2014 年 47 巻 6 号 p. 289-294
2014 年 47 巻 6 号 p. 289-294
Emerin is a LEM domain-containing integral membrane protein of the vertebrate nuclear envelope. Recently it has been reported that emerin regulates tissue-specific gene/protein expression. We studied the relationship between emerin expression and follicle function in normal and hyperplastic human thyroid tissues using immunohistochemistry and statistical methods. Emerin immunoreactivity was heterogeneous among follicular cells and follicles in normal thyroid tissue. It tended to be strong in the nuclei of tall follicular cells of small follicles and weak or negative in the nuclei of flat follicular cells of large follicles. Follicles with strong expression of emerin were also strongly positive for thyroglobulin (Tg) and thyroxine (T4) in follicular cells and colloid substance, suggesting active functioning follicles. In contrast, large follicles with weak expression of emerin were also weak or negative for Tg and T4. Emerin immunoreactivity was strong in almost all nuclei of hyperplastic follicular cells in Graves’ disease tissues. These findings suggest that emerin expression may be related with follicular function and may contribute to the understanding of hormonogenesis in normal thyroid follicles.
Emerin, a nuclear membrane protein encoded in the STA gene at the Xq28 locus, is a well-conserved protein among different mammalian species [3]. Emerin is expressed in essentially all tissues [21] and contributes to vital shared functions in various cells and tissues, including nuclear assembly, cell cycle progression, gene regulation, mRNA splicing, and signaling [7, 9]. It has been reported that the loss of function of emerin causes Emery-Dreifuss muscular dystrophy (EDMD) [3, 4, 15].
Emerin has a folded structure called a “LEM-domain,” which is a ~40-residue helix-loop-helix fold that is conserved in DNA/RNA-binding proteins [14]. Emerin and other LEM-domain proteins (e.g., Lap2, Man1) bind directly to lamins and to a conserved chromatin protein named BAF (barrier to autointegration factor) [13]. LEM-domain proteins and lamins are hypothesized to influence each other’s localization and dynamics, and also to mediate chromatin organization and tethering at the nuclear envelope [13, 18]. Emerin also binds directly to several other proteins such as BCL-associated transcription factor (Btf), germ cell-less (GCL), histone deacetylase 3 (HDAC3), and YT521-B that are implicated in gene regulation [5, 6, 8, 9, 22].
The primary function of the thyroid is the formation, storage, and secretion of thyroid hormone [11, 16]. Thyroid follicular functions in normal tissue are not completely synchronized by thyroid stimulating hormone (TSH), and are morphologically and functionally heterogeneous among follicles according to hormone synthesis and secretion stages. Our previous studies showed that these follicular functions can be controlled not only by TSH but also by follicular thyroglobulin (Tg) [19]. In contrast, thyroid tissue affected by Graves’ disease is diffusely hyperplastic and not heterogeneous in its appearance.
There are several studies on emerin distribution in neoplastic cells of thyroid tumors such as papillary carcinoma and follicular adenoma [1, 2, 10]. Immunohistochemical findings for emerin were described in normal and hyperplastic follicular cells. However, none of these studies discussed the relationship between emerin expression and follicular function. In the present study, we studied emerin immunoreactivity in normal and hyperplastic thyroid tissues and discussed its relationship to follicular function.
We used 25 normal thyroid tissue samples collected from areas adjacent to tumor masses that were surgically removed from patients with papillary thyroid carcinomas at Yamanashi University Hospital. All specimens were routinely processed after surgery, embedded in paraffin and stained with hematoxylin-eosin (HE). We also studied 8 Graves’ disease thyroid tissue samples in the same manner. The study protocols were approved by the Institutional Ethics Board of the University of Yamanashi.
Immunohistochemical analysisAntibodies used in this study are shown in Table 1. Formalin-fixed and paraffin-embedded tissues were cut into 3-μm thick serial sections, mounted on silanized slides and deparaffinized. For emerin staining, heat-induced epitope retrieval was carried out in citrate buffer in an autoclave, and endogenous peroxidase activity was blocked for 10 min with 3% (vol/vol) hydrogen peroxide. Sections were incubated in the primary antibody overnight at 4°C or for 1 hr at room temperature. To visualize the reaction, we carried out labeled polymer method (Envision detection system; Dako, Glostrup, Denmark) according to manufacturer’s instructions and used 3,3'-diaminobenzidine tetrahydrochloride solution (Dojindo, Kumamoto, Japan) followed by counterstaining with hematoxylin to visualize a positive reaction.
| Antibody | Vendor | Clone | Dilution |
|---|---|---|---|
| Emerin | Novocastra, Newcastle, UK | mouse monoclonal/4G5 | 1:50 |
| Thyroxine | Dako, Glostrup, Denmark | rabbit polyclonal | 1:1000 |
| Thyroglobulin | Original | rabbit polyclonal | 1:1000 |
To confirm emerin immunoreactivity, the immunofluorescence method was additionally performed. The 3-μm sections of thyroid tissue were stained by immunofluorescence methods using anti-emerin antibody and fluorescein isothiocyanate (FITC)-conjugated immunoglobulin (Dako, Glostrup, Denmark) was used as a secondary antibody. For a nuclear counterstain, slides were mounted with mounting medium containing 4',6-diamino-2-phenylindole (DAPI/H-1200, Vector Laboratories, Burlingame, CA, USA). We used the IX81-FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan).
Evaluations of immunohistochemistryWe randomly selected 100 follicles per section under the microscope (20× objective lens), and measured the diameter, area and perimeter–three parameters used by DP2-BSW software. We divided the follicles into three categories: negative (no immunoreactivity), positive 1+ (weak/focal positive nuclei) and positive 2+ (strong/diffuse positive nuclei).
Statistical analysisSPSS software was used for statistical analyses. We compared the 3 groups using the Kruskal-Wallis rank sum test to analyze categorical variables. Statistical significance was considered as P<0.05.
Immunofluorescence and immunoperoxidase methods using anti-emerin antibody in the sections after antigen-retrieval pretreatment revealed that emerin was restrictively localized in the nuclear membranes of almost all normal thyroid follicular cells, highlighting nuclear contours as a ring-like feature (Fig. 1). Immunoexpression of emerin was heterogeneous in intensity among follicular cells and follicles (Fig. 1).
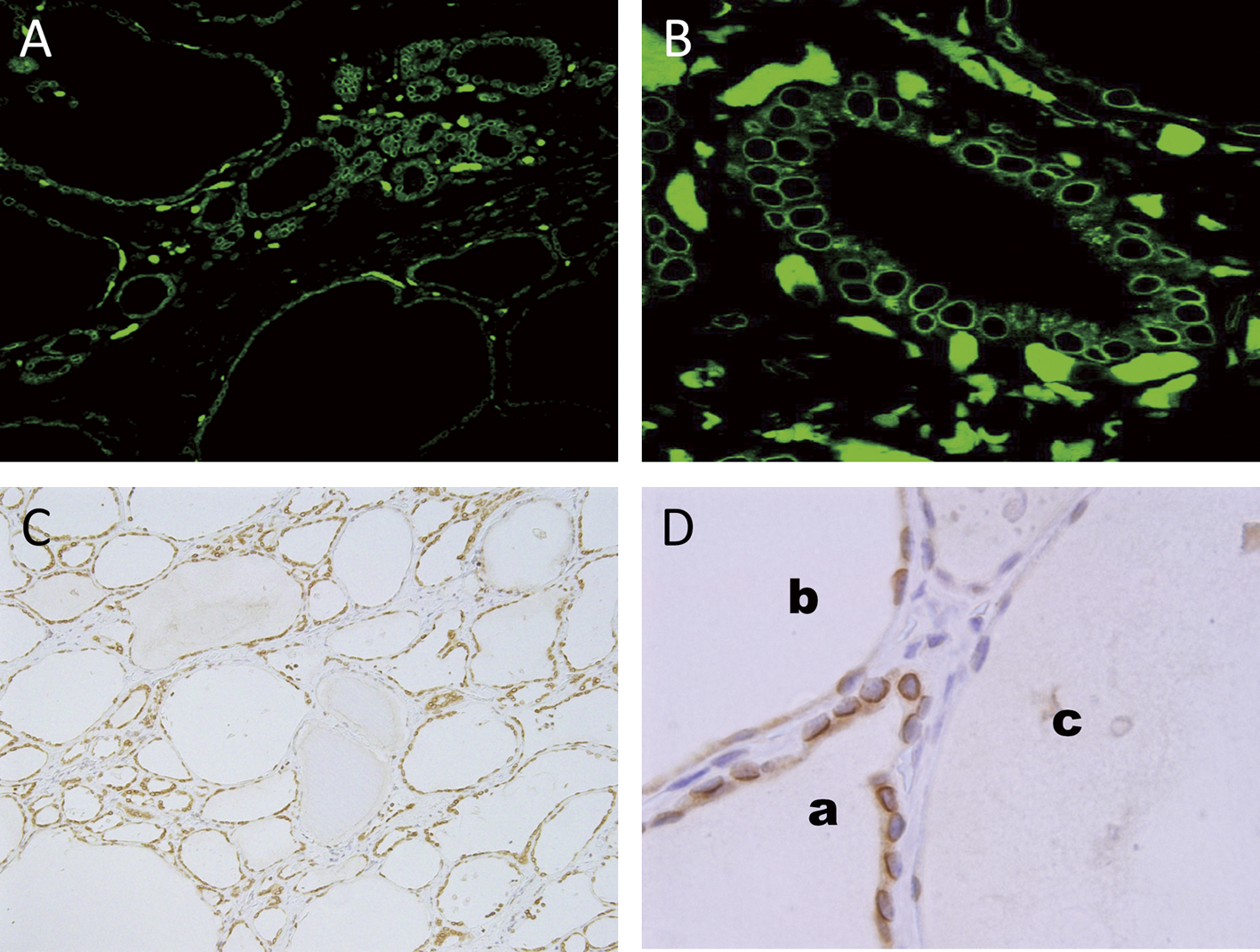
Emerin immunoreactivity in normal thyroid tissues. (A and C) Note the differential expression of nuclear emerin among follicles. Original magnification 10×. (B) Nuclear emerin in cuboidal follicular cells with a ring-like appearance. Original magnification 40×. (D) Large nuclei of cuboidal follicular cells are strongly positive (a) and flat nuclei of flat follicular cells are weakly positive (b) or negative (c). Original magnification 60×. (A and B) Immunofluorescent method; (C and D) Immunoperoxidase method.
Immunoexpression of emerin seemed to be related to follicle size (Fig. 2). We classified emerin expression into three degrees: negative, weak/focal positive (1+), and strong/diffuse positive (2+). To analyze the relationship between emerin expression and follicle size, we measured each follicle’s diameter, area, and perimeter (Table 2). Our results showed that the relationship between degree of emerin expression and all three parameters of follicle size was statistically significant (P<0.001). There was negative/weak expression of emerin in the rather flat nuclei of follicular cells of large follicles (Fig. 2A and B). We frequently observed strong expression of emerin in the large, round nuclei of cuboidal/tall follicular cells in small follicles (Fig. 2C). Cytoplasmic positivity of emerin was also observed in these cuboidal follicular cells (Fig. 2C).

Emerin expression and follicle size. (A) Nuclei of follicular cells in large follicles are negative (△). Original magnification 20×. (B) Nuclei of follicular cells in the large follicle are weakly positive. Original magnification 40×. (C) Nuclei of cuboidal follicular cells of small follicles are strongly positive. Also note the cytoplasmic positivity of emerin. Original magnification 60×. (A–C) Immunoperoxidase method.
| Parameter | Degree of emerin expression | |||
|---|---|---|---|---|
| negative | 1+ | 2+ | P value* | |
| Diameter (μm) | 249 ± 98 | 150 ± 60 | 70 ± 39 | <0.001 |
| Area (μm2) | 37474 ± 30176 | 13587 ± 10497 | 2778 ± 3299 | <0.001 |
| Perimeter (μm) | 736 ± 295 | 451 ± 173 | 202 ± 107 | <0.001 |
Negative, no positive nuclei; 1+, weak/focal positive nuclei; 2+, strong/diffuse positive nuclei
* K-M rank sum test
To elucidate thyroid follicle function, we performed immunohistochemistry with antibodies for thyroid specific proteins such as Tg and thyroxine (T4) (Fig. 3). Immunohistochemistry for Tg and T4 revealed that thyroid follicles were heterogeneous in their functional state. Some follicles were strongly positive in colloid and follicular cells and some were negative. The follicles showing a strong expression of Tg or T4 were rather small in size and lined with cuboidal/tall follicular cells. By contrast, the Tg- or T4-negative follicles were usually large and lined with flat follicular cells. Emerin immunoreactivity corresponded to the immunoexpression of Tg and T4 in the thyroid follicles. Emerin was strongly positive in the nuclei of follicular cells in follicles which were positive for Tg and/or T4, while it was weak and/or negative in the nuclei of follicular cells in follicles which were negative for Tg and/or T4 (Fig. 3A–C).

Immunohistochemical findings for emerin (A), T4 (B) and Tg (C) in serial sections. (A) Follicle lined with emerin-positive follicular cells (※) and with emerin-negative follicular cells (◇). (B) Emerin-positive follicle (※) is positive for T4 (※) and emerin-negative follicle (◇) is negative for T4 (◇). (C) Emerin-positive follicle (※) is positive for Tg (※) and emerin-negative follicle (◇) is negative for Tg (◇). Original magnification 20×. Immunoperoxidase method.
We examined emerin expression in hyperplastic thyroid tissues from the patients with Graves’ disease (Fig. 4). Unlike normal thyroid tissue the expression pattern of emerin from hyperplastic thyroid tissues was uniform and not heterogeneous. Almost all follicular cells in the Graves’ thyroid samples showed strong expression of emerin in their nuclei (Fig. 4B).

Immunoreactivity of emerin in Graves’ disease. (A) Thyroid tissue of Graves’ disease showing diffuse hyperplasia with large follicles lined with hyperplastic (columnar) follicular cells and numerous vacuoles in colloid substance. (B) Nuclei of hyperplastic follicular cells are strongly positive for emerin expression. Original magnification 40×. (A) HE staining; (B) Immunoperoxidase method.
We made a diagram to show the relationship between emerin staining and follicles. Emerin immmunoreactivity tended to be strong in the nuclei of tall follicular cells of small follicles that were strongly positive for Tg and T4. However, expression was weak or negative in the nuclei of flat follicular cells of large follicles that were negative for Tg and T4 (Fig. 5).

Schematic depiction of emerin expression in normal thyroid follicular cells.
Emerin, a member of the nuclear lamina-associated family, is a serine-rich nuclear membrane protein involved in mediating membrane anchorage to the cytoskeleton. In the present study, we provided analogous and complementary data on the staining expression of emerin in thyroid epithelial cells in parallel experiments using immunofluorescence and immunoperoxidase procedures. Using both methods in combination allowed us to obtain a higher resolution of images with immunofluorescence while we could gain a more direct appreciation of tissue features and areas with the immunoperoxidase method. These procedures revealed that emerin was clearly localized to the nuclear membrane of normal follicular cells, appearing as a ring-like feature. We also observed cytoplasmic positivity in tall follicular cells with strong nuclear staining. It is possible that the follicular cells with cytoplasmic positivity are actively synthesizing protein. Cytoplasmic positivity of emerin has been reported in the epithelial cells of other organs such as testicular interstitial cells and hepatic Kupffer cells [15].
In our present study, the distribution of emerin protein tended to be stronger in tall follicular cells of small follicles than in flat follicular cells of large follicles. This finding leads us to posit a the possible relationship between emerin expression and follicle size. Our image analyses confirmed that the expression grade of emerin was related to the morphological parameters of follicles such as follicular perimeter, diameter and area. The relationship between follicle size and iodine-131 uptake in the thyroid gland has been described by some investigators [12, 17]. Our study suggests that emerin, a nuclear membrane protein, may have a role not only in nuclear morphology but also in cell function.
Follicle functions can be controlled by serum TSH [11, 16]. Our previous studies reported that follicular Tg could also regulate follicle functions, and we hypothesized that Tg-initiated, transcriptional regulation of thyroid-restricted genes may be a normal compensatory feedback mechanism that limits follicular function and contributes to follicular heterogeneity [19, 20]. With this line of reasoning, to compare emerin expression and follicle function, we performed immunohistochemical stains for Tg and T4 together with emerin in serial sections of normal thyroid. In addition, we examined hyperplastic thyroid tissues taken from patients with Graves’ disease in the same manner. There are reports that emerin contributes to vital shared functions in various cells and tissues, including nuclear assembly, cell cycle progression, gene regulation, mRNA splicing, and signaling [7, 9]. Some studies showed that emerin participated in signal regulation binding with several partners [22]. We hypothesized that emerin expression was closely related with signal pathways and associated with follicular (cell) functions. Our immunohistochemical study would suggest that emerin might contribute to hormone production via signal pathways. However, further experiments are needed to clarify the actual mechanism.
We have no conflict of interest to declare.
The authors wish to thank Mikiko Yoda, Miyuki Ito, and Yoshihito Koshimizu for technical support and Kayoko Kono for executive assistance.