2014 年 47 巻 3 号 p. 103-112
2014 年 47 巻 3 号 p. 103-112
Granulosa cells form ovarian follicles and play important roles in the growth and maturation of oocytes. The protection of granulosa cells from cellular injury caused by oxidative stress is an effective therapy for female infertility. We here investigated an effective bioactive compound derived from Prunus mume seed extract that protects granulosa cells from hydrogen peroxide (H2O2)-induced apoptosis. We detected the bioactive compound, 3,4-dihydroxybenzaldehyde (3,4-DHBA), via bioactivity-guided isolation and found that it inhibited the H2O2-induced apoptosis of granulosa cells. We also showed that 3,4-DHBA promoted estradiol secretion in granulosa cells and enhanced the mRNA expression levels of steroidogenic factor 1, a promoter of key steroidogenic enzymes. These results suggest that P. mume seed extract may have clinical potential for the prevention and treatment of female infertility.
Granulosa cells form follicles in the mammalian ovary and are closely associated with the growth of oocytes. Indeed, the granulosa cells are indispensable for oocyte growth, differentiation, meiosis, cytoplasmic maturation and genomic transcriptional activity within the oocyte [31]. The granulosa cells are positioned around the oocyte to form a follicle, and estrogen is produced by the granulosa cells. Recently, it was suggested that the communication between the oocyte and granulosa cells may play an important role in oocyte maturation [23]. However, oxidative stress induced by reactive oxygen species (ROS) causes cellular damage related to ageing [7], which leads to apoptosis of granulosa cells [6, 12]. Subsequently, apoptosis of granulosa cells leads to follicular atresia [14, 20]. Thus, follicles decline gradually with ageing and disappear completely at the time of menopause. Thus, female age is the most significant determinant of success during fertility treatment, and an age-related increase in oxidative stress is correlated with a poor fertility treatment outcome [32]. Therefore, the prevention of follicular atresia by attenuating oxidative stress may contribute to infertility therapy.
Estradiol is an estrogen that plays a crucial role in the normal physiology, such as mammary gland proliferation, reproductive tract growth, vessel maintenance and bone formation. Estradiol secretion occurs via the following complex series of steps. First, cholesterol from blood capillaries is converted to androgen in theca cells. Androgen produced is then transferred to granulosa cells, where it is converted to estrogen by aromatase, which is activated by follicle-stimulating hormone (FSH) [24]. Estrogen produced in the granulosa cells acts on the whole body, including the granulosa cells, and increases FSH receptor abundance [21]. Follicles grow rapidly when there are increases in FSH receptor abundance and the estrogen secretion level. This process provides positive feedback, and a large amount of luteinizing hormone is secreted (luteinizing hormone surge), which leads to ovulation [8]. Therefore, estradiol secretion is the hallmark of the successful production of ovulatory follicles.
The Japanese apricot (Prunus mume Sieb. et Zucc., known as Ume in Japanese) is a traditional source of fruit in Japan. The fruit of P. mume is processed into a dried form, pickle, extract, liquor or a soft drink, and it is known to have various medical benefits. For example, an in vitro study showed that the concentrated fruit juice of P. mume may prevent cardiovascular diseases because of its anti-oxidative effect on angiotensin II-induced ROS generation [30]. In addition, P. mume extract may attenuate osteoporosis because it stimulates the differentiation of osteoblastic cells [16]. An epidemiological study in humans showed that P. mume had an inhibitory effect on Helicobacter pylori-related chronic gastritis [9]. Indeed, (+)-syringaresinol from unripe P. mume inhibits the motility of H. pylori [18]. With regard to reproduction, a previous study showed that P. mume extract improved the egg quality of hens [11]. Furthermore, P. mume seed extract inhibited the proliferation of adult T-cell leukaemia cells [15]. To the best of our knowledge, no previous reports suggest that compounds derived from P. mume seed are effective in treating reproductive diseases.
The COV434 line of immortalized granulosa cells was derived from a primary human granulosa cell tumor. The COV434 cells have many of the characteristics of normal granulosa cells [34]. Therefore, the COV434 cells are useful for studies of oxidative stress and granulosa cell function in vitro. The present study evaluated the effects of P. mume seed extract on hydrogen peroxide (H2O2)-induced death of COV434 cells and identified a bioactive compound. Furthermore, we demonstrated the effects of P. mume seed extract on estradiol secretion in COV434 cells.
H2O2 was purchased from Sigma-Aldrich (St Louis, MO, USA). 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) solution was purchased from Promega (Madison, WI, USA). 3,4-Dihydroxybenzaldehyde (3,4-DHBA) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Isolation and identification of the active compoundP. mume seeds were a gift from Okahata Farm Co., Ltd. (Wakayama, Japan). The seeds were washed in water and dried. Next, the seeds (300 mg) were refluxed with methanol (900 ml) for 3 hr to obtain a methanol extract. This extraction procedure was repeated three times. The methanol extract was filtered through a Whatman #1 filter paper and concentrated under reduced pressure. The methanol extract (48.8 g) was suspended in distilled water (1 L) and extracted with hexane (3 L), dichloromethane (3 L) and ethyl acetate (3 L) to obtain hexane (6.3 g), dichloromethane (1.5 g) and ethyl acetate (1.7 g) fractions. Each fraction was concentrated under reduced pressure to yield hexane (6.3 g), dichloromethane (1.5 g) and ethyl acetate (1.7 g) fractions. Each fraction was dissolved in dimethyl sulfoxide (Sigma-Aldrich) at a concentration of 200 mg/ml and used to analyze the protective effects against H2O2-induced cell death.
The ethyl acetate fraction exhibited a protective effect against H2O2-induced cell death. The ethyl acetate fraction was fractionated to fractions 1–5 by silica gel column chromatography using dichloromethane and methanol as the eluents. Fraction 3 had a potent protective effect and was fractionated to yield fractions 6–14 by C18 column chromatography using water and methanol as the eluents, and fraction 8 (compound 1) was isolated as a compound that protected against H2O2-induced cell death (Fig. 1). The chemical structure of compound 1 was identified as 3,4-DHBA by high-resolution electrospray ionization mass (HR-ESI-MS), 1H NMR and direct comparison with an authentic sample. The HR-ESI-MS spectrum was obtained using a micrOTOF mass spectrometer (Burker Daltonics, Bollerica, MA, USA). The 1H NMR spectrum was acquired using a Bruker AVANCE400 instrument (Bruker BioSpin, Bollerica, MA, USA) (400 MHz for 1H NMR).

Extraction procedure and chemical structure of the anti-oxidative stress compound isolated from Prunus mume seeds.
An amorphous powder. HR-EI-MS m/z 137.0254 ([M-H]-, calcd. for C6H6O3-H: 137.0244). 1H NMR (acetone-d6): δ 9.73 (1H, s, -CHO), 7.35 (1H, s, H-2), 7.34 (1H, d, J=8.0 Hz, H-6), 6.99 (1H, d, J=8.0 Hz, H-5).
Cell cultureCells of a human ovarian granulosa tumor cell line (COV434; DS Pharma Biomedical, Osaka, Japan) were grown at 37°C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (Life Technologies, Carlsbad, CA, USA), which was supplemented with 10% (v/v) fetal bovine serum (HyClone, Logan, UT, USA) and 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies). The COV434 cells were subcultured until they reached 80% confluence, before use in the assays.
Cell viability assaysMTS is converted to a formazan product by dehydrogenase enzymes found in metabolically active cells. The amount of formazan product measured as the absorbance at 490 nm is directly proportional to the number of viable cells in culture. Thus, an MTS assay was performed to assess the effects of 3,4-DHBA on cell viability and the protective effect of 3,4-DHBA against H2O2-induced cell death. COV434 cells were incubated at a density of 5×104 cells in 96-well plates (0.31 cm2; Sanplatec, Osaka, Japan) for 3 days. Next, the cells were treated with 50 μl of medium with or without seed extract for 5 min. To assess the effect of 3,4-DHBA on cell viability, 50 μl of fresh medium was added to each well. To assess the protective effect of 3,4-DHBA against H2O2-induced cell death, 50 μl of fresh medium containing H2O2 was added to each well at a final concentration of 0.5 mM. After incubating the cells for 18 hr at 37°C in a 5% CO2 atmosphere, the medium was replaced with 100 μl of fresh medium, and 25 μl of the MTS solution was added to each well. Next, the plates were incubated for 1 hr at 37°C. The absorbance of each well was recorded at 490 nm using a microplate spectrometer (Bio-Rad), where cells not treated with H2O2 were used as a control. Cell viability was expressed as a percentage relative to the control. The data are expressed as the mean±SD of three independent experiments (n=3).
Determination of the 8-OHdG levelAfter treatment of cells with 3,4-DHBA in the presence or absence of 0.5 mM H2O2, DNA was isolated from cell culture using a nucleic acid purification kit (Toyobo Co., Osaka, Japan) according to the manufacturer’s protocol. Isolated DNA was then digested with nucleases to obtain 8-hydroxy-2'-deoxyguanosine (8-OHdG) in the nucleoside form. 8-OHdG formation was determined using an oxidative DNA damage ELISA kit (Cell Biolabs Inc., San Diego, CA, USA) according to the manufacturer’s protocol. The absorbance of each well was recorded at 450 nm using the microplate spectrometer; cells not treated with H2O2 were used as the control. The 8-OHdG level was expressed as a percentage relative to the level in the control. The data are expressed as the mean±SD of triplicate samples. *P<0.05 compared with the H2O2-treated control cells.
Caspase-3/-7 activity and cytotoxicity assaysTo assess the effects of 3,4-DHBA on caspase activation events, cell viability and cytotoxicity, the activities of caspase-3/-7 were determined using a Caspase-Glo assay kit (Promega), according to the manufacturer’s protocol. In brief, COV434 cells were incubated with 3,4-DHBA and H2O2 using the same method as that used in the cell viability assay. Cell viability and cytotoxicity were determined as the viable and non-viable cell protease activities, respectively. The viable cell protease activity was measured using a fluorogenic, cell-permeant, peptide substrate (glycyl-phenylalanyl-aminofluorocoumarin; GF-AFC; Promega), which is cleaved by viable cell protease. The non-viable cell protease activity was measured using a fluorogenic, cell-impermeant, peptide substrate (bis-alanylalanyl-phenylalanyl-rhodamine 110; bis-AAF-R110), which is cleaved by non-viable cell protease. These substrates were added to each well, and the cells were incubated for 30 min at 37°C. The fluorescence signals produced were proportional to the numbers of viable or non-viable cells. The fluorescence intensity of GF-AFC and bis-AAF-R110 were observed at 400Ex/505Em and 485Ex/520Em, respectively, and the results are expressed in relative fluorescence units. Next, to measure caspase activity levels, the cells were incubated with Caspase-Glo buffer containing Caspase-Glo substrate for 30 min at room temperature. Luminescence emitted by the Caspase-Glo substrate cleaved by caspase-3/-7 in each well was recorded using the microplate spectrometer (Bio-Rad) with an integration time of 1 sec. Cells no treated with H2O2 were used as a control. The results are expressed as relative luminescence units.
Apoptosis detection and analysisTo detect the apoptotic cells, an Annexin V-FITC Apoptosis Detection Kit (Biovision, Milpitas, CA, USA) was used according to the manufacturer’s instructions. The COV434 cells (1.5×105 cells/well) were subcultured into 8-well chamber slides (Sarstedt, Tokyo, Japan) and pretreated with or without 3,4-DHBA for 5 min, before being incubated at 37°C for 18 hr in the presence or absence of H2O2. The cells were washed three times using phosphate-buffered saline (PBS) and incubated for 15 min with 300 μl of binding buffer, which contained 3 μl of annexin V-FITC, 3 μl of propidium iodide (PI, 50 μg/ml) and 0.3 μl of Hoechst 33342 (Life Technologies, 10 μg/ml). The cells were visualized by confocal laser scanning microscopy (CLSM; LSM700, Carl Zeiss MicroImaging GmbH, Jena, Germany). Annexin V-FITC was excited by a 488-nm wavelength argon–ion laser. Optical fluorescence signals of annexin V-FITC were observed using an emission filter (BP490–555, Carl Zeiss MicroImaging GmbH). PI was excited by a 555-nm wavelength helium–neon laser. Optical fluorescence signals of PI were observed without an emission filter. Hoechst 33342 was excited by a 405-nm wavelength argon–ion laser. Optical fluorescence signals of Hoechst 33342 were observed using an emission filter (BP 420–475+BP 500–610, Carl Zeiss MicroImaging GmbH). The confocal images were acquired using Plan Apochromat (×20, N.A. 0.8, Carl Zeiss MicroImaging GmbH) objective lens. On taking each confocal image (optical tomography), Z directional movement for optical sectioning of the entire specimen was controlled by the focus motor unit on axial scanning at 3.5-μm focus steps. CLSM image resolutions were 512×512 pixels. Triple-labeled data sets of these images taken using CLSM can be made into computer-assisted three-dimensional reconstructions using ZEN2009 software (version 6.0 SP2; Carl Zeiss MicroImaging GmbH). The three-dimensional images were converted to one-dimensional stacked images using ZEN2009 software. The cells were visualized by CLSM, where green (annexin V) and red (PI) staining represented early apoptotic (annexin V+/PI–), later apoptotic (annexin V+/PI+) and viable cells (annexin V–/PI–) based on double labelling with annexin V and PI, i.e., a phosphatidylserine (PS) and a cell-impermeant DNA stain, respectively [22]. Hoechst 33342, a cell-permeant DNA dye, was visualized as blue.
The percentage of apoptotic cells was analyzed by counting the number of annexin V-positive cells and Hoechst 33342-stained cells from CLSM images. The data are expressed as the percentage of apoptotic (annexinV-positive) cells relative to the Hoechst 33342-stained cells. Values in the bar graphs represent the mean±SD of four different areas of the images.
Scanning electron microscopyThe morphological characteristics of the COV434 cells were determined by scanning electron microscopy (SEM; Technex, Tokyo, Japan), where the cells were incubated according to the methods described above. Next, the cells were fixed with 2% glutaraldehyde in 0.2 M cacodylate buffer (pH 7.4) for 2 hr at 4°C. The cells were washed with PBS and dehydrated using a graded series of ethanol (50, 70, 90, 95 and 100%), 50% tert-butyl alcohol (in ethanol) and 100% tert-butyl alcohol. Then, the dehydrated cells were lyophilized. Samples were coated with palladium and platinum using an E102 Ion sputter (Hitachi, Tokyo, Japan), followed by observation using SEM.
HE stainingAfter incubating the cells with 3,4-DHBA in the absence or presence of H2O2 for 18 hr, the cells were stained with hematoxylin–eosin (HE). In brief, the cells were washed with PBS and fixed with formaldehyde (Sigma-Aldrich). The cells were then washed with water, stained with hematoxylin (Wako, Osaka, Japan) for 5 min, rinsed with 1% HCl in 70% ethanol for 20 sec and rinsed with water for 2 min. The cells were then stained with eosin (Wako) for 2 min and rinsed with water. Finally, the cells were dehydrated with ethanol and then mounted. The cells were observed under light microscopy.
Estradiol secretionCOV434 cells were seeded at a density of 5×105 cells/well in a 12-well microplate (3.8 cm2) and incubated for 4 days at 37°C in a 5% CO2 atmosphere. Next, the cells were incubated with or without 3,4-DHBA for 18 hr at 37°C, after which the medium was collected and centrifuged to obtain the supernatant. The estradiol levels in the supernatants were quantified using a chemiluminescent immunoassay with an ARCHITECT analyzer i2000 (Abbott Japan, Tokyo, Japan), according to the manufacturer’s instructions. An Architect Estradiol II kit (Abbott Japan) was used to analyze the chemiluminescent immunological reaction. The estradiol concentration was normalized on the basis of cell number, which was determined using the MTS assay. In brief, after treatment with 3,4-DHBA, the medium was replaced with 500 μl of fresh medium, and 125 μl of the MTS solution was added to each well. The plate was incubated for 2 hr at 37°C, and 125 μl of medium was transferred to a new 96-well plate. The absorbance of each well was recorded at 490 nm using the microplate spectrometer (Bio-Rad). The cell number was expressed as a percentage relative to the control. The data are expressed as the mean±SD of triplicate samples.
Gene expression analysisThe effect of 3,4-DHBA on the transcription of gene encoding steroidogenic factor 1 (SF-1), which is related to estradiol secretion, was investigated by quantitative real-time RT-PCR. COV434 cells were seeded at a density of 5×105 cells/well in a 12-well microplate (1.12 cm2; Sanplatec) and incubated for 4 days at 37°C in a 5% CO2 atmosphere. The cells were stimulated by replacing with medium that was supplemented (or not supplemented: control) with 3,4-DHBA (25 μg/ml) for 2 hr. Total RNA was collected from the cultured COV434 cells using the MagExtractorTM RNA kit (Toyobo). The RNA was reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad), according to the manufacturer’s protocol. Aliquots of cDNA were amplified in iQ SYBR Green Supermix (Bio-Rad) using a CFX96 real-time PCR detection system (Bio-Rad). The temperature profiles used for PCR comprised denaturation for 30 sec at 95°C, annealing for 30 sec at 60°C and elongation for 1 min at 72°C, with a total of 40 cycles. The target SF-1 mRNA was normalized against the housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. The results are expressed as the ratio relative to the control. The forward and reverse oligonucleotide primers were designed on the basis of published cDNA sequences. The primer sequences were as follows: GAPDH forward 5'-GAGTCAACGGATTTGGTCGT-3'; GAPDH reverse 5'-TTGATTTTGGAGGGATCTCG-3'; SF-1 forward 5'-GCTACCCAGAGCCCTTCTCT-3'; SF-1 reverse 5'-TCCAGCTCCTTGAAGACCAT-3'. The results are expressed as the mean±SD of triplicate samples (n=3).
Statistical analysisDifferences between groups were analyzed using a paired Student’s t test. P<0.05 was considered significant.
The methanol extract of P. mume seed was fractionated to identify the compound that suppressed oxidative stress-induced cell death. The ethyl acetate extract exhibited an inhibitory effect on H2O2-induced cell death. To isolate the active compound, the ethyl acetate extract was fractionated as shown in Figure 1 and assessed on the basis of its protective effect against H2O2-induced cell death. The active compound (fraction 8: compound 1) was purified by SiO2 column chromatography and C18 column chromatography, and compound 1 was identified as 3,4-DHBA on the basis of the spectral data (Fig. 1).
Protective effect of 3,4-DHBA againt H2O2-induced granulosa cell deathWe investigated the effects of 3,4-DHBA on granulosa cell viability using the MTS assay (Fig. 2). 3,4-DHBA did not affect the viability of COV434 cells at concentrations up to 50 μg/ml. Moreover, we investigated the protective effects of 3,4-DHBA against H2O2-induced cell death. 3,4-DHBA had protective effects. The 50 μg/ml 3,4-DHBA treatment group showed approximately 70% inhibition of H2O2-induced cell death compared with the control group. These results indicated that 3,4-DHBA is not cytotoxicity in the concentration range tested in the present experiment and has anti-oxidative effects.
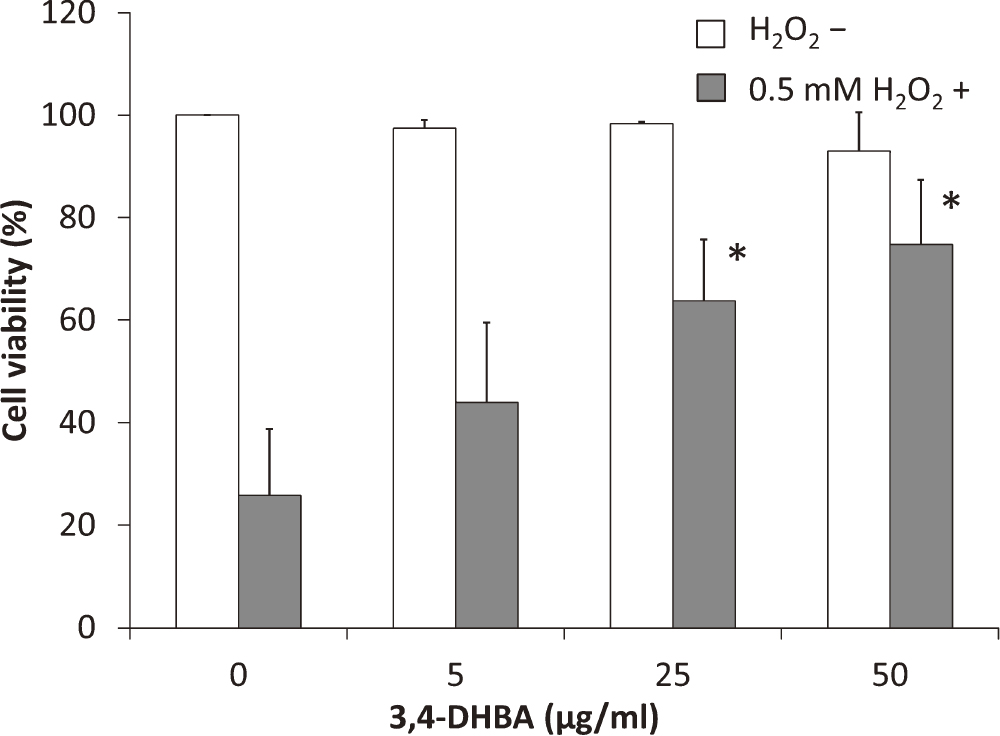
Effects of 3,4-dihydroxybenzaldehyde (3,4-DHBA) on granulosa cell viability. The cells were incubated with different concentrations of 3,4-DHBA in the presence or absence of 0.5 mM hydrogen peroxide (H2O2) for 18 hr. MTS assays were performed to measure cell viability after treatment. Cell viability was determined as the absorbance at 490 nm, which was expressed as a percentage relative to viability of the control. The data represent the mean±SD of triplicate samples (n=3). *P<0.05 compared with the control cells.
To determine the effects of 3,4-DHBA on H2O2-induced oxidative DNA damage in the COV434 cells, a competitive ELISA was performed for measuring the 8-OHdG level. The oxidation of 2'-deoxyguanosine to form 8-OHdG acts as a marker of oxidative DNA damage [4]. As shown in Figure 3, the 8-OHdG level in the H2O2-treated cells was higher than that in the control cells. The 8-OHdG level decreases with increasing 3,4-DHBA concentration. These results indicated that H2O2 causes DNA damage in the COV434 cells, and this DNA damage is attenuated by treatment with 3,4-DHBA.
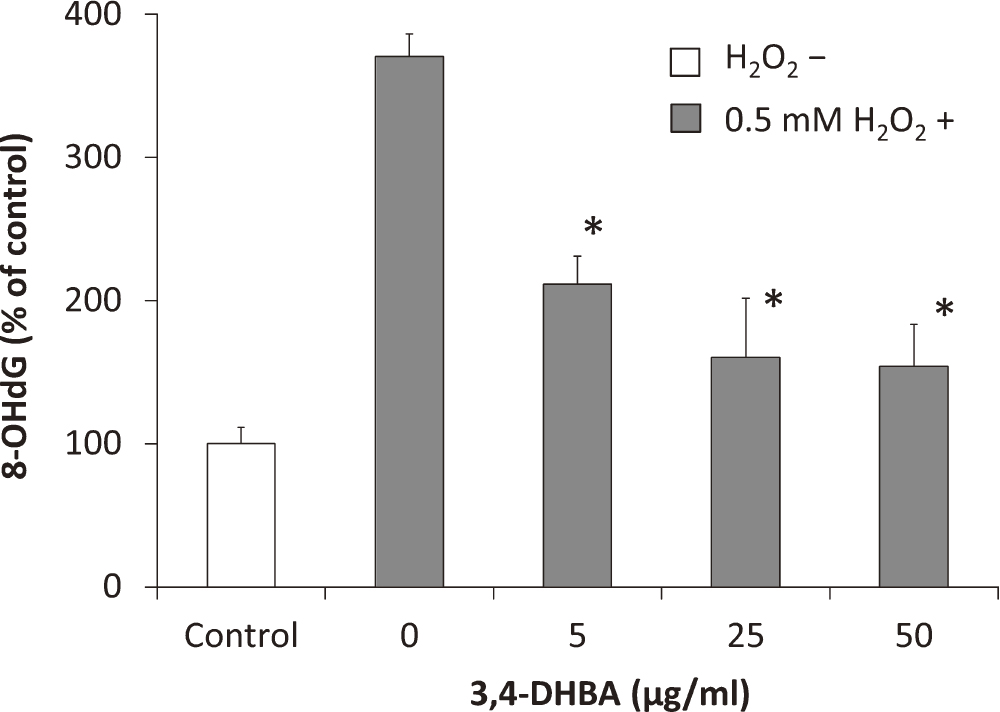
Effects of 3,4-DHBA on 8-OHdG formation in granulosa cells by H2O2 treatment. The cells were incubated with different concentrations of 3,4-DHBA in the presence or absence of 0.5 mM H2O2 for 18 hr. 8-OHdG formation in DNA was determined using a competitive ELISA kit. The data represent the mean±SD of triplicate samples. *P<0.05 compared with the H2O2-treated and 3,4-DHBA untreated cells.
We examined the effects of 3,4-DHBA on H2O2-induced caspase-3/-7 activation in COV434 cells (Fig. 4A). The caspase-3/-7 activity level in the H2O2-treated cells was higher than that in the control cells. The cytotoxicity level was also higher in the H2O2-treated cells than in the control cells. Therefore, H2O2-induced cell apoptosis occurred via caspase-3/-7 activation. In addition, 3,4-DHBA attenuated caspase-3/-7 activation and cytotoxicity in a concentration-dependent manner and increased cell viability. These results are consistent with the protective effects of 3,4-DHBA against H2O2-induced cell death detected by the MTS assay.

Effects of 3,4-DHBA on granulosa cell viability, cytotoxicity and apoptosis. The cells were incubated with different concentrations of 3,4-DHBA in the presence or absence of 0.5 mM H2O2 for 18 hr. (A) Cell viability, cytotoxicity, and caspase activities of H2O2-treated cells were determined on the basis of the viable and non-viable cell protease activities and caspase activity, respectively. The data represent the mean±SD as relative fluorescence units (RFU) or relative luminescence units (RLU) (n=3). (B) Apoptotic cells were detected by annexin V staining using CLSM. Annexin V-positive cells exhibited green staining. The nuclei and non-viable cells were stained with Hoechst 33342 (blue) and PI (red), respectively. Cells incubated in the presence or absence of H2O2 are shown in the upper and lower panels, respectively. Magnification: ×400; Bar=50 μm. (C) The percentages of apoptotic cells were analyzed from CLSM images. The data are expressed as the percentage of annexin V-positive cells relative to the Hoechst 33342-stained cells. Values in the bar graphs represent the mean±SD of four different areas of images.
We investigated the effects of 3,4-DHBA on H2O2-induced cell apoptosis. COV434 cells were stained with annexin V-FITC PI and Hoechst 33342 and examined by LSM (Fig. 4B). The annexin V-positive staining (green) indicated the exposure of PS in the cell membrane via apoptosis, and the membrane-impermeable PI-positive staining (red) indicated non-viable cells. The membrane-permeable DNA dye, Hoechst 33342, stained both viable and non-viable cells. The results showed that the H2O2-treated cells were positive for annexin V and PI, thereby indicating that the H2O2-treated cells were in a later apoptotic state. However, the number of apoptotic cells declined as the 3,4-DHBA concentration increased, and few cells were visible after treatment with 50 μg/ml 3,4-DHBA (Fig. 4C). A single treatment with 3,4-DHBA had no effect on cell apoptosis.
The morphological features of the COV434 cells were also observed by SEM (Fig. 5A) and HE staining (Fig. 5B). A lot of apoptotic cells, characterized as cell shrinkage, nuclear fragmentation and pyknosis, were easily recognized in the COV434 cells treated with 0.5 mM H2O2 alone, while no apparent morphological changes were found in the other two treatment (0.5 mM H2O2+ + 3,4-DHBA and 0.5 mM H2O2– + 3,4-DHBA) or control (H2O2–).
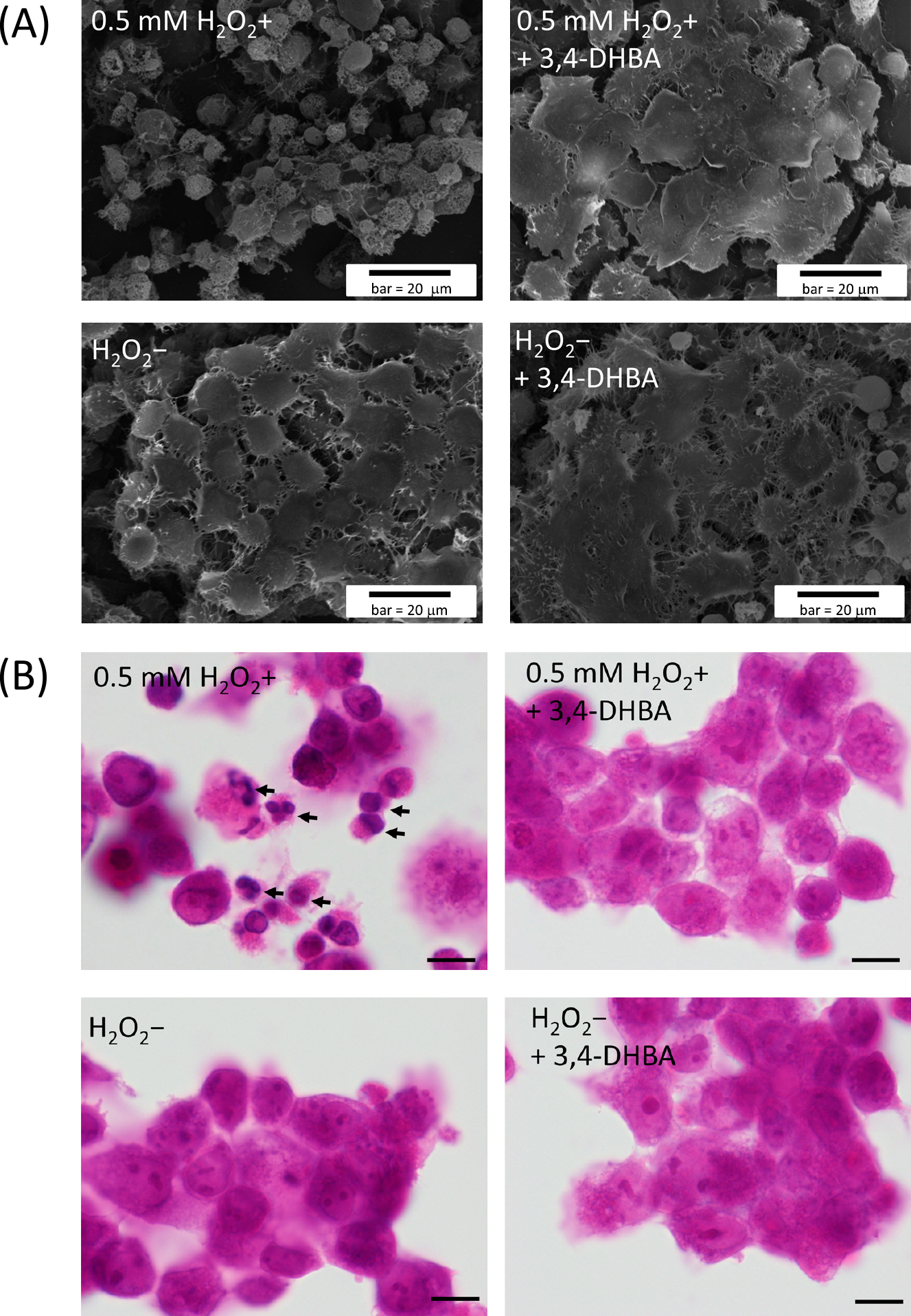
Effects of 3,4-DHBA on morphological changes in H2O2-treated granulosa cells and their nuclei. The cells were incubated with different concentrations of 3,4-DHBA in the presence or absence of 0.5 mM H2O2 for 18 hr. (A) Scanning electron microscopy images of granulosa cells. Bar=20 μm. (B) HE staining images of granulosa cells. Bar=10 μm. Arrows indicate typical apoptotic cells characterized as cell shrinkage, nuclear fragmentation and pyknosis.
To confirm the effects of 3,4-DHBA on COV434 cells, the estradiol secretion level was also measured. As shown in Figure 6A, the estradiol secretion levels were significantly higher in the 25–50 mg/ml 3,4-DHBA treatment groups than in the control group.

Effects of 3,4-DHBA on estradiol secretion levels and steroidogenic factor 1 (SF-1) mRNA levels in granulosa cells. (A) Granulosa cells were incubated in the presence of different concentrations of 3,4-DHBA for 18 hr. The values represent the mean±SD of the estradiol level, which was normalized against cell number (n=3). (B) Granulosa cells were incubated in the presence of different concentrations of 3,4-DHBA for 2 hr. The SF-1 mRNA expression level in granulosa cells was determined by real-time PCR. The SF-1 mRNA expression level was normalized against the GAPDH mRNA expression level. The data represent the mean±SD of triplicate samples (n=3). Statistical differences were observed between the 3,4-DHBA treatment groups and the control group (*P<0.05).
The nuclear receptor, SF-1, plays a key role in the expression of steroidogenic enzymes such as aromatase, which regulate estradiol secretion by granulosa cells [25]. Quantitative real-time PCRs were performed to evaluate the effects of 3,4-DHBA on SF-1 mRNA expression in COV434 cells. As shown in Figure 6B, the SF-1 mRNA expression level was increased in the 3,4-DHBA treatment groups compared with the control group.
In the present study, we demonstrated the effects of P. mume seed extract on H2O2-induced oxidative stress and steroid hormone secretion in cells of the human granulosa cell line COV434. We found that 3,4-DHBA fractionated from P. mume seed extract had a protective effect against H2O2-induced death of granulosa cells. The simple phenolic compound, 3,4-DHBA, is known as protocatechuic aldehyde, which is one of the major benzoic aldehyde derivatives found in plants and fruits. The anti-radical activity of 3,4-DHBA is approximately 2-fold higher than that of ascorbic acid [1]. Previous in vivo and in vitro studies have shown that 3,4-DHBA has clinical potential for preventing certain diseases related to ROS because of its high anti-oxidative activity. For example, 3,4-DHBA inhibits the abnormal migration and proliferation of vascular smooth muscle cells induced by the ROS generated by platelet-derived growth factor [19]. In addition, it has been reported that 3,4-DHBA has anti-inflammatory activities [3], neuroprotective effects against oxidative stress [10] and anti-cancer activities [13].
In the present study, we also demonstrated the protective effect of 3,4-DHBA from P. mume seed against H2O2-induced death of granulosa cells. Some of the most common ROS are H2O2, the superoxide anion (O2–) and the hydroxyl radical (OH–). If these ROS are present at high concentrations, they can damage cellular proteins and lipids or form DNA adducts that may promote carcinogenic activity [26]. The detrimental effects of oxidative stress, particularly DNA damage, are involved in ageing, degenerative diseases and cancer [5]. In human granulosa cells, treatment with H2O2 increases the percentage of apoptotic cells in a dose-dependent manner and increases the levels of activated caspase-3 [20]. In the present study, our results also showed that treatment of COV434 cells with H2O2 caused DNA damage and induced apoptosis on the basis of the increase in the caspase-3/-7 activity levels, exposure of PS in the cell membrane, nuclear fragmentation, pyknosis and visible cell shrinkage, which are typical of apoptosis. However, 3,4-DHBA reduced the levels of these characteristic signs of apoptosis. Therefore, apoptosis of granulosa cells is induced by treatment with H2O2; however, 3,4-DHBA suppresses H2O2-induced apoptosis via its high anti-oxidative effects. These results suggest that because oxidative stress increases with ageing, the inhibitory effect of 3,4-DHBA on oxidative stress-induced apoptosis of granulosa cells may be helpful for infertility treatment.
It is considered that the ROS-scavenging efficiency decreases with ageing [2]. The weakening of anti-oxidant defenses also occurs in granulosa cells where reproductive ageing is associated with the downregulation of genes encoding anti-oxidant enzymes such as copper/zinc superoxide dismutase, mitochondrial Mn2+-dependent superoxide dismutase and catalase, as well as the accumulation of oxidative damage mainly involving mitochondria [27]. Furthermore, antioxidant enzymes are known to play crucial roles in protection against superoxide anions and H2O2 generated during the synthesis of steroid hormones [28]. Thus, 3,4-DHBA has powerful anti-oxidative effects and may prevent cellular injury caused by the ROS generated during hormone synthesis.
We also found that estradiol secretion was enhanced in granulosa cells treated with 3,4-DHBA. There are few reports of the effects of plant extracts on estrogen biosynthesis in human granulosa cells. For example, icariin, the major compound in Epimedium brevicornum, which is a species of flowering plant in the family Berberidaceae, promotes estrogen biosynthesis in human ovarian granulosa-like cells by enhancing the mRNA and protein expression levels of aromatase, which is the only enzyme involved in the conversion of androgens to estrogens [17]. SF-1 plays an important role in steroidogenesis by regulating the transcription of steroidogenic enzymes such as aromatase [33]. Our results showed that treatment with 3,4-DHBA increased SF-1 mRNA expression levels. Therefore, it is likely that the increased estradiol level caused by treatment with 3,4-DHBA was mediated by an increase in SF-1 expression. In addition, previous studies suggest that estradiol may contribute to oocyte quality. For example, the addition of estradiol to oocyte maturation medium increased the fertilization and cleavage rates of in vitro-matured oocytes [29]. Therefore, it is considered that the increased estradiol secretion by granulosa cells may help to improve the quality of oocytes that interact with granulosa cells.
This is the first report of the effects of P. mume seed extract on oxidative stress and hormone secretion in human granulosa cells. 3,4-DHBA was isolated as the bioactive compound from P. mume seed extract, which had protective effects against H2O2-induced apoptosis of granulosa cells. Furthermore, 3,4-DHBA enhanced estradiol secretion by granulosa cells. The details of this mechanism are unknown, but it is suggested that estradiol secretion is enhanced via an increase in SF-1 mRNA expression, which is induced by 3,4-DHBA. Our results revealed that 3,4-DHBA protects granulosa cells from oxidative stress-induced apoptosis and enhances estrogen secretion via increased SF-1 expression. 3,4-DHBA may also contribute to the formation of good quality oocytes by activating granulosa cells. These functions of 3,4-DHBA may be effective for infertility treatment.
The authors thank Mr Y. Miyamura and Mr T. Nishioka for experimental assistance. The authors would like to thank Enago (www.enago.jp) for the English language review.