2013 Volume 36 Issue 6 Pages 1027-1031
2013 Volume 36 Issue 6 Pages 1027-1031
Mammalian sialidases (NEU1, NEU2, NEU3 and NEU4) that remove sialic acids from glycoconjugates have been implicated in diverse cellular functions. Human sialidases are involved in the development of various disease states such as cancer, diabetes and arteriosclerosis. Unregulated acidic sialidase NEU1 activity is associated with the pathogenesis of lysosomal storage disorder (LSD) sialidosis, abnormal immune responses and cancer progression. Obesity is closely related to several chronic diseases such as diabetes, cardiovascular diseases, hyperlipidemia or hypertension that are associated with metabolic syndrome. We examined fluctuations in mRNA levels and sialidase activities of NEU1 in two strains of obese and diabetic mice to assess the involvement of NEU1 in obesity. The activity of NEU1 was preferentially higher in epididymal fat and lower in the livers of two strains of obese and diabetic mice. Fluctuations in NEU1 activity might be associated with the pathological status of these tissues in obesity.
Sialic acids are generally located at a terminal position in glycoproteins, glycolipids and gangliosides. They play important roles in various biological processes possibly through influencing the conformation of glycoconjugates and by recognizing and masking the biological sites of these biomolecules as well as their cellular binding sites.1) Sialidases comprise a family of exoglycosidases that catalyze the removal of sialic acids from glycoconjugates. Therefore, the removal of sialic acids catalyzed by a sialidase significantly modulates many biological processes such as cell differentiation, proliferation, apoptosis and malignant transformation.2,3) Four types of mammalian sialidases (NEU1, NEU2, NEU3 and NEU4) have been identified and characterized and they have different major subcellular localization and enzymatic properties.4) Among them, NEU1 and NEU4 are acidic sialidases that catabolize oligosaccharides and glycopeptides. NEU1 is widely expressed at high levels in mammalian tissues and it is the only mammalian sialidase that has been linked to a human disease.5) On the other hand, NEU4 is predominantly expressed in the brain and little is known about its involvement in human diseases.3) NEU1 plays a catabolic role in lysosomes and genetic mutations at the NEU1 locus are the basis of lysosomal storage disorder (LSD) sialidosis, a severe, systemic condition characterized by broad clinical manifestations affecting most organs and nerve systems.5) In addition to its role in lysosomal catabolism, NEU1 is also expressed on the plasma membrane, where it regulates the activity of cellular signaling molecules involved in inflammation, elastogenesis, exocytosis, phagocytosis, cell adhesion and proliferation.6) Several studies have shown that the sialidase activity of NEU1 increases in cells of the immune system during activation or differentiation. The sialidase activity of cell surface NEU1 in activated T lymphocytes contributes to the hyposialylation of specific cell-surface glycoconjugates and to interferon-gamma (IFN-γ) production.7) In addition, NEU1 regulates cytokine production induced by lipopolysaccharide (LPS) in human dendritic cells,8) activates phagocytosis in macrophages and dendritic cells through the desialylation of surface receptors9) and regulates functioning of the hyaluronan receptor, which might be a target for treating asthma.10) Furthermore, NEU1 is a negative modulator of malignant phenotypes and cancer cell metastasis. Reduced NEU1 expression is associated with the low metastatic ability of mouse colon adenocarcinoma 26,11) murine B16 melanoma12) and human colon cancer cells.13) Thus, unregulated NEU1 activity might be associated with various pathological changes.
Obesity is associated with several pathological states including diabetes, dyslipidemia and atherosclerosis, which are risk factors for metabolic syndrome14) and unregulated NEU1 activity might be linked with obesity. Therefore, we assessed the involvement of NEU1 in obesity by comparing fluctuations in mRNA levels and sialidase activities of NEU1 of C57BLKS/J Iar-+Leprdb/+Leprdb (db/db) obese15) and Tsumura Suzuki Obese Diabetes (TSOD) mice16) with those in their lean counterparts.
Twenty eight-week-old db/db, TSOD, non-obese C57BLKS/J Iar-m+/+Leprdb (db/+) and Tsumura Suzuki Non-obese Diabetes (TSNO) mice purchased from the Institute for Animal Reproduction (Ibaraki, Japan) were housed at 24±2°C and provided with food and water ad libitum. The mice were sacrificed and then the liver, epididymal fat and kidney leaf fat were dissected, rapidly frozen and stored at −80°C. This study proceeded in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the Committee for the Care and Use of Laboratory Animals at Teikyo University.
Cell CulturePreadipocytes (3T3-L1; DS Pharma Biomedical Co., Ltd., Osaka, Japan) were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% calf serum (CS) (Cell Culture Technologies) and 1% antibiotics solution. Before each experiment, cells were seeded in DMEM containing 10% fetal bovine serum (FBS) (Biowest, Nuaillé, France) and 1% antibiotics, and incubated until they reached confluence. Two days later, cells were differentiated by incubation in DMEM containing 10% FBS, 5 µg/mL insulin (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 0.5 mm isobutylmethylxanthine (Sigma, St. Louis, MO, U.S.A.) and 1 µm dexamethasone (Wako) for 2 d. The medium was replaced with DMEM supplemented with 10% FBS and 5 µg/mL of insulin and changed every 2 d throughout the study. Cells were harvested on day 0 and on days 4 and 8 after inducing differentiation, and then sialidase activity in the cell homogenates was determined as described below. Cells were stained with Oil red O on day 8.
Transfection with Small Interfering RNA (siRNA)3T3-L1 preadipocytes were plated into 60-mm-diameter Petri dishes 1 d before transfection and incubated with NEU1-specific siRNA or negative control siRNA (Invitrogen, Grand Island, NY, U.S.A.) at 5 nm in OPTI-MEM (Invitrogen) medium using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol. Three days after transfection cells were also treated with similar siRNA (day 0) and at day 2 after differentiation into adipocytes.
Quantitative Reverse Transcription Polymerase Chain Reaction (PCR)Total RNA was extracted with Isogen (Nippon Gene Co., Ltd., Toyama, Japan) and then cDNA was synthesized using PrimeScript™ RT reagent kits (TaKaRa Bio Inc., Otsu, Japan) and amplified by real-time PCR using Power SYBR® Green PCR Master Mix and a 7500 Fast Realtime PCR system (Applied Biosystems, Foster, CA, U.S.A.). The amplification protocol was 95°C for 10 min, followed 45 cycles of 95°C for 5 s and 60°C for 1 min. The forward and reverse primer pairs for amplification were as follows (5′–3′): agagatgtttgcccctggac and cgtggtcatcactgaggaga, respectively, for murine NEU1 and tgtgtccgtcgtggatctga and cctgcttcaccaccttcttga, respectively, for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The expression of NEU1 mRNA was normalized to that of GAPDH as the internal control.
Measurement of Acidic Sialidase ActivityTissues were homogenized in ice-cold phosphate buffered saline (PBS) containing Complete Protease Inhibitor Cocktail (Sigma). The homogenate was centrifuged at 600×g for 15 min at 4°C and then enzyme activity was assayed in the supernatant (enzyme fraction). The assay mixture contained 50 µm 4-methylumbelliferyl N-acetylneuraminic acid (4MU-NeuAc) substrate (Sigma), 0.1 m citrate-phosphate (pH 4.6), 1 mm CaCl2, 10 µg of bovine serum albumin (BSA) and 16 µL of enzyme fraction in a final volume of 40 µL. After incubation at 37°C for 2 h, the reaction was terminated by adding 160 µL of 0.2 m glycine–NaOH (pH 10.6) and then the amount of released 4-methylumbelliferone was fluorometrically determined as described.12)
Statistical AnalysisAll values were analyzed by Student’s t-test for two groups and are expressed as means±S.D. p Values of <0.05 were considered significant.
We quantified NEU1 mRNA levels in epididymal fat, kidney leaf fat, and the livers from two strains of obese mice (db/db and TSOD) and their lean counterparts (db/+ and TSNO). Figures 1A and B shows that NEU1 mRNA levels were comparable in epididymal fat and kidney leaf fat from obese and non-obese mice. On the other hand, NEU1 mRNA levels were apparently lower in the livers from both strains of obese mice than from non-obese mice (Fig. 1C).
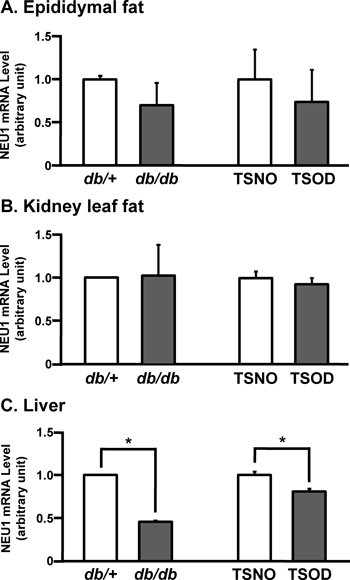
Expression of NEU1 mRNA was quantified by real-time RT-PCR in RNA isolated from epididymal fat (A), kidney leaf fat (B) and liver (C) of db/+, db/db, TSNO and TSOD mice. Expression of NEU1 mRNA is shown relative to that of GAPDH mRNA as internal standard. Bars represent means±S.D. (n=3). * p<0.05; significantly different from each counterpart.
We examined fluctuations in the activities of acidic sialidases that were mainly due to NEU1, as it was the most ubiquitously expressed at the highest levels among the NEU family in epididymal fat, kidney leaf fat and livers from obese db/db and TSOD mice, and their lean counterparts. Figure 2A shows NEU1 activities in epididymal fat from two strains of obese mice and their lean counterparts. More NEU1 activity was found in db/db, than db/+ mice (0.34±0.032 vs. 0.25±0.008), and in TSOD, than TSNO mice (0.24±0.037 vs. 0.14±0.010). These results show that despite NEU1 mRNA levels being comparable in both strains, NEU1 activity in epididymal fat was increased in obese, compared with non-obese mice (Fig. 2A). NEU1 is associated with carboxypeptidase-protective protein/cathepsin A (PPCA) and β-galactosidase in human and murine tissues, and NEU1 becomes activated upon binding to PPCA in lysosomes.17) Thus, the discrepancy between mRNA levels and NEU1 activities between obese and non-obese mice might be due to the association between NEU1 and this protective protein in epididymal fat. Adipocytokines secreted from adipose tissues are key mediators of arterial sclerosis and diabetes in metabolic syndromes18) and the relationship between adipocytokines and obesity has been widely studied in adipose tissues and cultured cell lines.19) The findings of these studies indicate that fluctuations in adipocytokine expression and secretion are associated with various complications of obesity. The secreted adipocytokines PAI-120) and adiponectin21) have terminal sialic acid residues and their sialylation status should affect levels of their secretion or activation. Whether NEU1 is involved in the desialylation of adipocytokines and the regulation of their activities has not been investigated. The present findings suggest that higher levels of NEU1 activity result in the aberrant glycosylation of some adipocytokines that affect secretion or other activities in adipose tissues of obese mice. On the other hand, NEU1 desialylates and modulates the activities of cell receptors involved in cell proliferation, phagocytosis, inflammation such as insulin receptors (IR), insulin like growth factor-1 (IGF-1) receptors,22) Toll-like receptors (TLR) 2, 3 and 4,23) Fc receptors for immunoglobulin G (FcRγ)9) and integrin β4.13) Considering its function as a modulator of signaling pathways, NEU1 might regulate the activities of some adipocytokines through the desialylation of receptors involved in their expression or secretion. We found that NEU1 activity predominantly increased during 3T3-L1 adipocyte differentiation (Fig. 3A) and that inhibiting NEU1 activity by transfection with an siRNA sequence that targets NEU1 did not block 3T3-L1 adipocyte differentiation as assessed by Oil red O staining (Figs. 3B, C). These findings suggest NEU1 activity is not necessary but is at least associated with adipocyte differentiation. The adipocytokine targets of NEU1 should be elucidated in vitro and in vivo to determine whether or not NEU1 is involved in adipocytokine secretion.

Sialidase activity of NEU1 in epididymal fat (A), kidney leaf fat (B) and liver (C) of db/+, db/db, TSNO and TSOD assayed using 4-methylumbelliferyl N-acetylneuraminic acid (4MU-NeuAc) substrate. Activity is defined as amount of 4-methylumbelliferone cleaved from 4MU-NeuAc by sialidase in 1 mg protein within 1 h. Values are presented as means±S.D. (n=5). * p<0.05, † p<0.01: significant differences compared with each counterpart.

Sialidase activity was determined in homogenates of 3T3-L1 cells after differentiation into adipocytes and harvesting at indicated times (A). 3T3-L1 cells treated with negative control or NEU1 siRNA were induced to differentiate as described in Materials and Methods. Sialidase activities (B) and Oil red O staining (C) of 3T3-L1 cells harvested on day 8. Bars represent means±S.D. (n=3). * p<0.05: significantly different from negative control.
We found that NEU1 activities did not significantly differ in kidney leaf fat from two strains of obese and non-obese mice (Fig. 2B). White (WAT) and brown (BAT) adipose tissues (AT) have different morphology, characteristic proteins and development24) and essentially antagonistic functions; WAT stores excess energy as triglycerides and BAT dissipates energy through heat production. Kidney leaf fat contains BAT whereas epididymal fat does not.25) Thus, differences in cell types and species that constitute each tissue might explain the difference in NEU1 activities between epididymal fat and kidney leaf fat.
Figure 2C shows NEU1 activities that were determined in the livers from obese db/db and TSOD mice, and their lean counterparts. Levels of NEU1 activity were similar in db/db and TSOD mice and lower than those in their respective non-obese controls (0.09±0.004 vs. 0.16±0.019 and 0.10±0.007 vs. 0.20±0.016, respectively). The liver is the largest organ in the body and it performs essential functions such as metabolism, detoxification and plasma protein production. The lysosomal system comprises a specialized network of organelles that are involved in the sorting, digestion, recycling and secretion of cellular components that are crucial to maintain cell and tissue homeostasis. NEU1 is one component of an established lysosomal multi-enzyme complex that catalyzes the cleavage of ketosidically-linked sialic acid residues on glycoproteins, glycolipids and oligosaccharides or polysaccharides. Autophagy is a lysosome-dependent degradative pathway that regulates the turnover of intracellular organelles, parasites and long-lived proteins, and deregulation of this pathway results in various pathological conditions.26) A low level of autophagy is associated with liver diseases involving fatty and alcoholic liver diseases, as well as hepatocellular carcinoma.27) Since NEU1 plays a crucial role in the degradation of substrates by the lysosomal system, the low level of NEU1 activity in the obese mouse liver might induce a decrease in the level of autophagy and impair several liver functions, resulting in fatty liver disease. Whether or not a low level of NEU1 activity directly or indirectly affects the level of autophagy and which factors and/or intracellular organelles of autophagy are NEU1 targets in the liver should be clarified.
In conclusion, we demonstrated that the mRNA levels and sialidase activities of NEU1 fluctuate in the epididymal fat, kidney leaf fat and livers of two strains of obese mice. The activity of NEU1 was higher in epididymal fat and lower in the liver of both strains of obese mice than in their non-obese counterparts. These results suggest that fluctuations in NEU1 activities are associated with the pathological states of these tissues in obese mice, although whether the up- or down-regulation of NEU1 activity causes these conditions, or is a phenomenon that results from obesity remains unclear. In addition to NEU1, the likelihood that other sialidases such as a novel acidic sialidase and NEU4 could be involved in obesity was not completely eliminated in the present study. However, aberrant NEU1 activity is associated with LSDs, autoimmune diseases and the malignancy and metastasis of cancer cells.3) Furthermore, therapeutic experimental trials of NEU1 for treating LSDs28) and studies of therapeutic approaches targeting NEU1 to treat several cancers29) and immune diseases10) have recently been conducted. A detailed investigation into the molecular mechanisms of NEU1 involvement in obesity might reveal novel physiological and pathological roles of NEU1 and contribute towards novel strategies for treating obesity.
This work was supported in part by a TSOD research meeting Grant in 2009 (to N. Ohkura) and a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan 22500774 (to N. Ohkura).