抄録
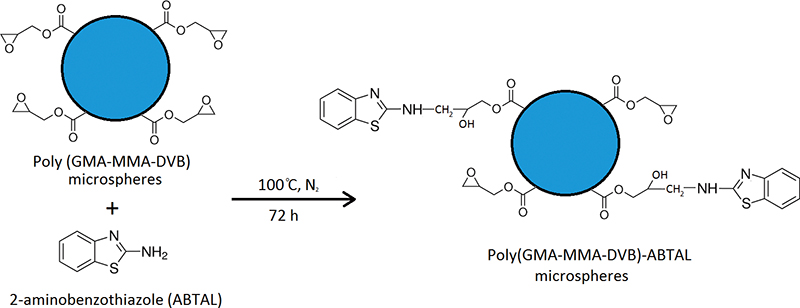
In this study, poly(glycidyl methacrylate-methyl methacrylate-divinylbenzene) was synthesized in the form of microspheres, and then functionalized by 2-aminobenzothiazole ligand. The sorption properties of these functionalized microspheres were investigated for separation, preconcentration and determination of Al(III), Fe(II), Co(II), Cu(II), Cd(II) and Pb(II) ions using flame atomic absorption spectrometry. The optimum pH values for quantitative sorption were 2 – 4, 5 – 8, 6 – 8, 4 – 6, 2 – 6 and 2 – 3 for Al(III), Fe(II), Co(II), Cu(II), Cd(II) and Pb(II), respectively, and also the highest sorption capacity of the functionalized microspheres was found to be for Cu(II) with the value of 1.87 mmol g−1. The detection limits (3σ; N = 6) obtained for the studied metals in the optimal conditions were observed in the range of 0.26 – 2.20 μg L−1. The proposed method was successfully applied to different beverage samples for the determination of Al(III), Fe(II), Co(II), Cu(II), Cd(II) and Pb(II) ions, with the relative standard deviation of <3.7%.