Abstract
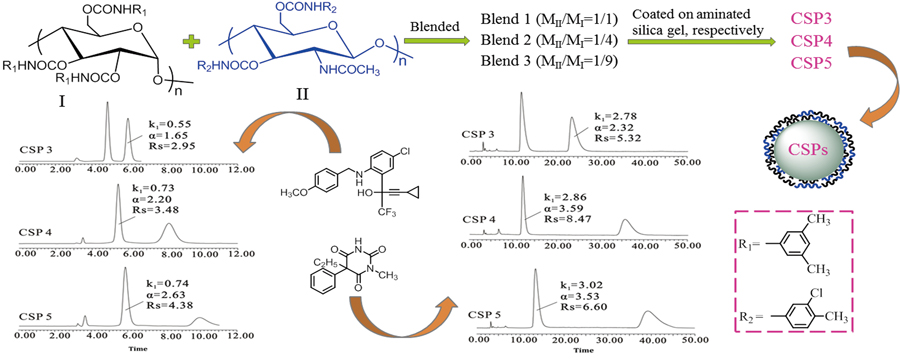
Amylose derivatives chiral stationary phases (CSPs), some of which are commercially available, are well known for their powerful enantioseparation performance. However, due to the dissolution or swelling properties of amylose derivatives, this type of CSPs prepared by coating method exhibit poor solvent tolerance and stability. In order to overcome the defect as well as maintain the chiral recognition capability of amylose tris(3,5-dimethylphenylcarbamate), chitin bis(3-chloro-4-methylphenylcarbamate) with good stability and good chiral recognition capability was blended with the amylose derivative at different ratios, and the resulting blends were further coated onto 3-aminopropyl silica gel to obtain three biselector CSPs. Meanwhile, the corresponding individual selector CSPs were also prepared, respectively, for the sake of comparison with biselector CSPs. The chiral recognition capacity and solvent tolerance of five CSPs were systematically investigated. In addition, the influence of composition and interaction of amylose tris(3,5-dimethylphenylcarbamate) and chitin bis(3-chloro-4-methylphenylcarbamate) in the biselector CSPs on the chiral recognition and the elute orders of enantiomers was also discussed in detail.