Abstract
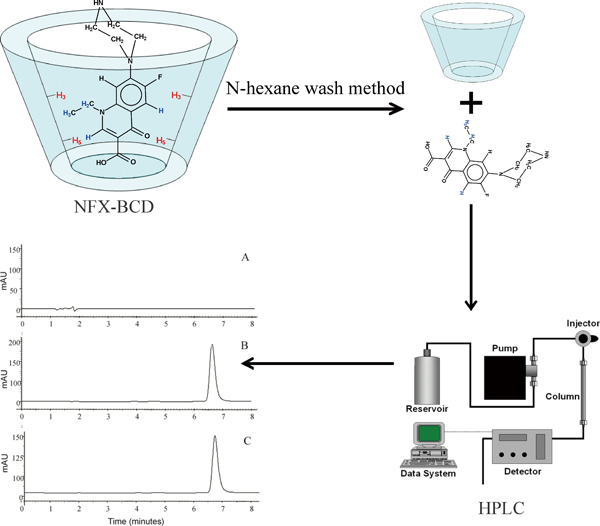
The aim of this study was to develop and validate a simple liquid-chromatography method, with good accuracy, reproducibility and sensitivity, for the quantification of norfloxacin in β-cyclodextrin inclusion complexes. In the method validation, the parameters evaluated were linearity, limits of detection and quantification, specificity, accuracy, precision and robustness. The stability-indication property of the method was evaluated through studies on the degradation under stress conditions. A method employing a simple mobile phase consisting of phosphate buffer (pH 3.0) and acetonitrile (86:14 v/v) was developed. Fluorescence detection was employed to minimize the influence of degradation products, due to its high sensitivity, selectivity and specificity. The method was specific, linear in the concentration range of 1 – 30 μg/mL, robust, precise and accurate. The proposed method was successfully applied in the determination of norfloxacin in inclusion complexes, thus aiding quality-control analysis in the future development of drug delivery systems.