2024 年 57 巻 1 号 p. 1-5
2024 年 57 巻 1 号 p. 1-5
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system, characterized by remyelination failure and axonal dysfunction. Remyelination by oligodendrocytes is critical for improvement of neurological deficits associated with demyelination. Rodent models of demyelination are frequently used to develop and evaluate therapies for MS. However, a suitable mouse model for assessing remyelination-associated recovery of motor functions is currently unavailable. In this review, we describe the development of the mouse model of internal capsule (IC) demyelination by focal injection of lysolecithin into brain and its application in the evaluation of drugs for demyelinating diseases. This mouse model exhibits motor deficits and subsequent functional recovery accompanying IC remyelination. Notably, this model shows enhancement of functional recovery as well as tissue regeneration when treated with clemastine, a drug that promotes remyelination. The IC demyelination mouse model should contribute to the development of novel drugs that promote remyelination and ameliorate neurological deficits in demyelinating diseases.
Myelin is composed of multilamellar lipid cell membranes that function as an insulator to increase the conduction velocity of nerve fibers, and is involved in various neural activities through its interaction with axons [2, 20, 21]. Numerous neurological diseases are associated with disruption of myelin in the central nervous system (CNS), including multiple sclerosis (MS) [5, 9, 14, 22]. MS is an intractable disease characterized by motor paralysis and sensory disturbance caused by demyelination. However, effective treatments are currently unavailable [5, 9, 14, 22]. A mouse model of demyelination induced by cuprizone toxicity has been widely used to evaluate treatments for demyelinating neurological diseases, and this model has also been used to examine remyelination [17, 26]. Moreover, the experimental autoimmune encephalomyelitis (EAE) model has been used in the development of immunomodulatory therapies [6, 23]. Studies using these animal models have contributed to the exploration of targeted signaling and the development of therapies that modulate the disease course of MS [4, 10, 19]. However, the EAE and cuprizone models are time-consuming to produce and often highly variable, both in the area affected by demyelination and neurological symptoms. In addition, demyelination-induced motor dysfunction and the subsequent functional recovery associated with remyelination are not always observed in these models. Therefore, new animal models that closely mimic the characteristic clinical features of MS are urgently needed. In this review, we introduce a mouse model of internal capsule (IC) demyelination that was recently developed to overcome the limitations of current models. Furthermore, we provide an overview of recent studies using this IC model.
Numerous studies have used the model of focal demyelination induced by injection of lysophosphatidylcholine (LPC) into major neural pathways, including the spinal cord, corpus callosum and sciatic nerve [3, 13]. The LPC-induced demyelination model is highly informative for the assessment of regeneration because remyelination is spontaneously induced in both the CNS and the peripheral nervous system (PNS) [12, 18, 28]. In contrast, the internal capsule (IC), a white matter structure, is a major pathway of the corticospinal tract, which regulates limb motor function [24, 25]. In clinical studies, asymmetric motor paralysis is frequently observed in MS patients with IC demyelination [15, 16]. Therefore, while motor deficits and paralysis associated with demyelination are not usually observed after conventional LPC injection, LPC injection into the IC to induce focal demyelination in this structure could be very useful for assessing motor recovery and remyelination.
Recently, we have reported a novel mouse model of focal demyelination induced by focal injection of LPC into the IC [27]. The mice were injected with 1% LPC (Fig. 1A), and acute IC demyelination was observed at 7 days post lesion (dpl) (Fig. 1B). The mice exhibited motor impairments, including asymmetric paralysis affecting the fore and hindlimbs [27]. Notably, the mice recovered their motor functions by 28 dpl [27]. Oligodendrocyte progenitor cells were recruited to the demyelinated lesions and differentiated into myelinating oligodendrocytes by 28 dpl, suggesting that this mouse model features remyelination and the resolution of inflammation (Fig. 1C) [27].
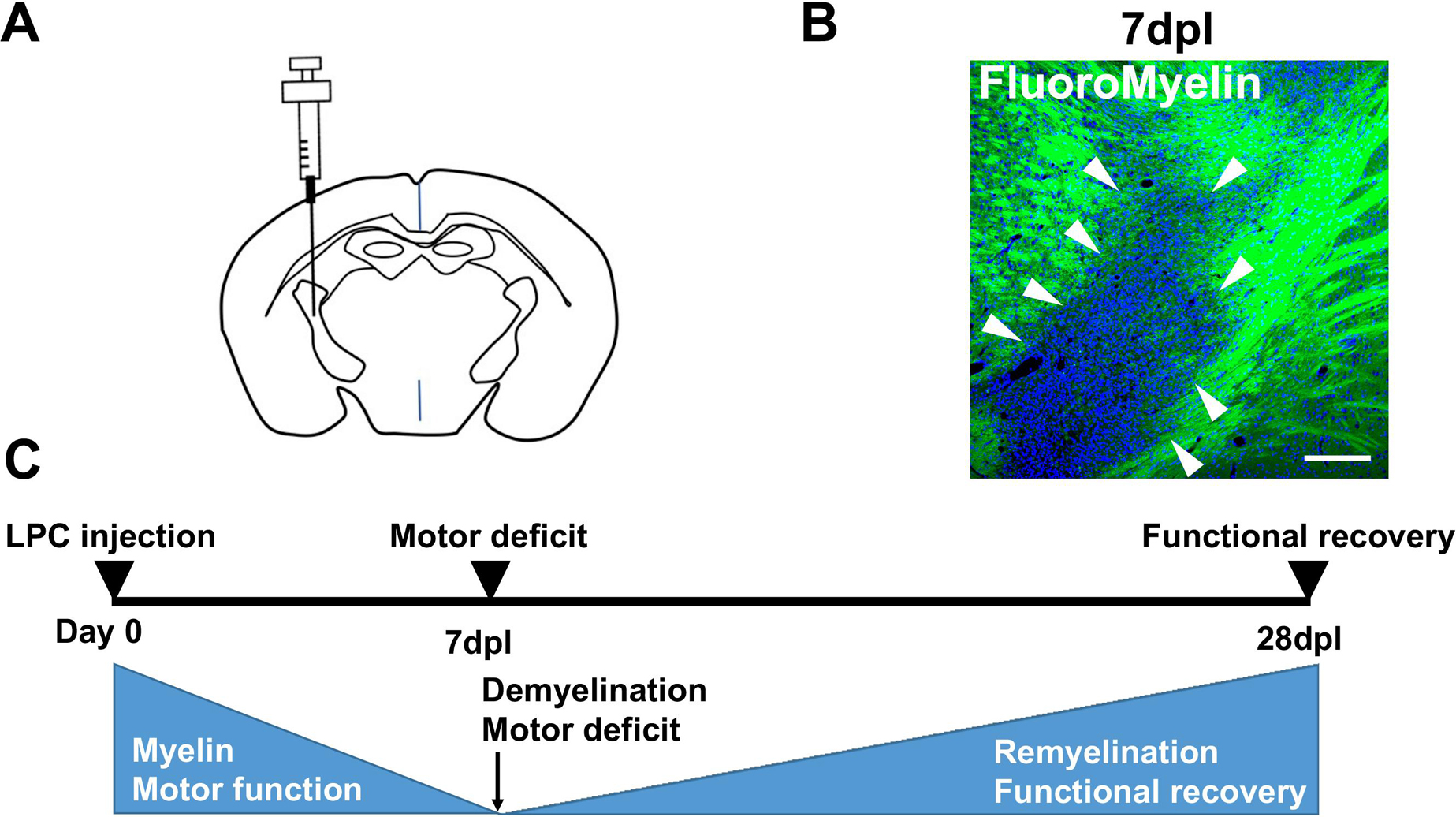
Summary of the production and analysis of the internal capsule (IC) demyelinating mouse model. (A) Lysophosphatidylcholine (LPC) is injected into the IC. (B) FluoroMyelin staining (green) of the IC demyelinating lesions at 7 days post lesion (dpl). Arrowheads show the demyelinated lesion lacking the FluoroMyelin staining. Bar = 200 μm. (C) Schematic diagram showing the time course of demyelination in the mouse model. Focal IC demyelination and acute motor deficits are observed by 7 dpl, and are followed by subsequent functional recovery through remyelination by 28 dpl.
Drug candidates for demyelinating diseases that promote remyelination have been identified using high-throughput screening platforms, such as micropillar array, and have been subsequently evaluated in clinical trials [11, 18]. Clemastine is an anti-muscarinic agent that promotes oligodendrocyte differentiation and remyelination, and has been reported as an effective drug for MS patients [11, 18]. Moreover, clemastine has been demonstrated to promote remyelination in rodent models [7, 8]. Therefore, clemastine is a promising drug candidate for MS.
We have recently investigated whether the IC demyelination mouse model can be used to evaluate drugs for functional recovery and remyelination [29]. In our study, the effect of clemastine on recovery of motor functions was examined using multiple behavioral tests. Clemastine (10 mg/kg) was administered from 3 dpl to 9 dpl for immunostaining or 3 dpl to 12 dpl for EM analysis by intraperitoneal injection after LPC injection into the IC. Clemastine treatment enhanced motor function and improved asymmetric motor paralysis after unilateral IC demyelination [29]. Histological analyses, including electron microscopy (EM), were performed to assess remyelination. The number of Olig2 and platelet-derived growth factor receptor α chain (PDGFRα)-double positive oligodendrocyte progenitor cells was significantly decreased by clemastine treatment at 10 dpl (Fig. 2A) [29]. In contrast, the ratio of mature oligodendrocytes double positive for the anti-Olig2 and anti-adenomatous polyposis coli (CC1) antigens was significantly increased in clemastine-treated mice at 10 dpl (Fig. 2B) [29]. We used neutral red staining, which is used to detect demyelinated lesions by light microscopy, for EM analysis [1, 28]. Neutral red-stained IC tissues were dissected from the IC demyelinating mouse model, and the structure of the myelin was observed by transmission EM (TEM). TEM analysis showed that the number of myelinated axons was greater in clemastine-treated mice than in mice treated with phosphate-buffered saline at 14dpl (Fig. 2C) [29]. These results demonstrate that clemastine treatment promotes remyelination and functional recovery after IC damage. Taken together, these findings suggest that the mouse model of IC demyelination is useful for testing drugs that promote remyelination and motor functional recovery.

Histological analysis of the internal capsule (IC) demyelinating mouse model given phosphate-buffered saline (PBS) or clemastine treatment. Clemastine was administered from 3 days post lesion (dpl) to 9 dpl for Fig. 2A and 2B or 3 dpl to 12 dpl for Fig. 2C by intraperitoneal injection after LPC injection. (A) Double immunofluorescence staining of PBS and clemastine-treated mice for Olig2 (green) and platelet-derived growth factor receptor a chain (PDGFRα) (red) in the demyelinated lesions in the IC at 10 dpl. Bars = 50 μm. (B) Double immunofluorescence staining of PBS and clemastine-treated mice for Olig2 (green) and CC1 (red) in the demyelinated lesions of the IC at 10 dpl. Bars = 50 μm. (C) EM images of the contralateral IC (right panel) of a clemastine-treated mouse and the ipsilateral IC of PBS (middle panel) and clemastine-treated (left panel) mice at 14 dpl. Bars = 2 μm.
It has been difficult to assess the recovery of motor functions associated with remyelination using conventional mouse models of demyelination. Therefore, to facilitate drug discovery, it was necessary to develop a new animal model of remyelination-induced functional recovery. The comparison of the demyelination mouse models are shown in Table 1. Although the IC demyelination mouse model has the disadvantage of being technically required, this is a simple model in which LPC is injected focally, and the area of demyelination and the degree of neurological symptoms can be modulated by adjusting the injection parameters. Therefore, it is a versatile mouse model that is simpler and easier to produce than the EAE and cuprizone models. In future studies, the IC demyelinating mouse model may be used in drug development pipelines to evaluate novel drug candidates for demyelinating diseases.
Comparizon of each demyelination mouse model
| Model | Method | Peak of demyelination (time) | Spontaneous remyelination | Motor dysfunction | Recovery |
|---|---|---|---|---|---|
| Cuprizone model | Feed the cuprizone diet | 4–6 weeks | Remyelination | Difficult to evaluate | Difficult to evaluate |
| EAE model | Immunization with peptide of myelin protein | 2–3 weeks | No remyelination | Motor deficit | No functional recovery |
| Internal capsule demyelination model | LPC injection into internal capsule | 5–7 days | Remyelination | Assymmetric motor deficit | Functional recovery |
| Corpus callosum demyelination model | LPC injection into corpus callosum | 5–7 days | Remyelination | Difficult to evaluate | Difficult to evaluate |
| Spinal cord demyelination model | LPC injection into spinal cord | 5–7 days | Remyelination | Difficult to evaluate | Difficult to evaluate |
There are no conflicts of interest.
We thank Dr. Jeffrey Huang at Georgetown University for research guidance, and members of the Ohno lab, especially Dr. Yasuyuki Osanai and Dr. Tom Kouki, for research support and valuable discussion. This work was supported by Jichi Medical University Young Investigator Award; Taiju Life Social Welfare Foundation; Japan Intractable Diseases (Nanbyo) Research Foundation; Japan MS Society; Takeda Science Foundation; YOKOYAMA Foundation for Clinical Pharmacology Grant Number #YRY-2210; Daiwa Securities Health Foundation; Kobayashi Foundation; Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number #20K22690 and #23K14432 to R.Y. and #21H05241, #21H04786 and #20KK0170 to N.O.; and a research grant from the National Center of Neurology and Psychiatry (No. 3–5). We also thank Barry Patel, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.