2013 Volume 36 Issue 10 Pages 1594-1601
2013 Volume 36 Issue 10 Pages 1594-1601
The effect of 2,3′,4,4′,5′-pentachlorobiphenyl (CB118) on serum total thyroxine (T4) level was comparatively examined between C57BL/6 and DBA/2 mice, which are sensitive and insensitive, respectively, to aryl hydrocarbon receptor-mediated biological changes. After 5 d of CB118 administration (50 mg/kg, intraperitoneally (i.p.)), the serum total T4 levels in both strains of mice were markedly decreased. However, significant decreases in serum thyroid-stimulating hormone levels were observed in DBA/2 mice, but not in C57BL/6 mice. In contrast, significant increases in the level and activity of hepatic T4-uridine 5′-diphosphate (UDP)-glucuronosyltransferase by CB118 treatment were observed only in C57BL/6 mice. Likewise, significant increases in the amounts of biliary [125I]T4 and [125I]T4-glucuronide after injection of [125I]T4 were observed only in the CB118-pretreated C57BL/6 mice. The CB118-mediated changes in the levels of [125I]T4 bound to transthyretin (TTR), albumin, and thyroxine binding globulin (TBG) were also observed in C57BL/6 mice, but not in DBA/2 mice. Despite such strain differences, significant increases in the liver-selective accumulation of [125I]T4 by CB118-pretreatment was observed in both C57BL/6 and DBA/2 mice. The present findings indicate that CB118-mediated decreases in levels of serum T4 in C57BL/6 and DBA/2 mice occur mainly through enhanced accumulation of hepatic T4.
Studies on polychlorinated biphenyls (PCBs)-mediated toxicities,1,2) such as body weight loss, endocrine disruption, teratogenicity, carcinogenicity and impairment of the reproductive and immune systems, have been performed over the last 40 years. Furthermore, Fukuoka Yusho (oil disease) and Taiwan Yucheng patients are reported to show various symptoms, such as acneform eruptions, hypersecretion of meibomian glands, hyperpigmentation of the face, and enhanced hormonal effects of thyroxine (T4), eyelids, and gingival.3,4)
PCBs consist of a series of 209 individual congeners that vary in the number and position of substituent chlorine on a biphenyl structure. These are classified into three types of inducers (i.e., 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-type, phenobarbital (PB)-type, and mixed (TCDD/PB)-type inducers) based on the induction pattern of hepatic drug-metabolizing enzymes, including cytochrome P450 (CYP) and uridine 5′-diphosphate (UDP)-glucuronosyltransferase (UGT) family enzymes.5,6) 3,3′,4,4′,5-Pentachlorobiphenyl (CB126) and 2,2′,4,4′,5,5′-hexachlorobiphenyls (CB153) are representative inducers of TCDD-type and PB-type, respectively.5) Namely, CB126 and CB153 can induce CYP1A/UGT1A and CYP2B/UGT2B subfamily enzymes, respectively.5,6) In contrast, 2,3′,4,4′,5′-pentachlorobiphenyl (CB118) is considered a mixed (TCDD/PB)-type inducer,5) which can induce CYP1A, CYP2B, UGT1A and UGT2B subfamily enzymes.5,6)
In contrast, PCB congeners, including CB126, CB153, and CB118, are well known to decrease the level of serum thyroid hormone.7–10) As a possible mechanism for the PCB-mediated decrease in serum thyroid hormone, the increase in hepatic T4-UGTs11) and the promotion of the release of T4 from a complex of T4-serum transthyretin (TTR) by a PCB congener and its hydroxylated metabolites have been proposed.10,12–14)
However, we have demonstrated that Kanechlor-500 (KC500), a commercial PCB mixture, shows an ability to decrease serum total T4 levels through enhancement of accumulation of T4 in several tissues, especially the liver, rather than via an increase in hepatic T4-UGT activity.15) Our study also indicated that CB126-mediated decrease in serum T4 levels occurs not only through the induction of T4-UGT, but also through enhanced accumulation of T4 in the liver.16) Likewise, the CB153-mediated decrease is demonstrated to be primarily dependent on enhanced accumulation of T4 in the liver and partially on the increase in the amount of excretion of biliary T4 and T4-glucuronide.17)
In the present work, therefore, to further clarify an exact mechanism for the decrease in serum total T4 levels in CB118-treated mice (Fig. 1), we examined whether there is a strain difference in the CB118-mediated decrease of serum total T4 levels between C57BL/6 mice and DBA/2 mice, which are sensitive and insensitive, respectively, to aryl hydrocarbon receptor-mediated biological changes.18,19) Based on the results in the present study, a possible mechanism for CB118-mediated decrease in serum total T4 level is herein discussed.

Panacete 810 (medium-chain triglycerides) was purchased from Nippon Oils and Fats Co., Ltd. (Tokyo, Japan). [125I]T4, radiolabelled at the 5′-position of the outer ring, was obtained from PerkinElmer, Inc., Life and Analytical Sciences (Waltham, MA, U.S.A.). CB118 was purchased from Cambridge Isotope Laboratories, Inc. (Cambridge, MA, U.S.A.). All other chemicals used herein were obtained commercially in appropriate grades of purity.
Animal TreatmentsMale C57BL/6 mice (16–31 g) and the DBA/2 mice (17–29 g) were obtained from Japan SLC, Inc. (Shizuoka, Japan). Male C57BL/6 and DBA/2 mice were housed three or four per cage with free access to commercial chow and tap water, maintained on a 12-h dark/light cycle (8 : 00 a.m. to 8 : 00 p.m. light) in an air-controlled room (temperature, 24.5±1°C, humidity, 55±5%), and handled with animal care under the guidelines of the University of Shizuoka (Shizuoka, Japan). Mice received a single intraperitoneally (i.p.) injection of CB118 (6.25, 12.5, 25, 50 or 100 mg/kg) dissolved in Panacete 810 (5 mL/kg). Control animals were treated with vehicle alone (5 mL/kg).
In Vivo StudyMice were sacrificed by decapitation without anesthesia at 5 d after administration of CB118. The thyroid gland and liver were removed and weighed. Hepatic microsomes were prepared according to the method of Kato et al.20) and stored at –85°C until use. Blood was collected from each animal between 10 : 30 and 11 : 30 a.m. After clotting at room temperature, serum was separated by centrifugation and stored at –50°C until use.
Analysis of Serum HormonesLevels of total T4, total triiodothyronine (T3), and thyroid-stimulating hormone (TSH) were measured by radioimmunoassay using a Total T4 kit (Diagnostic Products Corporation; Los Angeles, CA, U.S.A.), T-3 RIABEAD (Dainabot Co., Ltd., Tokyo, Japan) and the rTSH [125I] Biotrak assay system (GE Healthcare U.K., Ltd., Little Chalfont, Buckinghamshire, U.K.), respectively. These assays were performed in accordance with the manufacturer’s instructions. The intra-assay variation coefficients for total T4 and total T3 assays in this study were 5.7% and 3.1%, respectively, and their detection limits were 0.25 µg/dL and 0.1 ng/mL, respectively.
Hepatic Microsomal Enzyme ActivitiesThe amount of hepatic microsomal protein was determined by the method of Lowry et al.21) with bovine serum albumin as a standard. Microsomal O-dealkylase activities of 7-benzyloxy-, 7-ethoxy-, and 7-pentoxyresorufins were determined by the method of Burke et al.22) The activity of microsomal UGT toward T4 (T4-UGT activity) was determined by the method of Barter and Klaassen.23)
Western Blot AnalysisWestern blot analyses for microsomal UGT isoforms were performed by the method of Luquita et al.24) using polyclonal anti-peptide antibodies against the common region of rat UGT1A isoforms and specific antibody against rat UGT1A1.25,26) Mouse Ugt1a1, which corresponds to rat UGT1A1, was measured by use of an ECL detection kit (GE Healthcare U.K., Ltd.), and its protein level was densitometrically determined with LAS-1000 (GE Healthcare U.K., Ltd.).
Ex Vivo StudyAfter 5 d of CB118 treatment, the mice were anesthetized with saline solution (2 mL/kg) containing sodium pentobarbital (25 mg/mL) and potassium iodide (1 mg/mL). The femoral artery was cannulated (polyethylene tube SP8, Natsume Inc., Tokyo, Japan) and primed with heparinized saline (33 units/mL). Fifteen minutes later, the mice received a single i.v. injection of 1.5 µCi [125I]T4 (0.1 mL) dissolved in saline containing 10 mM NaOH and 1% normal mouse serum. Thereafter, bile was collected at the indicated times.
Biliary Excretion of Total [125I]T4 and [125I]T4 GlucuronideAfter administration of [125I]T4, bile was collected on ice for 2 h at 30-min intervals. Bile volume was gravimetrically determined. The amounts of total [125I]T4 and [125I]T4 glucuronide in bile were determined by the method of Vansell and Klaassen.27) In brief, an aliquot (10 µL) of each bile sample was used for determining total [125I]T4 level by a gamma counter (Cobra II Auto-Gamma 5002; PerkinElmer, Inc., Life and Analytical Sciences), and the assay was performed in duplicate. To measure the amount of [125I]T4 glucuronide in bile, a portion (10 µL) of each bile sample was added to 2 volumes of methanol and stored at −20°C for 1 h to precipitate the protein. After the mixture was centrifuged at 12000×g for 10 min at 4°C, the resultant supernatant was collected for HPLC analysis. The HPLC analysis was performed using a ChromSpher C18 column (10×0.3 cm) (Chrompack, Inc., Raritan, NJ, U.S.A.) in combination with ChromSep reverse phase guard column (10×2 mm) (Chrompack, Inc.) and Adsorbosphere C18 reverse-phase guard column (7.5×4.6 mm) (Alltech Associates, Inc., Deerfield, IL, U.S.A.). A solution of 0.02 mM ammonium acetate (pH 4.0) containing 16–45% acetonitrile was used to elute [125I]T4 glucuronide; 16% of acetonitrile was used as an initial solution for 6 min, and then the elution solution was changed by a linear increase to 27% over 12 min, held for 4 min, followed by a linear increase to 45% over 5 min and held for 11 min. The level of biliary [125I]T4 glucuronide was determined by a Radioisotope Detector 171 (Beckman Coulter, Inc., Brea, CA, U.S.A.).
Analysis of [125I]T4 Bound to Serum ProteinsThe levels of serum [125I]T4-thyroxine binding globulin (TBG), [125I]T4-albumin, and [125I]T4-TTR complexes were determined according to the method of Davis et al.28) In brief, after administration of [125I]T4, serum was prepared from a portion (0.08 mL) of blood, which was sampled from the femoral artery at the indicated times, and the serum was stored at –50°C until use. Serum was diluted in 100 mM phosphate buffer (pH 7.4) containing 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol, and 30% glycerol. The diluted serum was subjected to electrophoresis on 4–20% gradient native polyacrylamide gels PAG Mid “Daiichi” 4/20 (Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan). The electrophoresis was performed at 4°C for 11 h at 20 mA in 0.025 M Tris-buffer (pH 8.4) containing 0.192 M glycine. The human albumin and TTR, which were incubated with [125I]T4, were also applied on the gel as templates. After electrophoresis, a gel was dried and radioautographed for 20 h at room temperature using Imaging Plate 2040 (GE Healthcare U.K., Ltd.). The levels of [125I]T4-TBG, [125I]T4-albumin, and [125I]T4-TTR in serum were determined by counting the corresponding gel fractions identified from Bio Imaging Analyzer (BAS-2000II IP Reader, GE Healthcare U.K., Ltd.).
Tissue Distribution of [125I]T4Tissue distribution of [125I]T4 was performed according to the modified method of Oppenheimer et al.29) In brief, at 5 min after administration of [125I]T4 to CB118-pretreated and untreated mice, their cerebrum, cerebellum, pituitary gland, thyroid gland, sublingual gland, submandibular gland, thymus, heart, lung, liver, kidney, adrenal gland, spleen, testis, prostate gland, seminal vesicle, stomach, duodenum, jejunum, ileum and caecum were removed and weighed. Radioactivities in the tissues were determined by a gamma counter (Cobra II Auto-Gamma 5002; PerkinElmer, Inc., Life and Analytical Sciences).
StatisticsThe data obtained were statistically analyzed according to Student’s t-test or Dunnett’s test after analysis of variance. In addition, the amount of biliary [125I]T4 glucuronide and the binding level of [125I]T4 bound to serum proteins were statistically analyzed according to Newman–Keuls’ test after analysis of variance.
We first examined the changes in the level of serum total T4 at 2, 3, 4, 5, 6, 7 and 8 d after treatment with CB118 (50 mg/kg). Serum total T4 levels reached a minimum at day 5, and the decreased levels were maintained up to day 8. Therefore, the dose effect of CB118 on the level of serum total T4 was examined 5 d after the chemical treatment (Fig. 2). Serum total T4 level was significantly decreased by treatment with CB118 at doses over 6.25 mg/kg, and the clear decreases occurred in a dose-dependent fashion from 25 up to 100 mg/kg. Based on these results, the dose (50 mg/kg) and time (5 d after dosing) used in all the present experiments were determined. In addition, no significant effects on body weight gain, one of indicators for toxicity, in both C57/BL and DBA/2 mice were observed at 5 d after treatments with CB118 at the dose range used (data not shown).

Mice were sacrificed 5 d after i.p. administration of CB118 at the indicated doses, and the level of the serum total T4 was measured as described in Materials and Methods. Constitutive total T4 level: 2.12±0.11 µg/dL (n=4). Each point represents the mean±S.E. (vertical bar) for four to five mice. * p<0.05, significantly different from the control.
The effects of CB118 on the levels of serum thyroid hormones were examined in C57BL/6 and DBA/2 mice (Fig. 3). The serum total T4 level after CB118 treatment was markedly decreased in both C57BL/6 and DBA/2 mice. Likewise, significant CB118-mediated decreases in serum total T3 levels in both C57BL/6 and DBA/2 mice were observed (Fig. 3). The magnitude of the decrease in C57BL/6 mice was greater than that in DBA/2 mice. In contrast, a significant decrease in serum TSH levels mediated by CB118 was observed only in DBA/2 mice, but not in C57BL/6 mice (Fig. 3).

Animals were sacrificed 5 d after the administration of CB118 (50 mg/kg), and serum thyroid hormone levels were measured as described in Materials and Methods. Each column represents the mean±S.E. (vertical bar) for four to five animals. * p<0.05, significantly different from each control.
The effects of CB118 on hepatic microsomal activities of ethoxyresorufin O-dealkylase (Cyp1a1/2), benzyloxyresorufin O-dealkylase (Cyp2b1/2 and Cyp3a1/2), and pentoxyresorufin O-dealkylase (Cyp2b1/2) were examined in C57BL/6 and DBA/2 mice. Treatment of C57BL/6 mice with CB118 resulted in significant increases in hepatic microsomal enzyme activities as follows: 8.8-fold for ethoxyresorufin O-dealkylase; 4.0-fold for benzyloxyresorufin O-dealkylase activity; and 6.0-fold for pentoxyresorufin O-dealkylase activity (Table 1). In contrast, no such CB118-mediated increases were observed in DBA/2 mice.
| O-Dealkylase of alkoxyresolfin | C57BL/6 | DBA/2 | ||
|---|---|---|---|---|
| Control | CB118 | Control | CB118 | |
| 7-Ethoxy- | 0.17±0.02 | 1.50±0.17* | 0.14±0.009 | 0.14±0.01 |
| 7-Benzyloxy- | 0.13±0.03 | 0.52±0.03* | 0.06±0.006 | 0.09±0.01 |
| 7-Pentoxy- | 0.02±0.004 | 0.12±0.003* | 0.02±0.003 | 0.02±0.003 |
Animals were sacrificed 5 d after administration of CB118 (50 mg/kg). The activities of alkoxyresorufin O-dealkylase are represented as nmol of the resolfin formed/mg protein/min. Data represent the mean±S.E. for three to four mice. * p<0.01, significantly different from each control.
We first examined the effects of CB118 on hepatic microsomal T4-UGT activity in C57BL/6 and DBA/2 mice. The activity of hepatic T4-UGT was significantly increased by CB118 in C57BL/6 mice, but not in DBA/2 mice (Fig. 4).

Hepatic microsomes from individual animals were used for T4-UGT enzyme assay as described in Materials and Methods. Each column represents the mean±S.E. (vertical bar) for four animals. * p<0.05, significantly different from each control.
Because T4-glucuronidation is primarily mediated by hepatic UGT1A1 and UGT1A6 in the rat liver,11) the effects of CB118 on the amounts of the proteins responsible for T4-UGTs, such as total Ugt1a and Ugt1a1, were examined by Western blot analysis (Fig. 5A). CB118-mediated increases in the amounts of hepatic Ugt1a and Ugt1a1 proteins occurred in C57BL/6 mice, but not in DBA/2 mice (Fig. 5B).

Hepatic microsomes from individual animals were used for Western blot analysis as described in Materials and Methods. The separated bands (A) responsible for Ugt isoforms were densitometrically quantified as described in Materials and Methods. Molecular markers (Cell Signaling Technology, Inc.): 58 kDa, a maltose-binding protein–chitin binding protein; 46 kDa, a chitin binding domain-Mxe intelin-2 chitin binding domain. The data are represented as the mean±S.E. (vertical bar) for four animals (B). * p<0.05, significantly different from each control.
Furthermore, the amounts of biliary [125I]T4 and [125I]T4-glucuronide after intravenous (i.v.) injection of [125I]T4 were slightly increased by CB118-pretreatment in C57BL/6 mice, but not in DBA/2 mice (Fig. 6).

Bile was collected at 30-min intervals after i.v. administration of [125I]T4, and amounts of the biliary total [125I]T4 and [125I]T4-glucuronide were measured as described in Materials and Methods. Each point represents the mean±S.E. (vertical bar) for four mice. * p<0.05, significantly different from each control. ―○―, control; ―▼―, CB118.
The effects of CB118 on the binding of [125I]T4 to serum proteins, such as TTR, albumin, and TBG, were examined. In CB118-pretreated C57BL/6 mice, the levels of serum [125I]T4-TTR complex were significantly decreased. In contrast, the binding levels of [125I]T4 to serum albumin and TBG were increased. In DBA/2 mice, no such effects of CB118-pretreatment were observed (Fig. 7).

The amounts of [125I]T4 bound to serum proteins 5 min after [125I]T4 administration were assessed by the method described in Materials and Methods. Each column represents the mean±S.E. (vertical bar) for four to five animals. * p<0.05, significantly different from each control.
The effects of CB118-pretreatment on the tissue distribution level of [125I]T4 were examined. In the control C57BL/6 and DBA/2 mice, the accumulation of [125I]T4 was the highest in the liver among all the tissues examined (Fig. 8). In both strains of mice, pretreatment with CB118 resulted in significantly increased accumulation in the liver, and the hepatic levels in C57BL/6 and DBA/2 mice achieved more than 45% and 31% of the [125I]T4 dosed, respectively (Fig. 8). Furthermore, the accumulation levels per gram liver in the CB118-pretreated C57BL/6 and DBA/2 were increased 1.41- and 1.13-fold, respectively, compared with those in the corresponding control animals (Table 2). In addition, significant decreases in accumulation of [125I]T4 were observed in the testis of the CB118-pretreated C57BL/6 mice (Fig. 8). In the both strains of mice, little changes in the CB118-mediated levels of [125I]T4 occurred in any extrahepatic tissues examined.
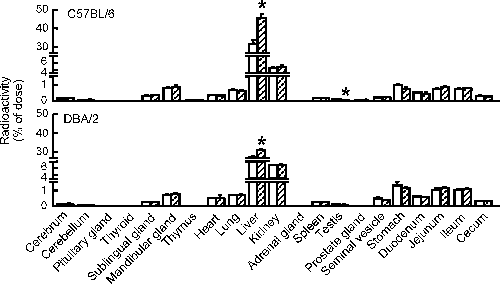
Five days after CB118-pretreatment, [125I]T4 was administered to mice, and at 5 min after administration, radioactivity in each tissue was measured as described in Materials and Methods. Each column represents the mean±S.E. (vertical bar) for four animals. * p<0.05, significantly different from each control.  , control;
, control;  , CB118.
, CB118.
| C57BL/6 | DBA/2 | |||
|---|---|---|---|---|
| Control | CB118 | Control | CB118 | |
| [125I]T4 | 22.64±1.35 | 32.00±2.85* | 19.88±0.46 | 22.37±0.52* |
[125I]T4 level is represented as % dose per g liver. Radioactivity in the liver was measured at 5 min after [125I]T4-administration. Data represent the mean±S.E. for three to four mice. * p<0.05, significantly different from each control.
Furthermore, approximately 10% increases in the liver weight by CB118-treatment were observed in C57BL/6 and DBA/2 mice, whereas no such increases in the weight of the thyroid gland were observed in either strain of mice (Table 3).
| Tissues | C57BL/6 | DBA/2 | ||
|---|---|---|---|---|
| Control | CB118 | Control | CB118 | |
| Thyroid | 0.007±0.0004 | 0.009±0.002 | 0.014±0.002 | 0.012±0.002 |
| Liver | 5.22±0.23 | 5.67±0.23 | 5.05±0.07 | 5.56±0.18* |
Animals were sacrificed 5 d after administration of CB118 (50 mg/kg). The values are represented as % body weight. Data represent the mean±S.E. for three to five mice. * p<0.05, significantly different from each control.
In the present study, we demonstrated that treatment with CB118 resulted in significant decreases in serum total T4 levels in both C57BL/6 and DBA/2 mice. Furthermore, we herein indicated that the CB118-mediated decrease occurred mainly through increased accumulation of T4 in the liver.
To date, as one of the explanations for the decrease in serum thyroid hormones by TCDD-type and mixed (TCDD/PB)-type PCBs, a hepatic T4-UGT-meidated mechanism has been considered, because PCBs including CB126 and CB118 show the ability to induce hepatic T4-UGT(s) responsible for the metabolism of thyroid hormones in rats.8,30,31) However, the change in hepatic T4-UGT activity by CB153, a PB-type inducer, does not necessarily correlate with those of serum total T4 in either rats or mice.10)
In this study, we further demonstrated that CB118, a mixed (TCDD/PB)-type inducer, showed a definite capacity for decreasing serum total T4 level in both C57BL/6 and DBA/2 mice, although the CB118-mediated increases in the level and activity of hepatic T4-UGTs, such as Ugt1a and Ugt1a1, and in the amounts of the biliary metabolite(s) including [125I]T4-glucuronide after i.v. injection of [125I]T4 occurred only in C57BL/6 mice (Figs. 4–6). Such strain differences in the amount of the biliary [125I]T4 metabolite(s) would be attributed to differences in CB118-mediated induction of hepatic T4-UGTs. In contrast, in both strains of mice, CB118-pretreatment resulted in a liver-selective significant increase of [125I]T4 accumulation, strongly suggesting that the decrease in the level of serum total T4 in CB118-treated mice occurs mainly through increased accumulation of T4 in the liver. These results were primarily identified with those previously reported on other PCB-treated rats and mice.15–17) Furthermore, the CB118-mediated decreases in serum T4 level in both C57BL/6 and DBA/2 mice were confirmed to occur through increase in T4 accumulation level per gram liver and/or development of liver hypertrophy. In addition, a slight CB118-mediated decrease in serum TSH level, one of the factors regulating a serum thyroid hormone level, was observed in DBA/2 mice, but not in C57BL/6, suggesting that CB118-mediated decrease in serum T4 level in DBA/2 mice is, at least in part, dependent on the decrease in serum TSH level.
As a possible mechanism for the CB118-mediated increase in T4 accumulation in the liver, a TTR-associated pathway might be considered because PCB and its hydroxylated metabolites act as competitors of T4, forming a T4-TTR complex12–14) and because the decrease in level of T4-TTR complex leads to increases in the levels of serum free T4 and hepatic T4 uptake.13,32) In addition, the concentrations of CB118 in the blood of Yusho patients are 10–1000-fold higher than those of serum free T4, suggesting that CB118 would inhibit a T4-TTR complex formation in the patients.4,33) However, CB118-mediated changes in the levels of the [125I]T4 bound to TTR, albumin, and TBG were observed in C57BL/6 mice but not in DBA/2 mice. Such strain differences would be dependent on differences in induction of hepatic CYP1A enzymes responsible for the formation of the hydroxylated CB118 metabolites, which show higher affinities to TTR than the parent PCB.12–14) Accordingly, in C57BL/6 mice, but not DBA/2 mice, a TTR-associated pathway might partially contribute to the CB118-mediated increase in [125I]T4 accumulation in the liver. Unfortunately, a principal mechanism for the CB118-mediated increase in the liver-selective accumulation remains unclear. Since a liver-selective function for T4-transportation might exist, further studies on hepatic T4-transporters would be useful for understanding a liver-selective increase in T4 accumulation by CB118.
In conclusion, we herein demonstrated that the CB118-mediated decrease in serum T4 levels in mice occurred primarily through increased accumulation of T4 in the liver and further suggested that the decreases in C57BL/6 and DBA/2 mice were partially dependent on an increase in the excretion of the biliary T4 metabolite(s), including T4-glucuronide, and a decrease in serum TSH level, respectively. Further studies on the effects of PCBs, including CB118, on hepatic T4-transporters would be necessary to understand the exact mechanism for the PCB-induced decrease in the serum T4 level.
This work was supported in part by the Grant-in-Aid for Scientific Research (C) [No. 23510083, Y.K.] from the Japan Society for the Promotion of Science.