2014 年 47 巻 6 号 p. 303-310
2014 年 47 巻 6 号 p. 303-310
Excretory organs contain epithelial cells that form a filtration membrane specialized for ultrafiltration to produce primary urine. In vertebrates, the filtration membrane is made up of slit diaphragm (SD) formed by glomerular podocytes. Basal metazoans such as flatworms are also known have filtration epithelial cells, called flame cells, which exhibit SD-like structures. The molecular components of podocyte SD have been studied in detail, while those of the SD-like structures in basal metazoans including flatworms remain to be clarified. To determine whether the SD-like structures in flatworms have molecular components common to the SD in vertebrate podocytes, we examined the expression of gene homologue for mammalian nephrin, which encodes an essential transmembrane protein that participates in the formation of the SD, in a species of flatworms, planarian (Dugesia japonica). Flame cells were distributed throughout the entire body of the planarian, but the nephrin-expressing cells identified by in situ hybridization were mainly detected at body periphery excluding head region. The distribution pattern of nephrin-expressing cells was similar to that of proliferating cell nuclear antigen-expressing neoblasts, which are pluripotent stem cells characteristic to planarians. These findings indicated that the SD-like structures can be formed without the Nephrin protein in planarian flame cells.
In multicellular organisms the excretory organ is an essential visceral system to maintain the homeostasis in virtually all metazoans. This excretory system is functionally divided into two compartments: primary-urine producing apparatus and modulating tubule. The primary-urine producing apparatus filters the primary urine from the body fluid (interstitial fluid or blood plasma) for the excretion of excess water with metabolites. The primary urine is subsequently transferred to the modulating tubule, where it is modified by epithelial secretion and reabsorption. The modified urine is finally discharged outside the body as terminal urine.
The main portion of the primary-urine producing apparatus is made up of an epithelium layer specialized for ultrafiltration. In eucoelomates including vertebrates, which possess a coelom lined with mesothelium, the filtration epithelium consists of podocytes and their basement membrane [20, 21]. These podocytes, together with several capillary loops, form a number of glomeruli within the mesonephric and metanephric kidneys in vertebrates. This basic cytoarchitecture of the podocyte is highly conserved among the taxonomic groups of eucoelomates. Neighboring podocytes, which are interdigitated with each other by their numerous foot processes and separated by filtration slits, are bridged by specialized intercellular junctions called slit diaphragms (SD) (Fig. 1A, C). The SD in the vertebrate podocytes serve as a highly selective filtration barrier in the glomerulus. Three kinds of SD-specific membrane proteins are essential to form and maintain the SD in vertebrate podocytes: Nephrin, Podocin, and Neph1 [3, 6, 12, 16]. Mutations in nephrin and podocin genes cause congenital nephrotic syndrome of the Finnish type and autosomal recessive steroid-resistant nephrotic syndrome, respectively [1, 10].

Ultrastructure of flame cells in the planarian D. japonica. Transmission electron micrographs of rat podocytes (A, C) and planarian flame cells (B, D, E), both of which form a primary-urine producing apparatus. The cell bodies of podocyte (A) and flame cell (B) are shown in low magnification view. Flame cell forms a flame bulb at the most proximal blunt end of protonephridial tubule, and possesses a bundle of motile cilia in the lumen (arrows in B, E). The slit diaphragm and slit diaphragm-like structure are recognized in podocytes and flame cells, respectively (arrowheads in C, D). Magnification of an inset in E is shown as D. CL, capillary lumen; GBM, glomerular basement membrane; IS, interstitial space; US, urinary space. Bars=1 μm (A, B, E); 200 nm (C, D).
In acoelomates and pseudocoelomates, both of which are invertebrate animals without the coelom lined with mesothelium, the primary-urine producing apparatus is also comprised of epithelial cells specialized for ultrafiltration of interstitial fluid at the most proximal end of the protonephridial tubules. Unlike podocytes in eucoelomates, the filtration epithelial cells exhibit diversity in shape among the acoelomates and pseudocoelomates. However, the basic cytoarchitecture of the filtration site is well conserved and similar to that of the podocyte. The filtration epithelial cells form a SD-like structure at the intercellular space between neighboring cells or at the fenestrations in the cells [11, 29]. Although gene homologues for mammalian nephrin and podocin are found in acoelomates and pseudocoelomates, it is unclear whether Nephrin and Podocin proteins are contained in the SD-like structures of any of the species.
Platyhelminthes (flatworms) are primitive acoelomate invertebrates, which possess flame bulbs as a primary-urine producing apparatus, and exhibit a basket-like structure formed by flame cells, which is one type of filtration epithelial cells [29]. The Platyhelminthes phylum is traditionally divided into four taxonomic classes: Turbellari (including planarians), Cestoda (tapeworms), Trematoda (flukes), and Monogenea [21], all of which possess flame cells. In freshwater and land planarians, the SD-like structures bridge numerous rectangular fenestrations perforating the cytoplasmic wall of the flame bulb [7, 13, 24]. To explore whether the filtration epithelial cells utilize Nephrin to make the SD-like structure in the basal metazoan such as flatworms, we examined and identified the nephrin-expressing cells in the freshwater planarian, Dugesia japonica.
Planarians D. japonica were collected at a branch of the Tedori River in Hakusan City, Ishikawa, Japan (GPS coordinates: 36.42960, 136.63722). No specific permissions were required for the collection in this place, and D. japonica was not endangered or protected. Planarians were maintained at 20°C in dechlorinated tap water and fed chicken liver. All animal works have been conducted according to the national guidelines. Approval of an ethics committee was not required in case of the planarians.
Transmission electron microscopyPlanarians were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer at 4°C for 24 hours (hr). The fixed samples were processed by modified cold dehydration method. This method enables detailed morphological observation of the extracellular matrices and cytoskeletons, as previously reported [5]. In brief, the samples were successively immersed in 0.4% OsO4 in 0.1 M phospate buffer for 1 hr, 2% low molecular weight tannic acid (Electron Microscopy Sciences, Hatfield, PA) in 0.05 M maleate buffer for 4 hr, and 1% uranyl acetate in 0.05 M maleate buffer for 3 hr. The samples were then dehydrated with a graded series of ethanol and embedded in Epoxy resin. The ultrathin sections were stained with uranyl acetate and lead citrate and observed with a JEM1230 transmission electron microscope (JEOL, Tokyo, Japan).
ImmunohistochemistryPlanarians were treated with cold 2% HCl in 5/8 Holtfreter’s solution for 5 min, and then fixed in Carnoy’s fixative at 4°C for 3 hr. To remove pigmentation, the specimens were immersed in 5% hydrogen peroxide in methanol under fluorescent light at room templeture (RT) for 15 hr. Bleached samples were rehydrated with a graded series of methanol, and washed with phosphate buffer saline (PBS) containing 0.5% Triton X-100 (PBSTx). Subsequently, the samples were blocked with incubation solution (PBSTx containing 1% BSA) for 2 hr at RT, and incubated with the anti-acetylated α-tubulin antibody (clone 6-11B-1, Sigma-Aldrich, St. Louis, MO) diluted with the incubation solution (working dilution 1:100) for 10–15 hr at 4°C. After washing with PBSTx, the samples were incubated with tetramethylrhodamine (TRITC)-conjugated donkey anti-mouse IgG F(ab’)2 (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted with the incubation solution (1:500) for 2 hr at RT. After being mounted in anti-fading mounting medium (90 ml glycerol, 10 ml PBS, 100 mg p-phenylenediamine), the samples were imaged with a LSM510 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
Synthesis of digoxigenin-labeled RNA probesOne clone of gene homologue to mammalian nephrin (comp12709_c0 seq1) was selected from a previously established D. ryukuensis expressed sequence tags (EST) database [8]. This EST sequence is predicted to contain the full open reading frame (ORF) sequence by searching for it with the ORF prediction tool (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi). The full length cDNA sequence for proliferating cell nuclear antigen (pcna) homologue of D. japonica was as previously reported [15]. Partial-length nephrin and pcna of D. japonica were amplified from cDNA of adult D. japonica using KOD-Plus-Neo-DNA polymerase (TOYOBO, Osaka, Japan) and the following primer sets: nephrin-3698F: 5'-AAA GAT CAC GAA CTG CCA AAT TA-3'; nephrin-4410RT7: 5'-GGT AAT ACG ACT CAC TAT AGG ATT ATT TGG ATC AAG GGT TCC AT-3'; pcna-107F: 5'-GCA ATT AAA GAT TTG ATC CAA GAA-3'; pcna-742RT7: 5'-GGT AAT ACG ACT CAC TAT AGG CAA AGA CAG TGA CAC GAT AGG-3'. The partial-length nephrin and pcna cDNA were subcloned into pCRII-Blunt-TOPO (Invitrogen, Carlsbad, CA), and verified by DNA sequencing. cDNA templetes for RNA probe synthesis were amplified from the pCRII-Blunt-TOPO plasmid using the same primer sets described above. Digoxigenin-labeled RNA probes were synthesized using T7 RNA polymerase (Roche Diagnostics, Mannheim, Germany) and DIG-RNA labeling kit (Roche Diagnostics).
Whole-mount in situ hybridizationWhole mount in situ hybridization was performed as described previously [26]. In brief, planarians were treated with cold 2% HCl in 5/8 Holtfreter’s solution for 5 min, and then fixed in Carnoy’s fixative at 4°C for 3 hr. To remove pigmentation, the specimens were immersed in 5% hydrogen peroxide in methanol under fluorescent light at RT for 15 hr. The specimens were incubated in a prehybridization solution of 50% formamide, 5×saline sodium citrate buffer (SSC), 10 μg/ml yeast tRNA, 10 μg/ml heparin, 0.1% Tween 20, and 10 mM dithiothreitol at 55°C for 1.5 hr, and hybridized with a digoxigenin-labeled anti-sense RNA probe (about 40 ng/ml) in the prehybridization solution supplemented with 10% dextran sulfate at 55°C for 15 hr. After hybridization, the specimens were washed three times with SSC buffer at 55°C for 1 hr. They were then incubated in a blocking solution (pH 7.5) consisting of 0.1 M maleic acid, 0.15 M NaCl, 0.1% Triton X-100, and 1% Blocking Reagent (Roche Diagnostics) at RT for 1 hr and incubated in the blocking solution with an alkaline phosphatase-conjugated Fab fragment against digoxigenin (Roche Diagnostics) at RT for 3 hr. NBT/BCIP (Roche Diagnostics) was used as the chromogenic substrate to produce the blue staining.
X-ray irradiationTo extinguish neoblasts from planarians, they were exposed to a single 20-Gy dose of soft X-rays using an MBR-1505R2 X-ray generator (Hitachi Medical Corp., Tokyo, Japan). The planarians which were cut at seven-days after irradiation could not regenerate at least for seven days.
RT-PCRTotal RNA was individually isolated from control and irradiated planarians seven days after irradiation (n=6, for each group). cDNA was synthesized from total RNA using SuperScript III reverse transcriptase (Invitrogen), and was used as a template for PCR using KOD -Plus- Neo-DNA polymerase. The primer set for nephrin, pcna, and beta-actin is following: nephrin-3698F; nephrin-4181R: 5'-CCT CCT TCA TGA ATT GGT GAT AA-3'; pcna-107F; pcna-817R: 5'-TTT CGG AGC CAG ATA ATA ACG TA-3'; beta-actin-330F: 5'-TCT CAA TCC AAA AGC AAA CAG A-3'; beta-actin-829R: 5'-CAT GAA TAC CGA CTG ATT CCA A-3'.
We first examined the ultrastructure of planarian flame cells in D. japonica. The flame cell is shaped like a test tube to form a flame bulb at the most proximal end of the protonephridial tubule (Fig. 1B). The cytoplasmic wall of flame bulb possessed numerous rectangular fenestrations, which were aligned in parallel and spanned by the SD-like structure (Fig. 1D, E) [13]. In comparison with the SD in mammalian podocytes, the SD-like structure is somewhat ambiguous. However, the SD-like structure was associated with an electron-dense cytoplasmic plaque, which was on the inner leaflet of the plasma membrane at the insertion site of SD-like structure (Fig. 1D), as seen in the podocyte SD (Fig. 1C).
Podocytes in eucoelomates possess a basement membrane. Especially in the vertebrate glomerulus, it is referred to as the glomerular basement membrane (GBM) (Fig. 1C) and serves as the glomerular filtration barrier together with the SD [16]. Unlike podocytes, planarian flame cells did not exhibit a distinct basement membrane (Fig. 1D), and appeared to be in direct contact with the interstitial space (Fig. 1B, D, E). Thus, the SD-like structure mainly formed a boundary between the interstitial space and the urinary space (the lumen of the flame bulb). The interstitial space contained amorphous materials, which were presumably aggregations of interstitial fluid proteins fixed by glutaraldehyde (Fig. 1B, D, E). Such materials were not observed in the urinary space (the lumen of the flame bulb), which the primary urine occupied, indicating that the SD-like structure played a role in the filtration barrier function in planarian flame cells.
Next, to determine the distribution of planarian flame cells, we visualized the cells using whole-mount immunohistochemical detection for acetylated α-tubulin (Fig. 2), since the flame bulb contained a bundle of motile cilia (arrows in Fig. 1B, E), whose axonemal α-tubulin was highly acetylated. The bundle of cilia in the flame bulb exhibited a characteristic fox-tail appearance (arrowheads in Fig. 2B–D), as reported in another planarian Schmidtea mediterranea [19]. The flame cells were situated beneath the epidermis and recognized to be distributed throughout the entire body.
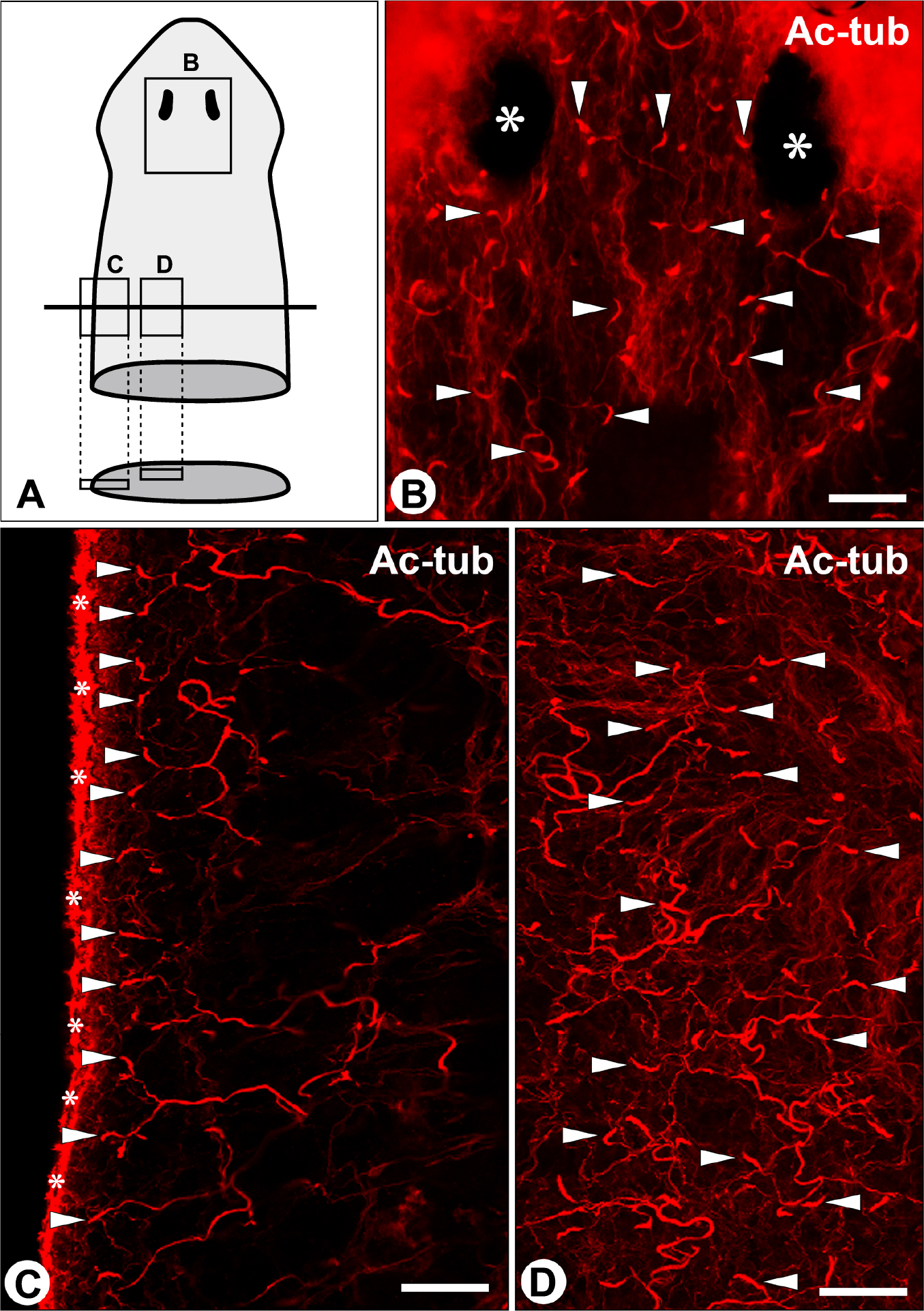
Distribution of flame cells in the planarian D. japonica. Distribution of flame cells is shown in the three regions which are indicated by rectangles in A. Motile cilia of flame cells were detected by whole-mount immunostaining with anti-acetylated α-tubulin (Ac-tub) antibody, and visualized as characteristic fox-tail-like signals (arrowheads in B, C, D). The flame cells were distributed throughout the entire body including head region. Asterisks in B, eyes; asterisks in C, motile cilia of epidermis. Bar=50 μm.
Amino acid sequences of Nephrin homologues were compared among planarian (D. ryukyuensis), blood fluke (Schistosoma mansoni), and human. Identity and similarity of the overall amino acid sequences are lower between planarian and blood fluke (6.3% and 16.0%, respectively), and between planarian and human (6.4% and 12.2%). However, the alignment pattern of functional protein domains was highly conserved among the three organisms (Fig. 3A). Nephrin homologues consisted of a type-1 integral membrane protein with several immunoglobulin and immunoglobulin-like domains and all three organisms had a fibronectin domain adjacent to the transmembrane domain in common.

Gene expression of planarian nephrin. (A) Structural similarity of Nephrin protein homologues among planarian (D. ryukyuensis, comp12709_c0 seq1), blood fluke (S. mansoni, Gene Bank Accession No. CCD77361), and human (No. AAG17141). All of the Nephrin homologues are a type-1 integral membrane protein with immunoglobulin domains (IG), immunoglobulin-like domains (IG like), immunoglobulin C2-type domain (IGc2), and fibronectin type-3 domain (FN3) close to the transmembrane domain (TM). To predict the protein domains, SMART was used. Whole-mount in situ hybridization for nephrin (B, B') and pcna (C, C') show that the localization pattern of nephrin mRNA is similar to that of pcna (arrowheads). Ph, pharynx. (D–D'') Double labeling for nephrin mRNA and acetylated α-tubulin (Ac-tub) protein. Longitudinal section of whole-mount double-labeled specimen. Motile cilia in flame cells are visualized as a fox-tail appearance of Ac-tub signals (arrowheads). The signals for nephrin (arrows) are not colocalized with the fox-tail signals of Ac-tub. (E) B', C', and D–D'' show the left-lateral peripheral region indicated by the rectangle. (F) RT-PCR for pcna, nephrin, and β-actin in control and X-irradiated planarians. The expression of nephrin is remarkably reduced in the planarian which exhibits the marked reduction of pcna expression. Bars=1 mm (C); 500 μm (C', D'').
The expression of nephrin was confirmed by PCR using cDNA templates of D. japonica planarians (Fig. 2F). Whole-mount in situ hybridization for nephrin mRNA was performed on D. japonica, and the nephrin-expressing cells were found mainly at body periphery excluding head region (arrowheads in Fig. 3B, B'). Some nephrin-expressing cells were also scattered at the central region of the trunk. The distribution pattern of nephrin-expressing cells was almost same among more than 50 planarians examined in this study.
This distribution pattern of nephrin-expressing cells was different from that of flame cells as shown in Figure 2. In particular, although numerous flame cells were found at the head region, the nephrin-expressing cells were not observed in the same region. Moreover, the double labeling for nephrin mRNA and acetylated α-tubulin protein clearly confirmed that most of the characteristic fox-tail signals for acetylated α-tubulin, which represented the bundle of motile cilia in flame bulb, was not colocalized with the nephrin signals (Fig. 3D–D''). The flame cells were predominantly found beneath the epidermis (arrowheads in Fig. 3D', D''), while the nephrin signals were recognized at the more medial region (arrows in Fig. 3D, D'). The above findings clearly indicated that flame cells did not express nephrin.
As to which cell type expressed nephrin in planarians, as described above, nephrin was expressed at the body periphery excluding head region. This characteristic expression pattern is similar to that of pcna as previously reported in D. japonica [15] (arrowheads in Fig. 3C, C'). pcna is predominantly expressed in neoblasts, which are pluripotent stem cells peculiar to planarians. The neoblasts possess high radiation sensitivity and can be extinguished from planarians using X-ray irradiation [15]. We thus examined the expression of nephrin in the planarians where neoblasts were extinguished using a single 20-Gy irradiation. The expression levels of pcna and nephrin corresponded well with one another, and in planarians which exhibited marked reduction of pcna expression, the expression of nephrin was also remarkably reduced (Fig. 3F). The above findings suggested that nephrin was expressed in the neoblasts, although further immunohistochemical examination using the specific antibody for planarian Nephrin protein is required to identify the Nephrin-expressing cell more exactly.
While vertebrate podocytes have a SD in the selective filtration barrier of its filtration membrane, planarian flame cells incorporate a SD-like structure. The SD-like structure was ambiguous in transmission electron microscopy in comparison with the SD, which allows for the possibility that the molecular components of the SD-like structure in flame cells may be markedly different from those of the SD in podocytes. In fact, planarians possess the gene homologue of nephrin, which encodes one of an essential transmembrane protein that participates in the formation of the SD in vertebrate podocytes, but it was expressed by a cell type other than the flame cell in planarians. This finding inevitably indicated that the SD-like structures can be formed without the Nephrin protein in planarian flame cells, unlike the SD in vertebrate podocytes.
Several instances of molecular diversity in podocyte-expressing proteins have been reported among vertebrate species. The most famous instances are α-actinin and drebrin, both of which are actin-associated proteins. In rodents, podocytes express α-actinin-2 and α-actinin-4, but not drebrin. On the other hand, in humans, podocytes express α-actinin-4 and drebrin, but not α-actinin-2 [4, 9, 17]. Recently, Volker and colleagues reported that the nephrin gene homologue was not registered in the chicken gene databases [27], indicating that the SD in chicken podocytes can be formed without the Nephrin protein [14]. It is highly possible that similar molecular diversity exists among the flame-cell-expressing proteins in the flatworm species. At the present time, we therefore cannot conclude that the SD-like structures in all classes of flatworms are formed without the Nephrin protein as found in planarians. The next issue is to determine whether flame cells express nephrin in flatworms other than planarians such as flukes, tapeworms, and monogeneans. Moreover, in the future study, we will investigate other SD-related genes such as podocin and neph-1 homologues in the flame cells of flatworms including planarians.
Diversity of nephrin-expressing cell type in invertebrate speciesIn some arthropods (insects and crustaceans), the podocyte-related cells, nephrocytes, serve to maintain the homeostasis of body fluid, and express two kinds of Nephrin homologue: Hibris and Sticks-and-stones (SNS) [28, 30, 31]. Both Nephrin homologues are essential components to form SD in the nephrocytes of fruit fly (D. melanogaster) as seen in the vertebrate podocytes, and are also involved in pupal eye development and axonal guidance [2, 18, 25]. Furthermore, in nematode worm (C. elegans), which do not possess the cell types performing ultrafiltration such as the podocyte, nephrocyte, and flame cell [21], the Nephrin homologue, SYG-2 is involved in synapse development and synaptic target recognition cooperatively with the Neph-1 homologue, SYG-1 [22, 23]. It is interesting that Nephrin homologue is also utilized for neural development in Arthropoda and Nematoda.
The present study revealed that the nephrin homologue was presumably expressed by neoblasts, but not by flame cells and neurons in planarians, although the function of Nephrin protein remains unclear in planarian neoblasts. On the basis of recent molecular taxonomy, Arthropoda and Nematoda belong to the superphylum Ecdysozoa, while Platyhelminthes including planarians belong to another superphylum Lophotrochozoa. Thus, it is possible that a great difference exists in the manner in which the Nephrin protein is utilized between Ecdysozoa and Lophotrochozoa.
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 23590226 to KI). This work was also supported in part by the Laboratory of Morphology and Image Analysis at Juntendo University Graduate School of Medicine.