2023 年 56 巻 2 号 p. 21-27
2023 年 56 巻 2 号 p. 21-27
Mitochondrial ferritin (FtMt) is an endogenous iron-storage protein localized in the mitochondria. FtMt is mainly observed in restricted tissues, such as those in the testis, islets of Langerhans, and brain. Further, it may protect cells from oxidative stress in neurodegenerative diseases, including Alzheimer’s disease and progressive supranuclear palsy. However, the role of FtMt in Parkinson’s disease (PD) remains unclear. Therefore, the current study investigated the localization and expression level of FtMt in the midbrain of patients with PD and healthy controls using immunohistochemical techniques. FtMt immunoreactivity was mainly detected in dopaminergic neurons in the substantia nigra pars compacta (SNc) in both healthy controls and patients with PD. In addition, FtMt-positive particles were observed outside the dopaminergic neurons in patients with PD. Based on a quantitative comparison, patients with PD had a significantly upregulated FtMt immunoreactivity in dopaminergic neurons than healthy controls. Our result might be helpful in future studies on the role of FtMt in PD.
Parkinson’s disease (PD) is a progressive neurological disorder characterized by motor dysfunctions such as tremor, rigidity, postural instability, and bradykinesia [3, 18]. PD pathology involves the loss of dopaminergic neurons and the formation of Lewy bodies, which comprise phosphorylated α-synuclein (p-α-syn), in the substantia nigra pars compacta (SNc) of the midbrain [3]. Although the mechanism of disease onset remains unknown, recent studies have shown that oxidative stress in dopaminergic neurons can trigger neurodegeneration and cause PD [7, 10]. Molecular genetics have revealed that genes such as SNCA, LRRK2, VPS35, and CHCHD2 play a role in autosomal dominant PD and PRNK, PNK1, DJ-1, ATP13A2, PLA2G6, and FBX07 in autosomal recessive PD. Moreover, in some cases, PD is associated with mitochondrial dysfunction, which causes oxidative stress [7]. Patients with PD have increased iron concentrations in the midbrain, and iron ions in the substantia nigra (SN) may catalyze the conversion of H2O2 to highly active hydroxyl radicals and induce oxidative stress [11].
Mitochondrial ferritin (FtMt) is an endogenous iron-storage protein localized in the mitochondria. FtMt is a 22-kDa protein and is encoded by an intronless gene on chromosome 5q23.1 [13]. Cytosolic ferritins, which comprise H and L subunits, are distributed ubiquitously. Meanwhile, similar to H-chain ferritin, FtMt is observed in restricted areas mainly in the testis, brain, and islets of Langerhans [2, 13, 14]. Hence, unlike cytosolic ferritins, it may have an important role. FtMt protects the mitochondria from oxidative damage, and it is associated with neurodegenerative diseases such as Alzheimer’s disease (AD) and progressive supranuclear palsy (PSP) [1, 6, 22, 23]. Recent studies have revealed that FtMt is observed in catecholaminergic neurons in the locus coeruleus of monkey pons and dopaminergic neurons in the SNc and the ventral tegmental area of human and monkey midbrains [24, 25]. In addition, a previous study showed that FtMt could have a neuroprotective role in the cell line model of PD [17]. Therefore, it may contribute to neuroprotection against dopaminergic neurodegeneration in PD. However, the detailed role of FtMt in human PD brains remains unknown.
The current study aimed to evaluate the localization and expression level of FtMt in the brain of patients with PD and healthy controls using immunohistochemical techniques.
This study was approved by the Ethics Committee of Shiga University of Medical Science (reference number: R2016-026). Human brain tissue samples were obtained from the brain bank of Shiga University of Medical Science. Consent for the use of brain samples for research purposes was obtained from the donors or next-of-kin. Brain tissue samples collected from controls clinically diagnosed with brain lesions were excluded. Meanwhile, samples from healthy individuals (n = 5) and those with PD (n = 5) were collected. Table 1 shows the details of cases used in this study.
| Cases | Age | Sex | Postmortem delay (h) | Pathological diagnosis |
|---|---|---|---|---|
| Control 1 | 47 | F | 3.2 | Breast cancer |
| Control 2 | 62 | M | 3.3 | Lung cancer |
| Control 3 | 83 | F | 6.5 | Malignant lymphoma |
| Control 4 | 60 | M | 5.0 | Pancreatic cancer |
| Control 5 | 52 | M | 10.0 | Malignant lymphoma |
| PD 1 | 71 | F | 2.6 | Parkinson’s disease |
| PD 2 | 71 | F | 2.3 | Parkinson’s disease and Alzheimer’ disease |
| PD 3 | 60 | M | 2.4 | Parkinson’s disease |
| PD 4 | 71 | F | 14.7 | Parkinson’s disease |
| PD 5 | 76 | F | 2.4 | Parkinson’s disease |
The postmortem human midbrains were fixed with formalin, as described in previous studies [1, 25]. After washing with water, the blocks were embedded in paraffin. The paraffin-embedded brain samples were cut to 5 μm.
AntibodiesThere are three primary antibodies used in immunohistochemistry. Human FtMt (C65-2) was developed in Molecular Neuroscience Research Center, Shiga University of Medical Science [24]. This is a mouse monoclonal antibody, with dilutions of 1:500 (multicolor fluorescence immunohistochemistry/IF) and 1:10000 (peroxidase/diaminobenzidine immunohistochemistry/DAB). The rabbit polyclonal antibody against tyrosine hydroxylase (TH) obtained from Merck Millipore (catalog number: AB152) was used at a dilution of 1:500. The rabbit monoclonal antibody against phosphorylated α-synuclein at S129 (p-α-syn) purchased from Abcam (catalog number: ab51253) was used at a dilution of 1:100.
Luxol fast blue stainingParaffin-embedded tissue sections were deparaffinized in xylene and rehydrated in 100% EtOH, 90% EtOH, and 70% EtOH. After washing with running tap water and rinsing with distilled water, sections were incubated in 0.1% Luxol fast blue (LFB) (Chroma-Gesellschaft Schmid, Köngen/N, Germany) in 95% EtOH containing 0.05% acetic acid (Nacalai-Tesque, Kyoto, Japan) for 90 min at 50°C–55°C. Then, they were rinsed with 95% EtOH for one min. After washing with running tap water for five min, the sections were differentiated using 0.05% lithium carbonate aqueous solution (Nacalai-Tesque) for 10 sec and then incubated twice in 70% EtOH for one min each time with constant agitation. After rinsing with distilled water, the sections were dehydrated in 70% EtOH, 80% EtOH, 90% EtOH, 95% EtOH, and 100% EtOH, processed in xylene, and coverslipped with Entellan new (Merck Millipore, Burlington, MA). Images were acquired using the IX83 inverted microscope (Olympus, Tokyo, Japan). The unstained area in LFB staining were considered as the area of the SN as LFB stains myelin sheath in the white matter.
Peroxidase/diaminobenzidine immunohistochemistryDeparaffinized sections were washed with running tap water, rinsed with distilled water, and washed once with 0.1 M phosphate-buffered saline (pH 7.4) containing 0.3% Triton-X100 (PBST). Next, they were incubated in 1% hydrogen peroxide solution for 20 min at room temperature (RT) to block endogenous peroxidase. After the sections were washed three times in PBST, antigen retrieval was performed in 1 mM EDTA (pH 8.0) by boiling for four min and then cooled down for 20 min. After washing three times in PBST, the sections were blocked with 2% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) in PBST for 30 min. Then, they were incubated overnight with mouse monoclonal anti-human FtMt antibody (C65-2; 0.1 μg/mL) at 4°C [24]. Further, they were washed three times in PBST and incubated with biotinylated anti-mouse IgM antibody (1:500; Vector Laboratories, Burlingame, CA) for 1 hr at RT. Next, they were washed three times in PBST and incubated with avidin–biotin complex (1:3000; Vectastain ABC Elite kit; Vector Laboratories) for 1 hr at RT. After washing three times in PBST, the dark purple immunoreaction product was developed in nickel ammonium sulfate-enhanced 3,3'-diaminobenzidine (200 μg/mL; Dojindo, Kumamoto, Japan) in 50-mM Tris-HCl (pH 7.6) and 0.0045% hydrogen peroxide. The sections were then washed with running tap water for 10 min and rinsed with distilled water for two min. After dehydration in 70% EtOH, 80% EtOH, 90% EtOH, 95% EtOH, and 100% EtOH and processing in xylene, the sections were cover-slipped with Entellan new. Images were collected using the Olympus BX50 light microscope.
Multicolor fluorescence immunohistochemistryDeparaffinized sections were washed with running tap water, rinsed with distilled water, and washed once with PBST. Antigen retrieval was performed in 1 mM EDTA (pH 8.0) by boiling for four min and then cooled down for 20 min. After washing three times with PBST, the sections were blocked with 2% bovine serum albumin in PBST for 30 min. Then, the sections were incubated overnight with anti-human FtMt antibody (C65-2; 1:500), rabbit polyclonal anti-tyrosine hydroxylase (TH) antibody (1:500; AB152, Merck Millipore), and p-α-syn at S129 (1:100; ab51253, Abcam, Cambridge, UK) at 4°C. Sections were washed three times with PBST and then incubated with the following fluorescent-labeled secondary antibodies for 1 hr at RT: Alexa Fluor 488-conjugated donkey anti-rabbit IgG antibody (ThermoFisher, Rockford, IL) and Alexa Fluor 568-conjugated donkey anti-mouse IgG antibody (ThermoFisher). After washing three times with 0.1 M phosphate-buffered saline (PBS), sections were incubated with Hoechst 33258 for 15 min at RT. After washing three times with PBS, TrueBlack (Biotium, Fremont, CA) was applied in a 1:40 dilution for 50 sec for quenching lipofuscin autofluorescence. Sections were washed three times in PBS and then coverslipped using antifade mounting media (ThermoFisher). Fluorescent images were taken using the Leica SP8 confocal microscope (Leitz, Wetzlar, Germany).
Absorption testThe brain sections of patients with PD3 were used in the absorption test. Deparaffinized sections were incubated overnight at 4°C with and without C65-2 antibody pre-absorbed with human FtMt peptide (antibody: peptide = 1:1000 [molar ratio]; overnight at 4°C) after peroxidase elimination and antigen retrieval procedures. The subsequent steps were performed using the same procedures as described above. See section “Peroxidase/diaminobenzidine immunohistochemistry”.
Quantitative analysis of FtMt immunoreactivity in dopaminergic neuronsThe four-neighboring tile images at three different areas of the SNc in a section immunolabelled with FtMt and TH antibodies were taken in each case using the Leica SP8 confocal microscope. The cell bodies of TH-immunoreactive neurons in the tile images were delineated using the ImageJ software, and the intensity of FtMt immunoreactivity was evaluated and normalized by the area of each cell body (pixel).
Statistical analysisStatistical analysis was conducted using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA). Statistical comparisons between two groups were performed using the Mann–Whitney U test. A p value of < 0.05 was considered statistically significant.
To determine the level of neurodegeneration in PD, the number of neuromelanin deposits in the SN was counted. Healthy controls had a higher number of neuromelanin-positive neurons in the SNc than patients with PD (Fig. 1A). Quantitative analysis revealed a significant difference in the number of neuromelanin deposits in the SN between healthy controls and patients with PD (p < 0.01; Fig. 1B).

Neuromelanin deposit in the substantia nigra (SN) in healthy controls and patients with Parkinson’s disease (PD). (A, B) Representative images of the neuromelanin deposit in the SN of healthy controls (A) and patients with PD (B). The midbrain section samples were stained with Luxol fast blue to identify the area of SN. Bar = 100 μm. (C) Quantitative analysis showed a significant decrease in the number of neuromelanin deposit in patients with PD compared with healthy controls. **P < 0.01 using the Mann–Whitney U test. Bar = 100 μm.
Immunohistochemistry using C65-2 antibody without preincubation with FtMt peptide showed dark purple-colored immunoreactivity in neuromelanin-positive neurons in the SNc. Conversely, C65-2 antibody pre-incubated with FtMt peptide did not show any immunoreactive signals, and only brown-colored neuromelanin was observed in the SNc (Fig. 2). These findings might indicate that the C65-2 antibody bound to FtMt in the human brain sections.
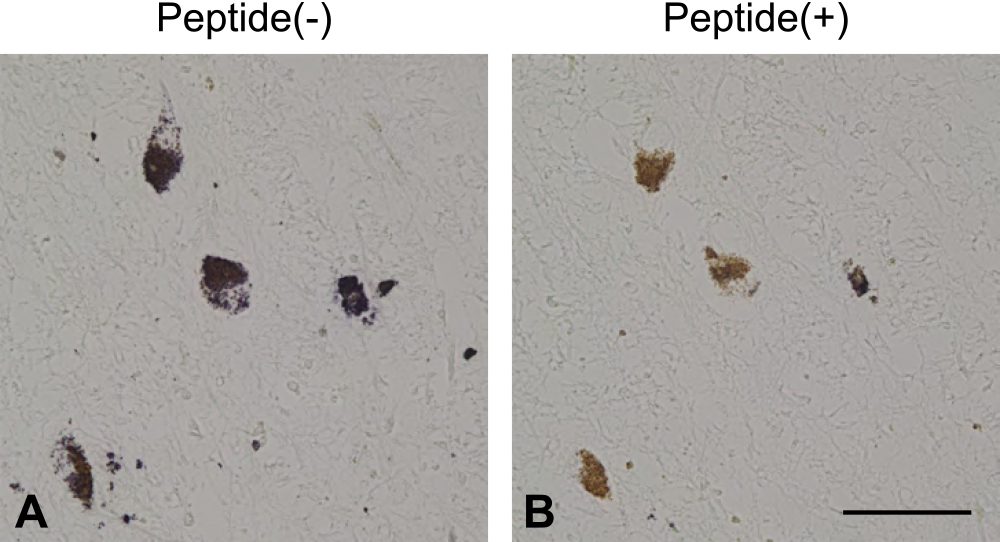
Absorption test for the evaluation of mitochondrial ferritin (FtMt) antibody in the substantia nigra pars compacta of patients with Parkinson’s disease. For absorption test, immunohistochemistry for FtMt was performed without (A) and with (B) the pre-incubation with FtMt peptides. FtMt antibody pre-incubated with FtMt peptides did not show specific signals in the midbrain sections. Bar = 20 μm.
Immunohistochemical analysis of FtMt using the C65-2 antibody was performed in the midbrain sections of healthy controls and patients with PD. FtMt immunoreactivity was depicted as dark purple color, which was well distinguished from neuromelanin pigment observed as light brown color. Strong FtMt immunoreactivity in the pigmental cells was observed predominantly in patients with PD (Fig. 3, black arrow). In contrast, weak FtMt immunoreactivity in the pigmental cells was observed in both controls and patients with PD (Fig. 3, white arrow). Some FtMt-positive non-pigmental cells were observed (Fig. 3, black arrowhead). In addition, FtMt-positive particles were remarkable in patients with PD compared with healthy controls (Fig. 3, white arrowhead).

Expression patterns of mitochondrial ferritin (FtMt) in the substantia nigra pars compacta. Representative images of FtMt immunohistochemistry using the C65-2 antibody in the midbrain sections in healthy controls (A) and patients with Parkinson’s disease (PD) (B). Four staining patterns were observed. Strong FtMt immunoreactivity was observed in the pigmental cells of patients with PD (black arrow). Meanwhile, weak FtMt immunoreactivity was noted in the pigmental cells of healthy controls and patients with PD (white arrow). Some non-pigmental cells were stained with the FtMt antibody (black arrowhead). FtMt-positive particles were noted particularly in patients with PD (white arrowhead). Bar = 20 μm.
TH is an enzyme that converts tyrosine to L-3,4-dihydroxyphenylalanine in the dopamine (DA) synthesis pathway [15] and is a marker of DA neurons in the SNc. Therefore, double immunofluorescence staining of FtMt and TH was performed to investigate the localization of FtMt in DA neurons in the SNc of healthy controls and patients with PD (Fig. 4).

Localization patterns of mitochondrial ferritin (FtMt) in dopaminergic neurons and other structures. Representative immunofluorescence images for tyrosine hydroxylase (TH) (A, D) and FtMt (B, E) in the midbrain sections in healthy controls (A–C) and patients with Parkinson’s disease (PD) (D–F). Merged images were shown in (C) and (F). Strong FtMt immunoreactivity was detected in the TH-positive neurons of patients with PD compared with healthy controls. Furthermore, FtMt-positive particles not co-localized with TH-positive neurons were observed particularly in patients with PD. Bar = 50 μm.
In both healthy controls and patients with PD, FtMt immunoreactivity was commonly co-localized with TH-immunoreactive neurons. Patients with PD had a higher level of FtMt immunoreactivity in TH-immunoreactive neurons than healthy controls (Fig. 4). In addition, some FtMt-immunoreactive particles were not co-localized with TH-immunoreactive neurons in patients with PD (Fig. 4F).
Distribution of FtMt immunoreactivity in the SNcThe intensity of FtMt immunoreactivity was evaluated and expressed as the value normalized by the area (pixel) of each cell body. Figure 5A shows the plots of intensity in each cell in individual cases. In control cases, the FtMt immunoreactivity was relatively low except in control 5. Meanwhile, in patients with PD, the FtMt immunoreactivity was relatively high particularly in cases 3, 4, and 5 (Fig. 5A). According to the mean values of individual cases, the FtMt immunoreactivity did not significantly differ between healthy controls and patients with PD (p = 0.22; Fig. 5B). Interestingly, significant differences were observed between healthy controls and patients with PD if the intensity of FtMt immunoreactivity in individual cell was plotted (p < 0.05; Fig. 5C).

Quantitative analysis of mitochondrial ferritin (FtMt) immunoreactivity in the substantia nigra pars compacta. (A) The intensity of FtMt immunoreactivity in individual cells in each case was plotted. (B) The graph shows the mean values of FtMt intensity in healthy controls and patients with Parkinson’s disease (PD). The mean values showed no significant difference between healthy controls and patients with PD. (C) The graph shows the intensity of FtMt immunoreactivity in individual cells in healthy controls and patients with PD. Statistical analysis showed a significant difference in FtMt immunoreactivity between healthy controls and patients with PD. ***P < 0.001 using the Mann–Whitney U test.
In PD, p-α-syn is a main component of Lewy body, which characterized the pathological feature of the disease [12]. Immunofluorescence staining of FtMt and p-α-syn was performed to assess the association between FtMt and the formation of Lewy body. FtMt was not co-localized with p-α-syn. However, three-dimensional modeling of immunoreactivities revealed that FtMt closely surrounded the p-α-syn (Fig. 6).

Immunofluorescence staining with mitochondrial ferritin (FtMt) and phosphorylated α-synuclein (p-α-syn) in the substantia nigra pars compacta (SNc) in patients with PD. (A) p-α-syn immunoreactivity in the SNc. (B) FtMt immunoreactivity in the SNc. (C) Merged images of p-α-syn and FtMt in the SNc. FtMt was not co-localized with p-α-syn. However, it was located in the region surrounding p-α-syn in patients with PD. Bar = 20 μm.
The current study investigated the localization and expression level of FtMt in the SNc in controls and patients with PD. Immunohistochemical analysis using C65-2 antibody showed that FtMt immunoreactivity was detected in dopaminergic neurons in healthy controls and patients with PD. Furthermore, the expression level of FtMt was highly observed in patients with PD compared with healthy controls. Hence, FtMt could be involved in PD pathology.
Shi et al. (2010) showed that FtMt maintained iron homeostasis and prevented neuronal damage in parkinsonian phenotype in vitro. Previous studies in our laboratory showed that FtMt immunoreactivity was observed in the locus coeruleus, SNc, and ventral tegmental area in the monkey brainstem [24]. In addition, FtMt immunoreactivity was detected in the neurons of SNc in human and monkey brains [25]. Therefore, FtMt could be correlated with PD pathology.
Results showed that FtMt was localized mainly in pigmental cells or dopaminergic neurons, and FtMt immunoreactivity was observed in non-pigmental cells or FtMt-positive particles particularly in the SNc of patients with PD. These results were consistent with those of our previous studies, which showed FtMt immunoreactivity inside and around TH-positive neurons in the substantia nigra of human and monkey brainstems [1, 25].
FtMt expression is induced by hydrogen peroxide treatment causing oxidative stress in the cells [19]. Oxidative stress is a harmful factor in the body and is associated with neurodegenerative diseases including AD, PSP, and PD [9, 16, 20]. Previous studies reported that patients with AD had an increased level of FtMt expression in the temporal cortex [19] and in the SNc of patients with PSP [1]. Hence, in the SN of patients with PD, the high FtMt expression is attributed to oxidative stress under PD pathology. In addition, FtMt has a neuroprotective activity against oxidative stress [8, 23]. Therefore, the induction of FtMt expression in the SN of PD indicates a compensative neuroprotection by FtMt against oxidative stress in PD pathology.
Ferroptosis, which was proposed in 2012, is an iron-dependent and non-apoptotic cell death initiated by inhibiting cystine uptake, and this phenomenon could promote the production of lethal lipid ROS [4]. Erastin inhibits cystine uptake and induces ferroptosis, and FtMt was found to inhibit erastin-induced ferroptosis [4, 21]. In addition, ferroptosis might be correlated with the cell death pathway of dopaminergic neurons in PD [5]. Therefore, FtMt might be associated with ferroptosis and might protect cells from iron-dependent oxidative damage. In future studies, the ferroptosis pathway could be a therapeutic target in specific diseases characterized by high FtMt levels, including PD.
The current study had several limitations. First, due to the limited number of samples, we used the immunohistochemical approach, but not the biochemical approaches, such as Western blot analysis, to analyze the expression level of FtMt. Second, it was challenging to achieve a convincing conclusion by performing statistical analysis. Finally, the SNc could not be selected in LFB staining. LFB stained the myelin sheath in the white matter, and the area not stained with LFB was considered as the SN area. Therefore, we could not distinguish the substantia nigra pars reticulata from the SNc.
There are various hypothesis and possible interpretations about neurodegenerative diseases, including PD. FtMt can be helpful in the assessment of neuroinflammation in PD pathology. However, future studies must be conducted to assess FtMt and its association with PD pathology in the human brains.
The authors declare that there are no conflicts of interest.
This study was supported by Grant-in-Aids for Scientific Research from Japan Society for the Promotion of Science (JSPS) (Grant Numbers 20K20588, I.T.). We appreciate the support from the brain bank at Shiga University of Medical Science for the brain samples used in this study. We would like to thank Mr. Yamamoto, belonging to Central Research Laboratory (CRL), Shiga University of Medical Science, for the technical support with microscopy. Additionally, we would like to thank all the members of the laboratory for advising the experiment and suggesting the solution strategy.