2013 Volume 36 Issue 6 Pages 925-930
2013 Volume 36 Issue 6 Pages 925-930
In the present study, the antinociceptive profiles of coumarin were examined in ICR mice. Coumarin administered orally (from 1 to 10 mg/kg) showed an antinociceptive effect in a dose-dependent manner as measured in the acetic acid-induced writhing test. Duration of antinociceptive action of coumarin maintained at least for 60 min. But, the cumulative response time of nociceptive behaviors induced by a subcutaneous (s.c.) formalin injection, intrathecal (i.t.) substance P (0.7 µg) or glutamate (20 µg) injection was not affected by coumarin. In addition, intracerebroventricular (i.c.v.) or intrathecal (i.t.) administration with coumarin (10–40 µg) attenuated acetic acid-induced writhing response in a dose dependent manner. Intraperitoneal (i.p.) pretreatment with naloxone (an opioid receptor antagonist) attenuated antinociceptive effect induced by coumarin in the writhing test. Furthermore, i.c.v. or i.t. pretreatment with naloxone (5 µg) reversed the decreased acetic acid-induced writhing response. However, methysergide (a 5-HT serotonergic receptor antagonist) or yohimbine (an α2-adrenergic receptor antagonist) did not affect antinociception induced by coumarin in the writhing test. Our results suggest that coumarin exerts a selective antinociceptive property in the acetic acid-induced visceral-derived pain model. Furthermore, the antinociceptive effect of coumarin may be mediated by activation of central opioid receptors, but not serotonergic and adrenergic receptors.
Currently, many drugs prescribed worldwide are obtained directly or indirectly from plants, and there is a growing interest in the use of plants for the search of new therapeutic agents.1) Coumarins form a large group of plant polyphenols. Thus far about 1500 coumarin derivatives have been isolated from plants.2) They occur ubiquitously in the plant kingdom, and coumarin derivatives show a chemotaxonomical tendency to accumulate in large amounts in Rutaceae and Apiaceae.3) Coumarin has been found to exhibit a wide range of bioactivities, such as hemoprevention against pathogens,4) herbivores,5) and abiotic stresses,6) suggesting that physiological roles of coumarins for plants for the adaption to environmental stresses. Some coumarin derivatives are also known to act beneficially on human health due to their therapeutic effects such as inhibitory activities against various tumor cells,7,8) mycobacteria,9) antioxidant,10,11) antihyperglycemic,12) antifungal,13) and antiasthmatic14) which have been extensively studied in the medical and pharmaceutical fields for the treatment of diseases of human.
Previous studies have demonstrated that some coumarins exert an antinociceptive action.15,16) However, exact antinociceptive profiles and mechanism of coumarin have not been well characterized. Thus, we, in the current study, tried to characterize antinociceptive profiles and mechanisms of coumarin in several pain models.
These experiments were approved by the University of Hallym Animal Care and Use Committee (Registration Number: Hallym 2009-05-01). All procedures were conducted in accordance with the ‘Guide for Care and Use of Laboratory Animals’ published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain.
Experimental AnimalsMale ICR mice (MJ Co., Seoul, Korea) weighing 20–25 g were used for all the experiments. Animals were housed 5 per cage in a room maintained at 22±0.5°C with an alternating 12 h light–dark cycle. Food and water were available ad libitum. The animals were allowed to adapt to the laboratory for at least 2 h before testing and were only used once. Experiments were performed during the light phase of the cycle (10:00–17:00).
Drug AdministrationOral administration was performed with gage in a volume of 500 µL/25 g body weight. Intraperitoneal (i.p.) injection was conducted to unanesthesized mice with volume of 250 µL. The intracerebroventricular (i.c.v.) administration followed the method described by Haley.17) The intrathecal (i.t.) administration was performed following the method of Hylden and Wilcox18,19) using a 30-gauge needle connected to a 25 µL Hamilton syringe with polyethylene tubing. The i.c.v. and i.t. injection volumes were 5 µL and the injection sites were verified by injecting a similar volume of 1% methylene blue solution and determining the distribution of the injected dye in the ventricular space or in the spinal cord. The dye injected i.c.v. was found to be distributed through the ventricular spaces and reached the ventral surface of the brain and the upper cervical portion of the spinal cord. The dye injected i.t. was distributed both rostrally and caudally but with short distance (about 0.5 cm from the injection site) and no dye was found visually in the brain. The success rate for the injections was consistently found to be over 95%, before the experiments were done.
Acetic Acid-Induced Writhing and Intraplantar Formalin TestsFor the writhing test,20) 1% acetic acid was injection i.p. and then, the animals were immediately placed in an acrylic observation chamber (20 cm high, 20 cm diameter). The number of writhes was counted during 30 min after the injection of acetic acid. A writhe was defined as a contraction of the abdominal muscles accompanied by an extension of the forelimbs and elongation of the body. For the formalin test,21) 10 µL of 5% formalin was injected subcutaneously under the plantar surface of the left hindpaw. Following injection of formalin, the animals were immediately placed in an acrylic observation chamber, and the time spent licking, shaking and biting the injected paw was measured with a stop-watch timer and considered as indication of nociception. The early phase of the nociceptive response normally peaked 0 to 5 min, and the late phase 20 to 40 min after formalin injection, representing the direct effect on nociceptors and inflammatory nociceptive responses, respectively.22) Animals were pretreated orally once with vehicle (control) or coumarin at various doses (from 1 to 10 mg/kg) 30 min prior to performing the acetic acid-induced writhing and formalin tests.
Substance P- and Glutamate-Induced Nociceptive Behavioral TestVehicle (control) or 10 mg/kg of coumarin was pretreated orally 30 min prior to performing i.t. injection of substance P (0.7 µg/5 µL) or glutamate (20 µg/5 µL). Immediately after i.t. injection with substance P or glutamate, the mice were placed in an observation chamber (20 cm high, 20 cm diameter) and their nociceptive behavioral responses were recorded during 30 min. The cumulative response time of licking, scratching and biting episodes directed toward the caudal parts of the body were measured with a stop-watch timer.19)
Pretreatment of AntagonistsIn the furst experiment, mice were pretreated i.p. with either saline, naloxone (10 mg/kg), methysergide (10 mg/kg), or yohimbine (5 mg/kg) 10 min before oral administration of vehicle as a control or a fixed dose of coumarin (10 mg/kg). In the second experiment, naloxone (5 µg) was pretreated i.c.v. or i.t. 10 min prior to oral administration of vehicle as a control or a fixed dose of coumarin (10 mg/kg). And then, the writhing response was tested 30 min after the treatment with either vehicle or coumarin.23–27)
DrugsAll drugs were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Coumarin, naloxone, methysergide and yohimbine were dissolved in saline. This solution excluding coumarin was used as vehicle control. All drugs were prepared just before use.
Statistical AnalysisData were presented as the mean±S.E.M. The statistical significance of differences between groups was assessed with one-way ANOVA with Bonferroni’s post-hoc test using GraphPad Prism version 4.0 for Windows XP (GraphPad Software, San Diego, CA, U.S.A.); p<0.05 was considered significant.
Coumarin attenuated the acetic acid-induced writhing numbers in a dose-dependent manner (Fig. 2A). Oral treatment with coumarin at the dose of 10 mg/kg led to 56% decrease in the acetic acid-induced writhing response compare to the control group of mice. In addition, the time–course study showed that pretreatment with coumarin for 30 or 60 min attenuated the acetic acid-induced writhing response compare to the control group of mice. However, pretreatment with coumarin for 120 min did not affect acetic acid-induced writhing response (Fig. 2B). I.c.v. or i.t. administration with coumarin (from 10 to 40 µg) attenuated acetic acid-induced writhing response in a dose-dependent manner as revealed in Figs. 2C and D. In addition, in vehicle-treated control group, injection of 5% formalin caused acute, immediate nociceptive formalin responses (i.e., licking/flinching and biting the injected paw) that lasted for 5 min (1st phase response). The 2nd phase nociceptive responses began about 20 min after formalin administration and lasted for about 20 min (20–40 min after formalin injection). In coumarin-treated mice, the nociceptive behaviors induced by a subcutaneous (s.c.) formalin injection were not affected by coumarin as compared with control group of mice during the both 1st and 2nd phases (Fig. 3A). In vehicle-treated control mice, i.t. injection of substance P (0.7 µg) or glutamate (20 µg) caused acute, immediate behavioral responses, i.e., licking, scratching and biting the lumbar or caudal region, which lasted about 30 min. As shown in Figs. 3B and C, the cumulative nociceptive response times for i.t. administration of substance P (Fig. 3B) or glutamate (Fig. 3C) was not affected by coumarin as compared with control group of mice.


Various doses (from 1, 5, 10 mg/kg) of coumarin were administered orally and then, 0.25 mL of 1% acetic acid solution was injected intraperitoneally 30 min after treatment. The number of writhing was counted for 30 min following acetic acid injection (A). Coumarin (10 mg/kg) was administered orally and then, 0.25 mL of 1% acetic acid solution was injected intraperitoneally 30, 60 and 120 min after treatment. The number of writhing was counted for 30 min following acetic acid injection (B). Various doses (from 10, 20, 40 µg/5 µL) of coumarin were administered intracerebroventricularly (C) or intrathecally (D) and then, 0.25 mL of 1% acetic acid solution was injected intraperitoneally 30 min after treatment. The number of writhing was counted for 30 min following acetic acid injection. The vertical bars indicate the standard error of the mean. The number of animal used for each group was 8–10 (* p<0.05; ** p<0.01; *** p<0.001, compared with control group).
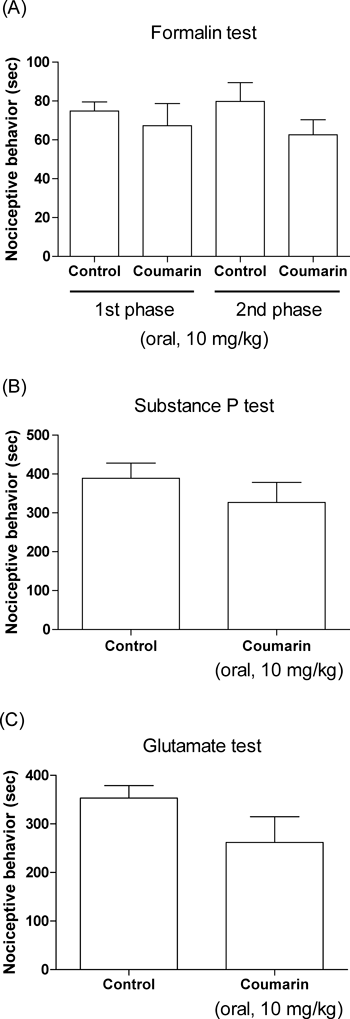
Animals were pretreated orally with coumarin (10 mg/kg) for 30 min prior to the formalin (5%, 10 µL) injection subcutaneously into the plantar aspect of the left side hindpaw. The cumulative response time of licking, biting and shaking the injected paw was measured during the period of 0–5 min (1st phase) and 20–40 min (2nd phase) (A). Coumarin (10 mg/kg) was administered orally for 30 min prior to the substance P (B; 0.7 µg/5 µL) or glutamate (C; 20 µg/5 µL) injection intrathecally. The cumulative response time of licking, scratching and biting episodes was measured for 30 min. The vertical bars indicate the standard error of the mean. The number of animal used for each group was 8–10.
We examined the possible involvement of opioidergic, serotonergic and adrenergic system in the coumarin-induced antinociception. The blockade of opioidergic receptor with systemic pre-administration of naloxone abolished the coumarin-induced inhibition of the writhing response (Fig. 4A). However, the pretreatment with methysergide (serotonergic receptor antagonist, Fig. 4B) and yohimbine (adrenergic receptor antagonist, Fig. 4C) did not affect coumarin-induced antinociception. The treatment of naloxone, methysergide or yohimbine itself did not affect the writhing response (Fig. 4).

Naloxone (10 mg/kg), methysergide (10 mg/kg) or yohimbine (5 mg/kg) was pretreated intraperitoneally for 10 min, before oral administration of vehicle or coumarin (10 mg/kg). Coumarin or vehicle was administered orally and then, 0.25 mL of 1% acetic acid solution was injected i.p. 30 min after treatment. The number of writhing response was counted for 30 min following acetic acid injection. The vertical bars denote the standard error of the mean. The number of animal used for each group was 8–10 (*** p<0.001, compared with control group; +++ p<0.001, compared with coumarin group).
To examine the possible involvement of central opioid system in the coumarin-induced antinociception, mice were pretreated i.c.v. or i.t. with naloxone (5 µg) for 10 min and the coumarin (10 mg/kg) was administered orally. The i.c.v. or i.t. pretreatment with naloxone caused a significant reversal of inhibition of the writhing response induced by coumarin as revealed in Figs. 5A and B.

Mice were pretreated i.c.v. (A) or i.t. (B) with naloxone (5 µg/5 µL) for 10 min prior to oral administration of coumarin (10 mg/kg). The number of writhing response was counted for 30 min following acetic acid injection. The vertical bars denote the standard error of the mean. The number of animal used for each group was 8–10 (*** p<0.001, compared with control group; ++ p<0.01, + p<0.05, compared with coumarin group).
In the present study, we found that coumarin administered orally produces a selective antinociception in the writhing test. The time–course study suggest that the duration of pharmacological action of coumarin is maintained at least 60 min. I.p. injection of acetic acid can produce the peritoneal inflammation (acute peritonitis), which cause a response characterized by contraction of the abdominal muscles accompanying an extension of the forelimbs and elongation of the body. This writhing response is considered as a visceral inflammatory pain model.20,28) I.p. administration of dilute acetic-acid produces a characteristic writhing response in mouse. This behavior is considered to be evidence of peritoneovisceral pain, since acetic-acid directly activates visceral and somatic nociceptors innervating the peritoneum and induces inflammation not only in subdiaphragmatic visceral organs, but also in subcutaneous muscle walls.29) To find the site of action of coumarin, we also administered coumarin i.c.v. or i.t. and the writhing test was performed. We found in the present study that supraspinal or spinal administration attenuates the number of acetic acid-induced writhing response, suggesting that coumarin administered orally may produce antinociception via acting on the brain or spinal cord.
Although we demonstrated in the present study that coumarin produces antinociception in the writhing test, no antinocieptive effect of coumain was observed in the formalin, substance P or glutamate pain models. It is widely agreed that the nociceptive behaviors manifested during the acute 1st phase may be caused by the direct effect on peripheral nociceptors activating primary afferent fiber. It is followed by the tonic 2nd phase, which may be resulted from the tonic inflammatory nociceptive response.21,22,30–32) Furthermore, it has been reported that i.t. injection of substance P or excitatory amino acid in mice can also elicit nociceptive responses, consisting of biting, scratching and licking the caudal parts of the body.19,33) Although the exact reasons for the lack of antinociception of coumarin in formalin, substance P or glutamate pain models are not clear at present time. It is speculated that a selective coumarin-induced antinociception in the writhing test may be due to the activation of differential neuronal-circuits stimulated by visceral pain and other inflammatory-induced pain modalities, respectively.
The roles of opioidergic, serotonergic and adrenergic receptors in the regulation of modulation of nociceptive processing have been demonstrated in many previous studies. For example, it has been reported that blockade of the spinal serotonergic or noradrenergic receptors by spinal injection of methysergide or yohimbine antagonize the antinociception induced by morphine administered supraspinally.34–36) Also, it is well known that opioid receptors are involved in the production of antinociception.37–39) We observed in the present study that the blockade of opioid receptors by naloxone reduces coumarin-induced antinociception, suggesting that opioid receptors are involved in the production of antinociception induced by coumarin. However, we found in the present study that the blockades of serotonergic or adrenergic receptors by methysergide and yohimbine, respectively, do not affect coumarin-induced antinociception, indicating that sertonergic or adrenergic receptors are not involved in coumarin-induced antinociception. To examine if central opioid receptors are involved in coumarin-induced antinociception, effects of naloxone pretreated supraspinally or spinally on coumarin-induced antinociception was further investigated. We found in the present study that i.c.v. or i.t. pretreatment with naloxone reduces coumarin-induced antinociception, suggesting that supraspinally or spinally located opioid receptors appear to be involved in the production of antinociception induced by orally administered coumarin.
In conclusion, the present study proposes that coumarin peoduces an antinociception, specially in the visceral inflammatory pain model. Furthermore, supraspinally or spinally located opioid receptors appear to be involved in the production of coumarin-induced antinociception.
This research was supported by Priority Research Centers (2012-R1A6A1048184) and Basic Science Research (2012-0000313) Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.