel BARBE, Shunichi FUKUZUMI
Session ID: A18
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
A trefoil-like porphyrin trimer linked by triphenylamine (TPA-TPZn3) was synthesized. Three-electron oxidation of TPA-TPZn3 forms radical trication (TPA-TPZn33+) where each porphyrin ring is one-electron oxidized. The radical trication TPA-TPZn33+ is spontaneously dimerized to afford (TPA-TPZn3)26+ in CH2Cl2. The characteristic charge-resonance band due to the charge delocalization over the pi-system of (TPA-TPZn3)26+ was observed in NIR region. The initial oxidation potential of TPA-TPZn3 is negatively shifted relative to that of corresponding monomer porphyrin, which results from the stabilization of the oxidized state of TPA-TPZn3 associated with the dimerization. The thermodynamic parameters for the formation of (TPA-TPZn3)26+ were determined by measuring UV-vis-NIR spectra at various temperatures. The formation constant of (TPA-TPZn3)26+ is significantly larger (e.g. over 200-fold at 263 K) than that of the radical cation dimer of corresponding monomer porphyrin. The electronic states were investigated using EPR and 1H NMR spectroscopies. The synergistically enhanced dimerization of TPA-TPZn33+ results from the multiple pi-bond formation between porphyrin radical cations.
View full abstract
-
Takeshi Sakano, Kenji Higashiguchi, Kenji Matsuda
Session ID: A19
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We focused on tetraphenylporphyrin rhodium chlorides (TPPRhCl) coordinated by 4-phenylpyridine and evaluated the effect of twisting angle of 4-phenylpyridine on molecular conductance by using STM. Both self-assembled monolayers of normal 4-phenylpyridine-coordinated TPPRhCl with four triacontyl groups (
C30-Rh-normal) and twisted 4-(2,6-dimethylphenyl)-3-methylpyridine-coordinated TPPRhCl with four docosyl groups (
C22-Rh-twisted) were observed as two different lamellar domains with different lattice constant at the same HOPG-octanoic acid interface in constant current mode. The larger and smaller lattices were attributed to
C30-Rh-normal and
C22-Rh-twisted, respectively. Apparent height analysis of both domains was separately performed. Statistical analysis revealed that the apparent height of
C30-Rh-normal is higher than that of
C22-Rh-twisted. The difference of apparent height reflects the difference of molecular conductance between normal and twisted 4-phenylpyridine.
View full abstract
-
Takeharu Haino, Eri Hirai, Yoshihisa Fujiwara, Kouki Kashihara
Session ID: A20
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Homoditopic host 2 selectively encapsulates C60 moieties grafted onto a polymer chain. Furthermore, this process creates a remarkably stable cross-linkage, leading to an increase in molecular weight and unique morphological changes to the polymer in its solid state. The stability of the supramolecular cross-linkage directly depends upon the solvent; thus, the supramolecular cross-linkage of the polymer can be reversibly regulated by the solvent system. The macroscopic solid-state morphologies of poly-1b are highly influenced by supramolecular cross-linking. Nanoparticle-like morphologies are probably favored by the immiscible nature of the C60 moiety, but supramolecular cross-linking makes the polymer bundle together to form uniform fibrils. These, in turn, align into a well-oriented 2D array on a HOPG surface.
View full abstract
-
Yuji Nakatani, Yoshio Furusho, Eiji Yashima
Session ID: A21
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
An optically-active amidine and an achiral carboxylic acid with a
m-terphenyl ligand bearing two olefin units at the both ends were prepared. When mixed in organic solvents, they quantitatively formed a supramolecular duplex, or precatenane, through salt bridge formation. The precatenane was subjected to ring-closing metathesis, and the corresponding [2]catenane was obtained in 68% yield after chromatographic purification. Moreover, we confirmed by CD and
1H NMR that the formation and dissociation of the salt bridge between the two macrocyclic components could be completely controlled by the addition of an acid or a base.
View full abstract
-
Yasuhiro Kohsaka, Kazuko Nakazono, Yasuhiro Koyama, Toshikazu Takata
Session ID: A22
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
To rotaxanes, consisting of dibenzo-24-crown-8-ethers as wheel components and sec-ammonium salts as an axle components, were introduced end-capping groups with complementary size to crown ether. While the rotaxane with cyclohexyl end-cap was unstable to solvation DMSO (t1/2 = 2.6 h), the rotaxanes with t-Bu or 4-t-BuC6H4 groups as the end-caps were sufficiently stable to maintain the structure in DMSO. However, even they were gradually dissociated via a counter anion exchange of ammonium salts with Bu4N+F-. The well-controlled stability and degradability of rotaxanes were applied to the creation of recyclable polymer materials: The polyrotaxane networks were synthesized from poly(crown ether)s and sec-ammonium salts with the end-capping group whose size are complementary to crown ether. The obtained gels showed similar reactivity to that of rotaxanes mentioned above. The polyrotaxane network with t-Bu or 4-t-BuC6H4 end-cap groups maintained the cross-linked structure in various organic solvents, but was easy to be decross-linked by the addition of Bu4N+F–. The decross-linked polymer gave a transparent solution, from which the trunk polymer was obtained by reprecipitation in CH3OH. The dissociation reaction of the rotaxanes would provide the efficient concept to design chemically recyclable polymer materials.
View full abstract
-
Hiroyoshi Sugino, Hidetoshi Kawai, Takeshi Umehara, Kenshu Fujiwara, T ...
Session ID: A23
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We have previously reported a novel rotaxane synthesis based on imine-bond formation between an axle molecule and a ring molecule, for which we can control the subunit mobility through reversible inverconversion between imine-bridged rotaxane and [2]rotaxane. Here we report structural effects of axle and ring units on mobility control, and preparation of [n]rotaxanes by degenerating the imine-bridged pseudorotaxane.
View full abstract
-
Michio Yamada, W. Bernd Schweizer, Franziska Schoenebeck, Francois Die ...
Session ID: A24
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We recently reported the synthesis of push–pull-chromophore-fullerene conjugates by [2+2] cycloaddition of electron-deficient olefins to the electron-rich CC triple bond of donor-substituted alkynylfullerene, followed by cycloreversion. During this work, we encountered an unprecedented thermal rearrangement yielding the formation of C1-symmetric 1,2,9,12-tetrakis-adducts of [60]fullerene, which will be presented.
View full abstract
-
Satoru Sato, Yutaka Maeda, Koji Inada, Hidefumi Nikawa, Michio Yamada, ...
Session ID: A25
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
The regioselective functionalization of La@C
82 by two different groups was conducted. We chose 1,2,3,4,5-pentamethylcyclopentadiene (Cp*) and adamantylidene carbene (Ad:) as reactants because cyclopentadiene and Ad: afford the corresponding regioselective mono-adducts of La@C
82, respectively. In this work, structural analysis of the mono-adduct of La@C
82 with Cp* was also performed. The bis-adducts of La@C
82 with Cp* and adamantylidene were synthesized by two different route and characterized. Furthermore, the addition position and the regioselectivity in functionalization of La@C
82 derivatives were also discussed on the basis of spectroscopic analysis and theoretical calculation.
View full abstract
-
Koji Harano, Akimitsu Narita, Eiichi Nakamura
Session ID: A26
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Bilayer vesicles made of amphiphilic fullerene Ph
5C
60K were photochemically modified in two ways, that is, post- and pre-modification to control membrane properties. Post-modification by UV irradiation to preformed vesicles reduced the water permeability due to photoaddition reactions in the fullerene membrane. On the other hand, pre-modification via photomodification of Ph
5C
60K prior to the vesicle formation enhanced the water permeation by photooxidation of the fullerene cores. Thermodynamic analyses of the water permeation by using the Eyring equation indicated the tightening and loosening of the membrane packing upon the post- and the pre-modification, respectively.
View full abstract
-
Yuta Morinaka, Michihisa Murata, Yasujiro Murata, Osamu Yoshikawa, Tak ...
Session ID: A27
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Previously, we have synthesized a cage-opened fullerene C
60 derivative (1) with a 13-membered-ring opening and introduced one molecule of H
2 into the cage. The compound (1) has two carbonyl groups directly connected to the fullerene pi system. Thus, we studied addition of methyl Grignard reagent to (1). Upon treatment of (1) with 5.0 equivalents of MeMgCl in THF at -20 .degree.C, 1,2-addition to one of the two carbonyl groups took place to give alcohol derivative (2) in 46% yield. Furthermore, in the presence of 2.9 equivalents of
p-toluenesulfonic acid, (2) underwent transannular isomerization in toluene at 110 .degree.C to afford ether derivative (3). The results of the electrochemical investigations demonstrated decreased electron affinity of (2) and (3) in accord with their elevated LUMO levels predicted by the DFT calculations. The performance of a solar cell device composed of (3) as an acceptor and poly-3-hexylthiophene (P3HT) as a donor was investigated.
View full abstract
-
Yuichi Yoshimoto, Hiroshi Ikeda, Kazuhiko Mizuno
Session ID: A28
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We investigated fluorescence properties of diaroylmethanatoboron difluorides in the solid state. As a result, it is suggested that the fluorescent domains in the solid state are not only excited monomer but also excimer and "multimolecular excited complex". Moreover, it is found that intermolecular interactions in the solid state depend on the bulkiness of substituent on phenyl groups. Thus, it was indicated that the steric substituent effects control a major fluorescent domain in the solid state. In addition, we will discuss fluorescence mechanochromism in the solid state.
View full abstract
-
Kenji Kobayashi, Ryota Ozawa, Yohei Hirumi, Kengo Suzuki, Daiki Onuma, ...
Session ID: A29
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Anthracene and its derivatives under photoirradiation conditions undergo photodimerization and/or photooxygenation, which are disadvantageous for uses related to optoelectronics. For the purpose of the protection of anthracene derivatives against photochemical reactions, we report two methodologies: (1) double alkyl-strapped 9,10-diphenylanthracenes and (2) encapsulations of 2,6-diacetoxyanthracene and 2,6-diacetoxy-9,10-bis(p-tolylethynyl)anthracene in a self-assembled boronic ester cavitand capsule.
View full abstract
-
Toshiki MUTAI, Hideaki SHONO, Tatsuya OHKAWA, Haruhiko TOMODA, Koji AR ...
Session ID: A30
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
6-Aryl, 8-aryl and 6,8-diaryl derivatives of 2-(2'-hydroxyphenyl)imidazo[1,2-a]pyridine (1) have been synthesized and properties of their fluorescence/luminescence properties are studied in THF solutions and in the solid state. While most of them show weak dual fluorescence due to direct and ESIPT processes in solution, they exhibit an efficient, blue-shifted ESIPT luminescence in the solid state. The luminescence quantum yield is 10 times larger compared to those observed in solution. Comparing substituents and substituted positions, the luminescence appears at longer wavelength in the order of methoxy, phenyl, then ester, and 6-, 8-, then 6,8-substitution, respectively.
View full abstract
-
Shunpei Hitosugi, Waka Nakanishi, Yusuke Shimada, Anna Piskareva, Hide ...
Session ID: A31
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We designed an anthracene derivative, disilanyl double-pillared bisanthracene (
SiDPBA) as a new structural motif for carrier transport material for OLEDs. We synthesized
SiDPBA in a one-step operation from 1,8-diiodoanthracene in 50% yield. The anti-geometry of the anthracene units in
SiDPBA was revealed by X-ray crystallographic analysis. The device performance of
SiDPBA as a carrier transport material was evaluated by employing a standard green phosphorescent OLED. The standard device with Alq
3 in electron transport layer (ETL) and a-NPD in hole transport layer (HTL) showed external quantum efficiency (EQE) of 3.4% at the constant current of 2.5 mA/cm
2, whereas the device with
SiDPBA in ETL showed superior performance of EQE = 11.0%. Notably,
SiDPBA functioned not only as an electron transporting material but also as a hole transporting material, and the device with
SiDPBA both in ETL and HTL effectively operated at EQE of 8.7%.
View full abstract
-
Iwao Okamoto, Yusuke Takahashi, Mika Sawamura, Nobuyoshi Morita, Osamu ...
Session ID: B01
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We have found that
N-methylation of aromatic amide caused its conformation cis-favored, and this conformational change influenced the function of the molecules as observed in retinoidal amide. In order to control the conformation and function with outer stimuli, we designed aromatic amide as conformational alteration unit bearing redox-responsive hydroquinone -
p-quinone system on the amide nitrogen. We synthesized
N,
N-diaryl amides
1 -
3 and investigated the conformational preference. The reduced forms
1 and
2 favor (
E)-amide conformation, whereas the oxidized forms
3 favor (
Z)-form. These conformational preferences are consistent with relative intensity of repulsion between aromatic π-electron and carbonyl oxygen lone pair. We also found the response in optical properties of
2 -
3c and
2 -
3d, according to the change of conformational preference.
View full abstract
-
Takumi Kato, Hirokazu Kamine, Masaaki Sato
Session ID: B02
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Unsymmetrically substituted quaterthiophenes with two terminal ferrocenyl groups were prepared as long pai-conjugated systems. Anisotropy of the electronic structures of the pai-conjugated systems was introduce by substitution of methoxy and hexyl groups. Electrochemical and spectroscopic studies were carried out to evaluate the oxidation states of the pai-conjugated systems and interaction between the two terminals. The ferrocenes at both ends of 1 are interactive, but these of 2 are not interactive. These results show that the influence of substituted groups on the oxidation state of pai-conjugated systems is important.
View full abstract
-
Shin-ichi TAKEKUMA, Manami YAMAMOTO
Session ID: B03
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Wittig reaction of (
E)-2-(3-guaiazulenyl)ethylene-1-carbaldehyde (
5) with (3-guaiazulenylmethyl)triphenylphosphonium bromide (
3) in ethanol in the presence of sodium ethoxide at 25
oC for 48 h gives the target extended -electron system, (2
E,4
E)-1,4-di(3-guaiazulenyl)-1,3-butadiene (
6), in 33% isolated yield. The title studies: namely, a facile preparation and crystal structure as well as spectroscopic, chemical, and electrochemical properties of
6 are discussed in detail.
View full abstract
-
Eisuke OHTA, Hiroyasu SATOH, Shinji ANDO, Atsuko KOSAKA, Yuki SUNA, Ta ...
Session ID: B04
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
o-Phenylene is an elusive class of pi-conjugated polymers due to its synthetic difficulty. We have successfully established the method for synthesis of long-chain o-phenylene oligomers and polymers. The structures and dynamic behaviors of the new pi-system will be discussed.
View full abstract
-
Hiroki Ito, Satoshi Ogawa, Noriyuki Yoshimoto, Hiroki Muraoka, Kazuaki ...
Session ID: B05
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We attempt to construct of novel
π-extended planar molecules applied to p-type organic semiconductor materials for OFETs. Distyrylthiophene derivatives having styryl groups as
π-extended fragments were synthesized by Wittig reactions. The electrochemical properties of the distyrylthiophene derivatives were furnished by cyclic voltammetric studies. The voltammograms of all compounds showed one electron oxidation waves derived from the corresponding radical cation species. Target molecules having styryl groups bound to fused thiophene cores showed surface carrier density in the OFETs.
View full abstract
-
Tomohiro Fujita, Jyun-ichi Nishida, Yoshihide Fujisaki, Shizuo Tokito, ...
Session ID: B06
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Novel donor-accepter type organic semiconductors composed of diazaborole and quinone units have been synthesized by the reaction of boronic acids with diamino groups. They are planar molecules and showed amphoteric redox properties owing to the quinone unit and amino groups. Compounds 1a, 2 and 3 exhibited good electron mobilities ( ~ 10-2 cm2/Vs ) in the FET devices ( bottom-contuct ). The XRD measurements show the formation of crystalline films.
View full abstract
-
Koji Nakano, Natsuko Chayama, Kyoko Nozaki
Session ID: B07
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
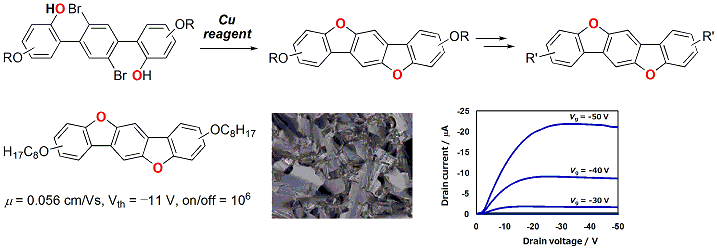
Efficient synthesis of dibenzo[d,d']benzo[1,2-b:4,5-b']difuran (DBBDF) derivatives was achieved by using copper-mediated
carbon−oxygen bond-forming reaction. The effects of the substituents on their physical properties and their packing structure were investigated. In particular, DBBDF derivatives with linear alkyl or alkoxy chains in their long molecular axes were found to be liquid-crystalline molecules. The DBBDF derivatives can be applied to organic semiconducting materials for organic field-effect transistors (OFET).
View full abstract
-
Toshihiro Okamoto, Katsumasa Nakahara, Hideyuki Tanaka, Joon-Hak Oh, H ...
Session ID: B08
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We have introduced aryl and perfluoroaryl groups as substituents into a series of fused acene core at several central positions. From the single crystal analysis, it is found that the aryl-perfluoroaryl interactions have successfully played important role in the induction of pi-pi interaction among acene core. The OTFT measurement reveals that
FPPAs exhibited much higher hole mobilities than
PPAs which do not have any aggressive interaction. This result strongly demonstrates that aryl and perfluoroaryl system could open up general and versatile strategy to control the molecular ordering, resulting in the creation of new semiconducting materials with high charge mobilities.
View full abstract
-
Tsuyoshi Murata, Yuki Kanzaki, Daisuke Shiomi, Kazunobu Sato, Takeji T ...
Session ID: B09
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Trioxotriangulene (TOT) is a neutral pi-radical having 25-pi electronic system established by oxo-substitution and two-dimensional pi-extension of phenalenyl system. The
tert-butyl derivative TOT(
t-Bu)
3 is highly stable under air, and exhibits intriguing functions based on the multi-stage redox ability and self-assembling ability. The chemical modification on TOT system is a promising chemical approach to control the electronic and structural properties, and to demonstrate new functions. In the present study, we have designed and synthesized halogenated derivatives of TOT, and their physical properties were investigated focusing on the substituent effects by means of electrochemical, structural, and magnetic measurements.
View full abstract
-
Shin-ichi Fuku-en, Tomoyuki Yano, Hiroyuki Higashikawa, Ryo Kawamura, ...
Session ID: B10
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
During our attempts at the synthesis of stable triplet carbenes from allene
1 bearing two acridene skeletons, we found that the oxidation of
1 gives a product of interesting properties. Allene
1 was prepared from 3-iodoanisole and 3-anisidine in 4 steps in 16% overall yield. The oxidation of
1 by aminium salts resulted in a color change of the solution (to dark purple) and afforded an unexpected species as the main product. The structure of the product was confirmed by X-ray analysis to be that of a dimer with demethylation of one of the four methoxy groups. DFT calculations indicated that the dimer has open-shell singlet biradical character. The synthesis and properties of
1 and the dimer will be discussed.
View full abstract
-
Toru Saito, Akihito Konishi, Yasukazu Hirao, Kozo Matsumoto, Hiroyuki ...
Session ID: B11
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
10-(9-anthryl)anthroxyl radical was prepared and was isolated in a crystalline form. This species were found to be stable in air and could be purified by silica-gel column chromatography. X-ray crystallographic analysis revealed that the radical has a short C-O bond of 1.242(3) Å, suggesting appreciable contribution of the structures containing the C=O double bond to the ground state. In addition to the isolation of the anthroxyl radical, a solution containing both the radical and the alcohol precursor afforded brown single crystals of a hydrogen-bonded complex consisting of radical/alcohol (1:1). X-ray crystallographic analysis at 100 K revealed that a hydrogen atom locates on one side of the O-H-O hydrogen bond. The complex can be regarded as an analogue of the intermediate in phenoxyl/phenol hydrogen self-exchange reaction.
View full abstract
-
Yuki Morita, Naoto Nagaya, Yuji Shibata, Kenkichi Sakamoto, Mitsuo Kir ...
Session ID: B12
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Nanocrystals of several oligosilanes were fabricated using a reprecipitation method. The water dispersions of nanocrystals obtained were stable for several weeks. The average particle size was investigated by SEM of a filtrate sample and found to be 32 nm for permethyldecasilane. Powder XRD patterns showed the nanocrystals and bulk-crystals of oligosilanes had almost similar molecular and crystal structures. The electronic absorption bands of nanocrystals in water were substantially narrow and red-shifted. This behavior shows the main chains of oligosilanes were regulated to be all-anti conformation by the nanocrystallization process. Moreover, we found significant excitonic interaction occurred among the oligosilane molecules on the basis of fluorescence studies.
View full abstract
-
Takaya Matsuura, Soichiro Kyushin
Session ID: B13
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
We carried out theoretical calculations of molecular orbitals of ladder polysilanes and ladder polygermanes, and discuss transition and transition moments in UVvisible spectra. The lowest energy absorption bands were found to be due to the transition moments generated by the double helix structure.
View full abstract
-
Koh SUGAMATA, Takahiro SASAMORI, Norihiro TOKITOH
Session ID: B14
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
Aryltellurenyl(II) halides, RTeX, are known to be highly reactive, and there hace been only a few examples of their isolation as stable compounds. We report hare the synthesis and isolation of ArTeX (X = halogen) and ArTeX
3 bearing a Bbt group as a steric protection group. The reactivity of the obtained tellurennyl halides will be described.
View full abstract
-
Yuki Yamaguchi, Norio Nakata, Akihiko Ishii
Session ID: B15
Published: 2011
Released on J-STAGE: March 29, 2012
CONFERENCE PROCEEDINGS
FREE ACCESS
3-Methylene-2,3-dihydrothiophene derivative
2 was synthesized by the thermal reaction of disulfide
1 with DMAD in the presence of 2 equivs. of Pd(PPh
3)
4. The structure of
2 was determined by NMR, IR, and X-ray crystallography. The UV/vis spectrum of
2 in CH
2Cl
2 showed the longest absorption maximum at 359 nm (ε = 7400). In the fluorescence spectrum,
2 exhibited an intense emission at 488 nm with a large Stokes shift (7360 cm
-1), and the quantum yield (Φ
F) of
2 was 0.91, which is much larger than that of selenium analogue (Φ
F = 0.66).
2 also showed relatively strong, blue fluorescence in the solid state (λ
em = 443 nm, Φ
F = 0.30). We also examined transformations of ester groups in
2.
View full abstract