2013 Volume 36 Issue 12 Pages 1883-1890
2013 Volume 36 Issue 12 Pages 1883-1890
The present study was conducted to investigate the effects of probucol on the progression of diabetic nephropathy and the underlying mechanism in type 2 diabetic db/db mice. Eight weeks db/db mice were treated with regular diet or probucol-containing diet (1%) for 12 weeks. Non-diabetic db/m mice were used as controls. We examined body weight, blood glucose, and urinary albumin. At 20 weeks, experimental mice were sacrificed and their blood and kidneys were extracted for the analysis of blood chemistry, kidney histology, oxidative stress marker, and podocyte marker. As a result, 24 h urinary albumin excretions were reduced after probucol treatment. There were improvements of extracellular matrix accumulation and fibronectin and collagen IV deposition in glomeruli in the probucol-treated db/db mice. The reduction of nephrin and the loss of podocytes were effectively prevented by probucol in db/db mice. Furthermore, probucol significantly decreased the production of thiobarbituric acid-reactive substances (TBARS), an index of reactive oxygen species (ROS) generation and down-regulated the expression of Nox2. Taken together, our findings support that probucol may have the potential to protect against type 2 diabetic nephropathy via amelioration of podocyte injury and reduction of oxidative stress.
Diabetes mellitus is an increasingly prevalent disease around the globe. Diabetic nephropathy (DN) is the most common renal complication of diabetes mellitus and a leading cause of end-stage renal disease. Increased urine albumin excretion is not only an indication of diabetic renal injury but an important factor in the progression of DN.1) Multiple factors are involved in the evolution of DN, including metabolic disturbance, abnormal renal haemodynamics, chronic inflammation and the oxidative stress. Rectification of metabolic disturbance is a basic strategy in the treatment of DN.2) Angiotension converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) are internationally accepted drugs in the treatment of DN by improving renal haemodynamics. Although the current therapy by inhibition of rennin angiotensin system (RAS) slows down the progression of DN, it is not enough to prevent completely the progression of DN. In recent years, more attention has been paid to the anti-oxidative treatments, which have been found to delay the progression of DN.3,4) All these suggested that rectification of metabolic disturbance and renal haemodynamics combined with anti-oxidative treatments may provide new strategies for comprehensive treatment to improve the therapeutic efficacy in patients with DN.
Probucol is a compound with two phenolic groups, which has been used for years as an anti-hyperlipidemic agent. Over the last several years, intensive studies have shown that this drug acts as a potent oxygen radical scavenger and can efficiently prevent tissue and organ damage caused by oxidative stress.5,6) In vitro and in vivo studies have demonstrated that the antioxidant potential of probucol is comparable to or even higher than that of well-known antioxidant vitamin E, the advantage of the former being its higher lipophilicity and tendency to concentrate in cellular membranes, efficiently protecting them against damage caused by lipid peroxidation.7,8) The addition of probucol to candesartan normalized urinary protein excretion, glomerular cell number and glomerulosclerosis, and completely eliminated reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-associated reactive oxygen species (ROS) production in rat progressive mesangioproliferative glomerulonephritisin.9) Probucol suppressed oxidative stress and improve glomerulosclerosis in the kidneys of streptozotocin (STZ) induced type 1 diabetic rats.10) Moreover, interestingly, recent clinical trial indicates that probucol reduce urinary protein, prevent the progression of DN, and extend the interval to initiation of hemodialysis therapy in type 2 diabetic patients with clinical albuminuria.11) However, the potential mechanism explaining the findings obtained by the clinical trial is still unknown. Moreover the efficacy of probucol on type 2 diabetic nephropathy remains to be poorly understood in not only humans but also animal models. Given that type 2 diabete is much more prevalent in humans than type 1 diabetes, it is important to test this strategy in type 2 diabetes models. In this study, using obese and type 2 diabetic db/db mice, a useful model of human diabetic nephropathy, we examined the effects of probucol on type 2 diabetic nephropathy in detail.
Probucol was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.), all antibodies including Fibronectin (rabbit polyclonal), Collagen IV (rabbit polyclonal), Nephrin (rabbit polyclonal), WT-1 (rabbit polyclonal), desmin (monoclonal), Nox2 (monoclonal), and Nox4 (monoclonal) from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.).
Animals and TreatmentAnimal experiments were performed according to the Guidelines for Animal Experimentation approved by the Ethics Committee of the China Medical University. C57BL/KsJ-db/db and age-matched db/m male mice at 7 weeks of age were obtained from China Medical University Laboratories (Shenyang, Liaoning, China). The mice were allowed free access to food and water in a specific pathogen-free environment. At 8 weeks of age, the db/db mice were divided into two groups, with one group (n=9) kept on a regular diet and the other group (n=9) started on a probucol-containing diet (1.0%). The db/m mice (n=9) served as the non-diabetic control. Blood glucose and 24 h urinary albumin were performed every 4 weeks. At the 20 weeks of age, mice were sacrificed, and blood samples and renal cortex were collected.
Determination of Urine AlbuminMice were housed in metabolic cages throughout 24 h. Urine specimens were obtained, and the volumes were determined. Urine albumin concentrations were measured by enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions (Exocell Inc., U.S.A.).
Determination of Blood Biochemical ParametersEthylenediaminetetraacetic acid (EDTA) anti-coagulated heart blood specimen was obtained. The levels of cholesterol (Chol), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), alanine transaminase (ALT), aspartatetransaminase (AST), blood glucose, glocosyated hemoglobin (HbA1C) and creatinine (Cr) in the plasma were determined using a automatic biochemical analyzer (Hitachi7080).
Periodic Acid Schiff (PAS) StainingThe kidneys were fixed in 10% formaldehyde, embedded in paraffin, cut into 2-µm sections and stained with PAS. The pathological changes were observed under light microscope. Photographs were obtained and glomerular matrix expansion was quantified in a blinded fashion by Image J software. At least 20 glomeruli from each individual mouse were assessed. The result was present as percentage of PAS-positive area in mesangium divided by the glomerular tuft.
ImmunofluorescenceCryosections of the renal cortex tissues were obtained followed by blocking with 10% bovine serum albumin (BSA). Then, these sections were incubated with rabbit polyclonal antibody: fibronectin (1 : 100), collagen IV (1 : 100), nephrin (1 : 150), WT-1 (1 : 200) at 4°C overnight followed by fluorescein isothiocyanate (FITC) or TRIC conjugated secondary antibody. The slides were observed under fluorescence microscope and photographs were obtained. The optical density and area were quantified in a blinded fashion by Image J software. At least 20 glomeruli from each individual mouse were assessed.
Western BlotRenal cortex tissue were homogenized in lysis buffer (Cell Signaling Technology, Beverly, MA, U.S.A.) with 1% NP40, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 tablet/10 mL protease inhibitor mix (Complete, Mini; Roche Diagnostics Corp., Indianapolis, IN, U.S.A.). Protein samples (60 μg, determined by the BCA protein assay) were separated in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose membrane, which was then blocked and incubated with specific antibodies: Nox2 (1 : 1000), Nox4 (1 : 1000) or β-actin (1 : 2000). Membranes were subsequently washed, incubated with specific secondary horseradish peroxidase-conjugated antibodies, and developed with an ECL chemiluminescence detection kit (Santa Cruz). Membranes were visualized by exposure to X-ray film. The films were then scanned and quantified with Image J software. The results were represented relative to β-actin.
Real-Time Quantitative Polymerase Chain Reaction (PCR)Total RNA was extracted immediately from renal cortex tissue by TRIzol Reagent according to the manufacturer’s instructions. Two micrograms of total RNA was reverse-transcribed using the superscript III first-strand synthesis system (Invitrogen). Real-time reverse transcription (RT)-PCR was performed using a SYBR green dye I (Applied Biosystems, Foster City, CA, U.S.A.) with the ABI 7900 Sequence Detection System (PE Applied Biosystems). Fluorescence signals were recorded in each cycle. Relative quantification of gene expression was carried out using 2ΔΔCT method. Samples were run in triplicate in separate tubes to permit quantification of the target gene normalized to β-actin. Sequences of primers used are listed below (nephrin: sense 5′-GGC TCC TGA AGA CAC AGA CC-3′, anti-sense 5′-CAT TCC TGA TTC GAT CCT CCT-3′; WT-1: sense 5′-AGT TCC CCA ACC ATT CCT TC-3′, anti-sense 5′-ATT CAA GCT GGG AGG TCA TTT-3′).
Measurement of Thiobarbituric Acid-Reactive Substances (TBARS)The measurement of TBARS in the mouse kidney was based on the formation of malondialdehyde by using a commercially available TBARS Assay kit (Cayman Chemical) according to the manufacturer’s instructions.
Statistic AnalysisData were expressed as mean±standard deviation (S.D.). Multiple comparisons were initially subjected to one-way analysis of variance (ANOVA). Immunofluorescence and PAS staining were subjected to non-parametric statistical methods. Values of p<0.05 were considered significant.
The levels of Chol, TG and LDL were markedly reduced by probucol. More over, the level of HDL in diabetic mice was significantly higher than normal mice. After administration with probucol, the HDL level was reduced but still higher than in normal mice. Blood glucose and HbA1C levels were not changed by probucol treatment. Kidney weights and body weights were significantly greater in db/db mice. Probucol treatment had no effect on kidney weights and body weights in db/db mice. Renal function measured as plasma creatinine concentration of the db/db mice was identical to those of the normal mice. There was no difference in liver function (AST and ALT) among three groups (Table 1).
| db/m | db/db | db/db+PB | |
|---|---|---|---|
| Blood glucose (mmol/L) | |||
| 0 weeks | 119.2±42.1 | 456.9±95.2* | 485.3±86.5* |
| 4 weeks | 132.4±26.5 | 499.3±102.3* | 502.7±110.5* |
| 8 weeks | 140.1±39.4 | 576.4±107.5* | 590.4±148.3* |
| 12 weeks | 128.0±13.5 | 596.7±105.2* | 588.1±132.5* |
| HbA1c (%) | 3.31±0.28 | 10.56±2.23* | 11.09±1.45* |
| Chol (mmol/L) | 1.92±0.33 | 4.43±1.02* | 3.32±0.82# |
| TG (mmol/L) | 0.81±0.22 | 2.25±1.13* | 1.56±0.94# |
| LDL (mmol/L) | 0.15±0.05 | 0.31±0.04* | 0.19±0.07# |
| HDL (mmol/L) | 1.38±0.16 | 2.32±0.28* | 1.73±0.12# |
| ALT (U/L) | 33.52±2.13 | 29.35±3.64 | 31.25±1.28 |
| AST (U/L) | 23.45±4.41 | 30.26±3.12 | 28.53±5.18 |
| Plasma creatinine (mg/dL) | 1.025±0.31 | 0.958±0.27 | 1.024±0.22 |
| Kidney weight | 289.2±21.4 | 365.5±66.7* | 358.3±26.6* |
| Body weight | 27.6±1.38 | 50.07±4.69* | 52.53±2.12* |
* p<0.05 vs. db/m normal mice; # p<0.05 vs. db/db diabetic mice
At the onset of intervention (8 weeks of age), albumin excretion of db/db mice was identical and was significantly higher compared with db/m mice (p<0.01). After 4 weeks of treatment, probucol markedly decreased the urine albumin level (p<0.05). The therapeutic effects were more evident with the prolonging of treatment. Albumin excretion increased by 171% between 8 weeks and 20 weeks in diabetic db/db mice. This increase was totally prevented by probucol treatment (Fig. 1).
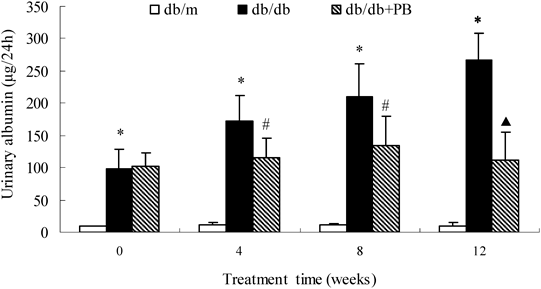
* p<0.01 vs. db/m normal mice; # p<0.05, ▲ p<0.01 vs. db/db diabetic mice. db/db+PB, db/db diabetic mice treated with probucol.
The glomeruli of diabetic mice demonstrated increased accumulation of PAS-positive matrix in the mesangium compared with glomeruli of normal mice. Treatment with probucol significantly ameliorated the degree of ECM deposition by 41.67% from the diabetic glomeruli (Figs. 2A, B, p<0.05). The levels of both fibronectin and collagen IV, two major components of glomerular ECM, were further determined by immunofluorescence. In consistent with the accumulation of glomerular PAS-positive matrix, levels of fibronectin and collagen IV in glomeruli were significantly increased in diabetic mice. Probucol treatment substantially attenuated the protein levels of fibronectin and collagen IV (Figs. 2C, D, p<0.05).
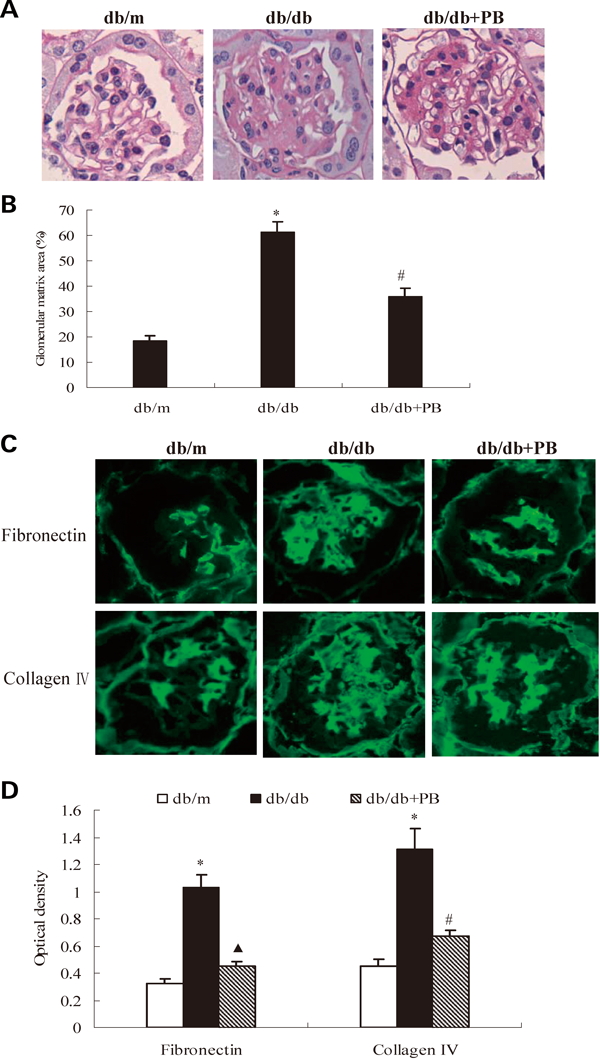
(A) Representative photomicrographs of PAS staining (×400). (B) Comparision of PAS-positive area (matrix area). (C) Representative photomicrographs of immunofluorescence staining of fibronectin and collagen IV (×400); (D) Comparision of optical densities of fibronectin and collagen IV. * p<0.01 vs. db/m normal mice; # p<0.05, ▲ p<0.01 vs. db/db diabetic mice. db/db+PB, db/db diabetic mice treated with probucol.
WT-1 positive cells were significantly reduced in the glomeruli of diabetic mice compared with those of nomal mice. However, probucol preserved WT-1 positive cells. The average number of cells stained with anti-WT-1 antibody per glomeruli was higher in the probucol treated group than db/db group (Figs. 3A, B, 14.52±1.58 vs. 8.47±1.76, p<0.05). The nephrin-positive staining is lined along the glomerular capillary loops with even distribution in normal mice. However, the expression of nephrin was markedly decreased, and the absence of nephrin expression was noted in partial region. The expression of nephrin protein was dramatically increased by 1.73 fold after 12 weeks of probucol treatment. (Figs. 3C, D, p<0.05). Real time PCR results showed that the mRNA expression of nephrin and WT-1 were markedly reduced in db/db diabetic mice. Probucol increased the mRNA expression of nephrin and WT-1. Western blot result showed that there was low expression of desmin in db/m mice, which, however, was markedly increased in diabetic db/db mice. Probucol decreased the expression of desmin to normal level (Figs. 3E, F, p<0.01).

(A) Immunofluorescence staining of kidney slides for WT1 (×400). (B) Comparision of average WT1-positive podocytes per glomerulus. (C) WT-1 mRNA expression. (D) Representative photomicrographs of nephrin by immunofluorescence staining in glomerulus (×400). (E) Comparision of optical densities of nephrin. (F) Nephrin mRNA expression. (G) Desmin protein level analysis by Western blot. (H) Comparision of band intensity of desmin. * p<0.01 vs. db/m normal mice; # p<0.05, ▲ p<0.01 vs. db/db diabetic mice. db/db+PB, db/db diabetic mice treated with probucol.
The level of kidney thiobarbituric acid-reactive substances (TBARS), an index of reactive oxygen species (ROS) generation, significantly increased in diabetic mice and this increase was completely abolished by probucol treatment (Fig. 4A, p<0.01). The increased ROS generation in diabetic mice was associated with elevated renal expression of Nox2 and Nox4, the two major subunits of NADPH oxidase. The stimulation of the expression of Nox2, but not Nox4, was concomitantly suppressed in the probucol treated db/db mice (Figs. 4B, C, p<0.05).

(A) Measurement of renal cortex thiobarbituric acid-reacrive substances (TBARS). (B) Nox2 and Nox4 protein level analysis by Western blot. (C) Comparision of band intensity of Nox2 and Nox4. * p<0.01 vs. db/m normal mice; # p<0.05, ▲ p<0.01 vs. db/db diabetic mice. db/db+PB, db/db diabetic mice treated with probucol.
When TBARS and Chol were tested for simple linear correlations agaist albuminuria, ECM area and nephrin, the strong correlation were found between TBARS and albuminuria (r=0.92; p<0.01), ECM area (r=0.76; p<0.01), nephrin (r=0.69; p<0.01). A statistically significant correlation was also observed between Chol and albuminuria (r=0.79; p<0.01). However, there are no correlation between Chol and ECM area (r=0.32; p>0.05), nephrin (r=0.43; p>0.05).
In the present study, we showed that probucol could improve diabetic nephropathy by reducing proteinuria and attenuating glomerulosclerosis in db/db mice. It seemed to exert protective effect via anti-oxidative stress and protecting podocyte injury.
The innate obese db/db diabetic mouse provides an ideal model for type 2 diabetes, and the disease course is extremely similar to that of humans.12) The significant metabolic disturbance has been observed in db/db mice aged 8 weeks with increased albuminuria.13) Therefore, db/db diabetic mice aged 8 weeks were chosen for experiment. Microalbuminuria is a major risk factor for progressive renal function decline in diabetic nephropathy14) and is thought to be the first step in an inevitable progression to proteinuria and renal failure.15,16) Thus, reduction of albuminuria is a major target for renoprotective therapy in both type 1 and type 2 diabetes. Probucol could markedly decrease the urine albumin excretion in a time-dependent manner without affecting blood glucose. The alleviated albuminuria reflected the protective effects of probucol on renal lesion. In the present study, we found that probucol obviously improved metabolic disturbance, which characterized by decreased levels of Chol, TG and LDL, and the Chol level was strong correlated with albuminuria. The meta-analysis of clinical studies showed that active control of hyperlipidemia was beneficial for albuminuria.17) The effects of probucol on decreasing levels of Chol, TG and LDL might contribute to attenuate albuminuria. In this study, probucol reduced HDL-C levels. The decreased HDL level was also one of characteristics of improved metabolic disturbance. Generally, low HDL-C levels aggravate atherosclerosis. However, probucol is known to increase reverse cholesterol transport and improve HDL function.18,19) Furthermore, the antiatherogenic effects of probucol have been reported.20,21) These data suggest that despite the HDL-C lowering action, probucol has anti-atherogenetic effects. We speculate that reverse cholesterol transport by probucol might also contribute to prevent progression of atherosclerosis in the kidney, and is a mechanism by which probucol suppresses diabetic nephropathy. It should be noted that our study used 1% probucol-containing diet in mice and plasma concentration of probucol in the treated db/db mice was similar to that in humans given 750 mg/d probucol.22) Therefore, it is likely that the clinical dose of probucol (500–1000 mg/d) also reduces urine albumin in humans.
Besides albuminuria, glomerulosclerosis is another important pathological hallmark of DN.23) The accumulation of extracellular matrix (ECM) in glomeruli contributes to diabetic glomerulosclerosis. Fibronectin and collagen IV are the main components of ECM, which are key cytokine responsible for diabetic glomerulosclerosis.24) The increase of glomerular fibronectin and collagen IV are associated with the appearance of significant mesangial matrix expansion in db/db mice.25) Treatment decreasing fibronectin and collagen IV preserves the creatinine clearance, and prevents the expansion of mesangial matrix.26) In the present study, glomerular expression of fibronectin and collagen IV was significantly increased in db/db mice, being consistent with previous data.25,26) Of note, probucol significantly restored the increase in glomerular fibronectin and collagen IV in db/db mice. Taking together with the fact that fibronectin and collagen IV are implicated in the development of mesangial matrix expansion in db/db mice, our present observations demonstrate that the protective effect of probucol against glomerulosclerosis in db/db mice is at least in part attributed to the inhibition of the increase in glomerular fibronectin and collagen IV.
Podocyte injury is a typical characteristic in DN. Drugs that have beneficial effects on podocyte can improve our ability to treat DN. Podocyte have a key role in maintaining the integrity of the glomerular filtration barrier, and diabetic renal injury is known to lead to loss of podocytes and slit diaphgram protein. The magnitude of albuminuria correlates with the podocyte number and slit diaphragm proteins in the kidney. Nephrin, a transmembrane protein forming the molecular substrate of the slit diaphragm, plays an important role in the glomerular filtration barrier.27,28) The loss of nephrin causes foot process effacement of podocytes, leading to the development of albuminuria.29) In present study, we surveyed the podocyte number with anti-WT-1 antibody, a podocyte-specific antibody, and the expression of nephrin and desmin (a marker of podocyte lesion). Results showed that, glomerular WT-1-positive cells and the nephrin expression were decreased in db/db mice, accompanied by increased expression of desmin, findings consistent with previous reports.30,31) Very importantly, treatment of db/db mice with probucol significantly restored the decline of podocyte and nephrin as well as reduced the expression of desmin, suggesting the protective effects of probucol against podocyte injury, which, to our knowledge, has not been reported before. Considering the pivotal role of podocyte in pathogenesis of albuminuria, the amelioration of urinary albumin excretion by probucol seems to be mediated by the restoration of podocyte injury in db/db mice.
There is increasing evidence that oxidative stress is major factors in the progression of DN.3,32,33) ROS acts as a signal amplifier in diabetes.34) NADPH oxidase is a major source of ROS in phagocytes as well as non-phagocytic cells, including fibroblasts, vascular smooth muscle cells, and glomerular cells.35–39) Chronic inhibition of NADPH oxidase with apocynin prevents podocyte apoptosis and mesangial matrix expansion in db/db mice.40) A role for Nox-derived ROS in diabetic nephropathy is now widely accepted. Notely, in this work, we found that probucol treatment markedly improved oxidative stress, which is characterized by decreased production of TBARS, an index of ROS generation. Therefore, we obtained the evidence that the protective effects of probucol against diabetic nephropathy were attributed to the suppression of ROS production. Furthermore, to elucidate the molecular mechanism underlying the inhibition of ROS, we examined the effect of probucol on NADPH oxidase subunits, Nox2 and Nox4. NADPH oxidases are composed of gp91phox (Nox2)-homolog, called Nox protein and several subunits that contain p22phox, p47phox, p67phox, and small GTPase rac. At least three different Nox isoforms are expressed in the kidney cortex: Nox4, Nox2, and Nox1. Nox1–Nox3 represent an evolutionarily closely related subgroup of Nox enzymes, Nox4 is more distant, sharing only ca. 39% identity to Nox2.41) Although no strict comparisons have been performed, based on mRNA levels it appears that Nox4 is most abundant in kidney.42,43) Interestingly, we found that both Nox2 and Nox4 expression were up-regulated in db/db mice, but probucol significantly attenuated the protein expression of Nox2 without affecting Nox4. This result was consistent with the previous study that probucol inhibited Nox2 but not Nox4 in progressive mesangioliferative glomerulonephritis.9) Upregulation of Nox2 or subunits have also been reported in other several studies about diabetic nephropathy.44–47) However, increased expression of Nox4 mRNA and protein in diabetic nephropathy has also been implicated,46,48) and treatment of diabetic animals with Nox4 antisense RNA decreases kidney pathology.46) Recently, Sedeek et al. observed renal mRNA expression of Nox4, but not of Nox2 increased in db/db mice at 24 weeks of age.49) The reason for this discrepancy on the activation of Nox2 and Nox4 is not clear but may be related to the different observation ages of mice (20 weeks vs. 24 weeks). Actually, there is still a debate regarding the Nox isoforms involved in mediating ROS-dependent tissue damage in diabetic nephropathy. It is tempting to speculate that Nox2 and Nox4 act synergistically in generating ROS dependent damage in diabetic nephropathy, but more studies will be necessary to clarify this point. Given that Nox2 is implicated in the enzymatic activity of NADPH oxidase, our results support that the elemination of ROS by probucol is at least partially mediated by the down-regulation of Nox2.
Probably, progression of diabetic nephropathy might be affected by not only oxidative stress but also abnormality of lipid metabolism. Our results showed that both TBARS and Chol were correlated with albuminuria, and TBARS also was correlated with the ECM area and nephrin in glomeruli. It has been reported that inhibition of oxidative stress prevents podocyte injury and mesangial matrix expansion in db/db mice.40) These data suggest that probucol has the beneficial effects on podocytes and glomerular matrix expansion and these effects might be mediated through the elimination of ROS generated from NADPH oxidase. The lowering-lipid effect of probucol may also contribute to slowing down the progression of DN .
During the experiment, no adverse effects of probucol on AST, ALT and Cr levels were observed, which suggested that probucol treatment in diabetic patients might be safe without liver or kidney toxicity. In conclusion, our study provides important evidence that probucol arrested proteinuria and the progression of DN. Clinical study have reported that probucol can delay progression of diabetic nephropathy and exert the antiatherogenic effect in patients.11,20,21) Probucol, which is frequently used in daily clinical practice, may represent a novel route of therapy for patients with DN.