2013 Volume 36 Issue 11 Pages 1683-1687
2013 Volume 36 Issue 11 Pages 1683-1687
Organic–inorganic hybrids of poly(dimethyl siloxane), gelatin, and chitosan with such silanes as tetraethoxysilane and 3-glycidoxytriethoxysilane are derived via the sol–gel routes. Their biomedical applications are discussed from biomimetic deposition of bone-like apatite, cell culture, and in vivo behavior.
The sol-gel procedure1) for silicate-related materials commonly uses alkoxysilanes such as tetraethoxysilane (TEOS) or its derivatives in which the alkoxy groups are partially replaced by groups including aminopropyl, vinyl, or 3-glicydoxypropyl to form, for example, (RO−)3Si–X where RO and X stand for the alkoxy and substituted organic groups, respectively. The alkoxy groups are subjected to step-wise hydrolysis due to an acid or base catalysis:
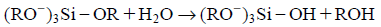 | (1) |
 | (2) |
Thus, in stoichiometry, 1 mol of Si(OR)4 leads to SiO2 and 4ROH, consuming 2H2O. In a conventional sol–gel synthesis, the system consists of silanes, an excess amount of H2O, catalyst such as HCl, HNO3, CH3COOH, or NH4OH, and an alcohol as the co-solvent for H2O and the silanes. Some additives such as surfactants and block co-polymers can be involved when necessary. For example, Nakanishi2) employed poly(ethylene oxide), form amide, poly(acrylic acid), and others to prepare meso-porous silica gels under the HNO3 catalysis from the systems TEOS and tetramethoxysilane (TMOS), H2O, and ethanol. Since Si is one of the biologically active elements in metabolism as Carlisle showed,3) introduction of Si in the form of silanes into our living systems is acceptable as far as the amount remains within the necessary level. When combined with natural polymers such as gelatin or chitosan, the silane bridges control biodegradation of those polymers, whereas the use of (RO−)3Si–X, like aminopropyltriethoxysilane (APTES) modifies those polymers. Table 1 lists some examples of the hybrids systems, a few of which are introduced below in more detail.
| Components | Biodegradable hybrids | Sustainable hybrids |
|---|---|---|
| Polymers | Natural polymers Chitosan, gelatin, collagen | Synthetic polymers PDMS, poly(lactic acid) |
| Inorganic | X−Z(OR)3: Z=Si, Ti OR: alkoxy groupsX: vinyl, glycidoxy, methacryloxy, amino group | |
| Additives | Poly(ethylene oxide), co-polymers like PEO–PPO–PEO | |
| Applications | ||
| Solid bodies | Resorbable fillers | Tissue defect fillers |
| Porous bodies | Scaffolds | Scaffolds |
| Tissue guides | Bioreactors | |
| Beads, granules | Drug delivery | Fillers |
| Sheets, films | Protector of cell invasion | |
| Tubes, fibrils | Wound healing agents | Fillers |
| Coatings | Tissue guides | Tissue guides |
| Gelling sols, gels | Injectable sols | Injectable sols |
| Carriers | ||
| Tissue defect fillers | Tissue defect fillers |
Organic–inorganic hybrids are denoted as Ormosils4) or Ceramers.5) Inorganic bonds Si–O and Ti–O form the hybrid skeleton, from which other organic or inorganic modifying groups branch off. Ormosils are employed to denote the hybrids specifically from the system TEOS and poly(dimethyl siloxane) (PDMS).6,7) They consist of nano-sized silica blocks from the hydrolysis and condensation of TEOS and PDMS chains of varied length, most of which are grafted to relevant silica blocks. The ratio of mixing TEOS and PDMS as well as the molecular length of PDMS can change in the fractions of the blocks and chains, giving rise to rubbery or ceramic-like brittle hybrids.7) Adding calcium ions in the precursor solution derived from TEOS–H2O–HCl–PDMS oligomer, Tsuru et al.6) synthesized strange Ormosil-type hybrids that spontaneously deposit apatite similar to that in bone in terms of Ca-deficiency and carbonate ion incorporation. They used an aqueous solution that contained only the same inorganic ions as human plasma in similar concentrations: Kokubo’s simulated body fluid (SBF).8) SBF is accepted to reproduce well in vivo behavior of materials under in vitro conditions. This result suggests that the Ca-involved Ormosil should spontaneously deposit bone-like apatite on the surface when implanted in bone. Such apatite is known to deposit in vitro on Bioglass®, Bioverit®, or Cerabone A–W® when those glass and glass-ceramics form strong and direct bond with bone.9) Those are called “bioactive” materials, and it is taken for granted that the spontaneous deposition of the bone-like apatite is equivalent to the bone-bonding characteristics of the relevant materials. Therefore their Ca-Ormosils are bioactive, and are employable as a bone-repairing material. In addition, Yabuta et al.10) applied approximately 2-mm-large sucrose granules as the porogen to fabricate Ormosils with 3-dimensionally porous microstructure. Figure 1 shows that those hybrids with approximately 0.5-mm-large pores responded well when cultured with several cell-line and primary cells, suggesting the possibility of application to sustainable scaffolds for artificial organs or bioreactors that take advantage of their chemical stability and biocompatibility. Indeed, Kataoka et al.11) set the porous hybrid in a radial-flow bioreactor to which human hepatocellular carcinoma (HepG2) cells were attached. They found that the cells were actively proliferated and formed cell clusters, and that the cells secreted albumin more efficiently than they did in a poly(vinyl alcohol) (PVA) scaffold.11)

(a) Keratinocyte (HaCaT), (b) osteoblast-like cell-line (HuO–3N1), and (c): hepatocyte (OUMS-29). (d) hepatocellular carcinoma (HepG2). Hematoxyline and eosin (H&E) staining. Courtesy of Dr. Huh Nam Ho, Department of Cell Biology, Okayama University Medical School.
The components can be replaced with many similar compounds to modify the hybrid properties. Titanium tetraisopropoxide12,13) is one of the good substituents for TEOS, while a variety of polymers can replace PDMS, such as poly(ether ketone), poly(methyl methacrylate), polystyrene, poly(ethylene oxide) (PEO), epoxy, to name a few. Other polymers such as poly(vinyl alcohol), or polyurethane can be accommodated in the system, whereas, Wen and Wilkes postulated,14) no bonding interactions between them and silanes were likely. It should be noted here that Nakanishi,2) mentioned above, added PEO to his TEOS–H2O–HNO3 system, but he found the spinodal-type phase separation into silica-rich and polymer-rich phases. Thus the bonding or no bonding interactions are likely to depend on the other ingredients and compositions (mixing ratio of the components).
Gelatin has been long considered for a carrier of some growth factors and proteins,15–17) although it degrades very quickly. Silanes18) are good candidates, as well as poly(L-lactic acid),19) etc., to be combined with gelatin, because appropriate involvement of a silane in the system should optimize the stability or biodegradability. Furthermore, hybrids with calcium ions can release these ions into plasma when embedded in vivo, and they should stimulate spontaneous deposition of apatite on the hybrid surface. Thus hybrid gel with calcium ions is fixed to bone or other tissues that involve apatite as an inorganic component. Ren et al.20) hybridized 3-glycidoxypropyltrimethoxysilane (GPTMS) with gelatin and controlled the degradation in TRIS buffer solution. These researchers also considered21) that the calcium ions broke the helical arrangement of the gelatin fibers and uncoiled the strands into random coils, as Wüstneck et al. suggested.22) Their amino-residue analysis indicated that the epoxy group of GPTMS preferably was grafted to the lysine (Lys) and histidine (His) residues whereas the Si–OH groups on a GPTMS molecule from the hydrolysis of Si–OCH3 groups are condensed with that on the other molecule to bridge two adjacent gelatin fibrils. With an increase in the hybridization ratio, i.e., molar mixing ratio of gelatin unit/GPTMS, over 0.5, the hybrid sustained in TRIS buffer solution longer than 40 d with weight decrease of <25%. When the ratio was kept 0.33, the hybrid was completely dissolved by 30 d. Moreover, the Ca-containing hybrids interestingly deposited apatite in SBF within 1 d as Bioglass® and Cerabone A–W®. Freeze-drying the wet hybrids introduced pores, but the porosity and average pore size decreased with the freezing temperature: frozen at −17°C: approximately 80%, 300–500 µm; at −80°C: 63%, 30–50 µm; −196°C: approximately 50%, 5–10 µm. When −17°C freezing was applied after drying the porous gel frozen at −196°C, bimodal pores were introduced: that is, so many smaller pores 5–10 µm in diameter were formed in the walls of the 300–500-µm pores. MC3T3-E1 osteoblast-like cells were vigorously proliferated on those hybrids with the bimodal pores, and a large number of Ca-rich globules covered the surface in 2 weeks. Bundles of collagen fibers were also observed after cell culture for 3 weeks. Figure 2 shows that the hybrid induced calcification within 2 weeks when embedded in the muscle of mice hind legs, indicating that the gelatin hybrids are excellent in osteoconduction or induction. Deguchi et al. applied this porous hybrid for brain nerve cell regeneration.23) They observed that the specimen implanted into the lesion remained at the same site for 60 d, kept integrity of the brain shape, and attached well to the surrounding brain tissues without any damage. Yet, their experiments indicated that the materials were insufficient in the ability to induce better regeneration of brain cells and required basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF).

Pellet: f: 6 mm, thickness: 3 mm, pore size 120 μm. a) Surgery. The insert shows the pellet specimens. X-Ray images after the surgery: b) 0 week, c) 2 weeks.
Chitosan, like collagen and gelatin, is one of the materials long used in biomedical applications because of excellent biocompatibility and biodegradation. For tailoring the characteristics of chitosan, one of the key issues is to bridge the chitosan fibrils with some cross-linking agents. Since the amino groups on the chitosan chain are present in the form of –NH3+ under physiological pH conditions, silane coupling agents, like GPTMS and TEOS, are likely to bridge two chitosan fibrils. In contrast, glycidylmethacrylate (an epoxy group on one side and a vinyl group on the other) behaves as a monovalent coupler in terms of the covalent interaction with chitosan fibril, because the vinyl groups would not be polymerized with each other. Cross-linking is hence unlikely to occur. Shirosaki et al.24) hybridized chitosan and GPTMS to fabricate xerogel membrane specimens coded as ChGx with x (0–20 mol%) representing the hybrids of compositions (100−x)chitosan+ xGPTMS, and examined the effects of GPTMS on the proliferation of MG63 cells. Even a small addition of GPTMS was highly effective to stimulate cell proliferation. Figure 3 shows that the cells partly covered the surface of sample Ch, while sample ChG01 was almost confluent with MG63 after culture for 6 d. Sample ChG05 was fully covered with the cells, and ChG10 and ChG20 similarly stimulated cell proliferation.

Bar: 20 µm.
The same precursor solutions were freeze-dried to fabricate 3-dimensionally porous chitosan–siloxane hybrids ChGx (x: 0–20),25) where the pore sizes for specimens frozen at −20 and −85°C were approximately 100 and 50 µm, respectively, and the porosity was roughly 90% irrespective of the freezing temperature. MG63 cells were well proliferated on the surface, and migrated throughout the porous membrane. Although they showed good cell proliferation, those xerogel membranes with little porosity caused inflammation of the surrounding tissues when implanted. Yet, no such inflammation was observed around the porous membrane.26) Nutritious substances are not always supplied into every part of a scaffold even if their porosity is high. The porous hybrids also well cultured primary cells such as human osteoblasts (HOB).24,25) The above results strongly indicate that these ChGx hybrids should be suitable for scaffold applications. Indeed, Amado et al.27) and Simoes et al.28) employed these porous hybrids for repairing rat sciatic nerve defects that were taken as a model for peripheral nerve injury. The withdrawal reflex latency data were taken from Amado et al.27) and plotted in Fig. 4. The group without any treatment naturally showed the slowest recovery, and the two groups treated with the porous hybrid ChG10 membrane showed the fastest recovery. The N1E-115 cell culture prior to implantation caused marginal effects on recovery from injury, indicating that the porous microstructure of the hybrid membrane was the key factor.

The sciatic nerve of rats was crushed, and the recovery was measured in terms of WRL. See ref 27 for the detail of the experiments. Symbols: open circle: as crushed; open square: solid membrane; filled square: solid membrane with N1E-115 cell culture prior to implant; open triangle: porous hybrid; filled triangle: porous hybrid with N1E-115 cell culture prior to implantation. The materials leading to the shorter WRL are better in the nerve repairing.
Hybridization of organic components with inorganic components, especially with silane coupling agents via the sol–gel procedure, yields several hybrids that exhibit some interesting biological characteristics. For example, Ormosils from TEOS and PDMS were very compatible with many cells and induced marked proliferation. The porous Ormosils are applicable as bioreactors or artificial organs. Gelatin was hybridized with GPTMS for bridging gelatin fibrils to control biodegradation. The porosity and pore sizes were controllable by the freezing temperature before vacuum drying. The gelatin hybrids induced calcification when implanted in the muscle of mice hind legs, suggesting their osteoconduction or induction ability. Those with optimum pore structure are applicable to repairing the central nervous system (CNS) although some growth factors are still necessary for sufficient nerve cell regeneration. The chitosan–GPTMS hybrid xero gel membranes markedly proliferated osteoblasts, but caused severe inflammation when they were subcutaneously implanted into mice. In contrast, the porous hybrids actively stimulated tissue regeneration, even nerve cells. Many other hybrids have been synthesized and reported in the literature: with those experiences, organic-inorganic hybrids are highly promising materials in the biomedical field.