2013 Volume 36 Issue 7 Pages 1118-1125
2013 Volume 36 Issue 7 Pages 1118-1125
Telekin, a eudesmane-type sesquiterpene lactone compound isolated from Chinese folk medicine Carpesium divaricatum, has been reported to strongly inhibit the proliferation of cancer cells. In this study, the involvement of a mitochondria-mediated pathway in the pro-apoptotic action of telekin was investigated in human hepatocellular carcinoma cells. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays showed that telekin exhibited excellent anti-proliferation activity in hepatocellular carcinoma cells and low cytotoxicity to normal hepatocyte cells. Telekin-induced apoptosis was characterized by chromatin condensation, formation of apoptotic bodies, and exposure of phosphatidylserine on the extracellular surface, as revealed by 4,6-diamidino-2-phenylindole (DAPI) nuclear staining and flow cytometry. Flow cytometry analysis showed that telekin induced the loss of mitochondrial membrane potential (MMP), as well as increased the levels of intracellular free calcium and reactive oxygen species (ROS). Additionally, Western blot results demonstrated that telekin induced the decrease in Apaf-1 and Bcl-2 expression, increase in Bax expression, release of cytochrome C, and activation of caspase-9 and caspase-3 in HepG-2 cells. These findings indicate that telekin activates the mitochondria-mediated apoptotic pathway in hepatocellular carcinoma cells and may merit further investigation as a potential therapeutic agent for the treatment of hepatocellular carcinoma.
Hepatocellular carcinoma (HCC) is one of the most frequent primary tumors in adults with a high mortality rate worldwide.1,2) Chemotherapy is currently the most common and effective strategy against HCC in the clinical field. A number of anticancer drugs derived from natural products, such as taxane, vinca alkaloid, and hydroxyl-camptothecin, have exhibited excellent anticancer potential.3) Telekin is isolated from Carpesium divaricatum, which has long been used as Chinese folk medicine given its antipyretic, analgesic, vermifugic, and anti-inflammatory properties.4) In China, the whole C. divaricatum plant is used in medicine as febrifuge and astringent. It is also used to treat colds, wind syndrome of the head, sore throat, toothache, mumps, hemorrhoids, and malaria.5) Telekin has also been reported to exhibit strong antitumor activity in several human carcinoma cell lines.6,7)
Apoptosis is a genetically controlled and evolutionarily conserved process of cell death. Cells undergoing apoptosis are characterized by membrane blebbing, cytoplasmic shrinkage, DNA fragmentation, and apoptotic body formation.8–10) This process is also most frequently taken as a major pathway for chemotherapeutics to combat cancer cells. Therefore, searching for agents that can trigger tumor cells apoptosis has become an attractive strategy in anti-cancer drug discovery.
Molecular biological studies indicate that extrinsically and intrinsically mediated pathways cause apoptosis. The extrinsically mediated pathway is characterized by the activation of cell surface ligand-gated death receptors. The intrinsically medicated pathway is also called the mitochondria-mediated pathway. In this process, mitochondria are disrupted by cell stress,11) thereby releasing cytochrome c into the cytoplasm, inducing apoptosome formation with Apaf-1, activating caspases, and triggering an irreversible apoptotic process.12,13) In addition, Bcl-2 family proteins, including anti-apoptotic proteins (such as Bcl-2 and Bcl-X1) and pro-apoptotic proteins (such as Bax and Bak), play important roles as critical checkpoints that control the mitochondria-dependent intrinsic pathway.14) Two major members of the Bcl-2 family, Bcl-2 and Bax, may form a heterodimer complex that causes the mutual neutralization of their respective functions, thereby triggering apoptosis.15) Moreover, Bax was shown to serve as a requisite gateway for the mitochondria-dependent apoptotic pathway.16,17) Therefore, the balance between the expression levels of Bcl-2 and Bax is critical for determining the fate of cells (survival or death). In this study, we determined the pro-apoptotic effect and mitochondria-mediated pathway of telekin on HCC.
Telekin and its analogs were isolated from C. divaricatum in the previous reports. The purified telekin (over 98% pure) was dissolved in dimethylsulfoxide (DMSO) as 10 mmol/L stock solution and diluted according to experimental requirement. 3-(4,5-Dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) and 4,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma, U.S.A. JC-1, Fura-2 AM and DCFH-DA were purchased from the Beyotime Institute of Biotechnology, China. Caspase-3, caspase-9, cytochrome c, and Bax antibody were purchased from Santa Cruz Biotechnology, U.S.A. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Hsp60, and Bcl-2 antibody were purchased from Bioworld Technology, U.S.A. Apaf-1 antibody and Annexin V-fluorexcein isothiocyanate (FITC) Apoptosis Detection Kit were brought from BD Biosciences, U.S.A. All chemicals used in this study were commercial products and reagent grade.
Cell CultureThe human hepatocarcinoma cell lines Smmc-7721, HepG-2, LX-2, human lung cancer cell line A549, human glioblastoma cell line U87 along with normal hepatocyte cell line HL-7702 were purchased from the Shanghai Institute for Biological Sciences (SIBS), Chinese Academy of Sciences (China). A549 cells were cultured in HAM’S/F-12 Medium (Hyclone). Smmc-7721, HepG-2, HL-7702 cells were cultured in RPMI 1640 Medium (Hyclone) and LX-2, U87 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen) containing 10% fetal bovine serum supplemented with 100 units/mL of penicillin and 100 µg/mL of streptomycin. All cells were cultured in a humidified atmosphere of 5% CO2 at 37°C.
MTT AssaysCytotoxicity of telekin was evaluated by MTT assays. Cells (3–4×103 per well) were plated onto 96-well plates in 100 µL of medium. After incubating for 24 h, the medium was removed and replaced by fresh medium containing telekin at various concentrations (1.9–30 µmol/L) for 24–72 h at 37°C. Next, 20 µL of MTT (5 mg/mL) was added to each well for 4 h. The medium with MTT was removed and 150 µL/well of DMSO was added to dissolve the formazan. Optical density of each well was measured by a microplate reader (Bio-Rad 680) at 570 nm. Cell viability ratio (%)=(A570 sample−A570 blank)/(A570 control−A570 blank)×100%. IC50 values for each cell line were evaluated at a dose of drug causing 50% absorbance reduction in comparison with DMSO-treated control cells. Each test was conducted in triplicate.
Flow Cytometry Analysis of ApoptosisWe used Annexin V-FITC Apoptosis Detection Kit to evaluate telekin-induced apoptosis according to the manufacturer’s instructions. Cells were seeded at a density of 5×104/mL onto 6-well plates. After 24 h incubation, cells were treated with different concentrations of telekin. Then, cells were washed twice with cold phosphate buffered saline (PBS) and transferred to individual tubes and centrifuged at 2000 rpm/min for 5 min. The supernatant was removed, and the cells were suspended in 400 µL of Annexin V-FITC binding buffer and incubated with 5 µL Annexin V-FITC and 5 µL propidium iodide (PI) for 20 min at room temperature in dark. The apoptotic ratio was analyzed by flow cytometry (Becton Dickinson, U.S.A.) and Win MDI 2.9 analysis software.
DAPI StainingIn this part, we put 12-mm round glass cover slips in 24-well plates. Cells were seeded on these slips at a density of 5×104/mL. After 24 h incubation, cells were treated with telekin (2.5–10 µmol/L) for 48 h. After the cells were washed by cold PBS, cold-methyl alcohol–acetone (1 : 1) was used to fix cells. Then washed by PBS and stained with DAPI (4 µg/mL) for 10 min at room temperature. Cover slips containing the cells were then washed by PBS-TX (10 mL PBS+10 µL 10% Triton X-100) three times and mounted using mounting medium (PBS–glycerol=1 : 1 (v/v)) and analyzed by fluorescence microscopy (Leica DM IRB).
Measurement of Mitochondrial Membrane PotentialWe evaluated the changes in mitochondrial membrane potential (ΔΨm) in hepatocarcinoma cells using JC-1 staining. Cells were grown into 6-well plates and then treated with 2.5–10 µmol/L telekin for 24 h. Collecting cells and washing two times, suspending cells with 200 µL PBS. Then, adding JC-1 into cells (10 µg/mL) and incubating 20 min at 37°C, staining cells were washed with PBS at 2000 rpm/min for 5 min. JC-1 dye enters selectively into mitochondria and reversibly changes color from green to orange as membrane potential increase. In a flow cytometric analysis, the green emission was analyzed in fluorescence channel 1 (FL1) and the orange-red emission in channel 2 (FL2). ΔΨm was expressed by the ratio of FL2/FL1.
Measurement of ROS Generation2′,7′-Dichlorofluorescein diacetate (DCFH-DA) was used to quantify the change of intracellular reactive oxygen species (ROS) levels. DCFH-DA could change into fluorescent dichlorofluorescein (DCF) when intracellular DCFH reacts with ROS. In order to detect telekin-induced intracellular ROS accumulation, Smmc-7721, HepG-2 cells were grown in 6-well plates and then treated with 2.5–10 µmol/L telekin at 37°C for 24 h. After incubation, the culture medium was removed and the cells were washed three times with PBS. The cells were incubated with DCFH-DA (3 µmol/L) at 37°C for 20 min. Cellular fluorescence was measured by flow cytometry with a FACS-SCAN apparatus. Increased values compared to control were considered to represent the increase of intracellular ROS levels.
Measurement of Intracellular Free Calcium LevelsThe intracellular free calcium levels were quantified by Ca2+ indicator dye Fura-2 AM. Cells pretreated telekin for 6–24 h were collected and washed by PBS. Then, cells were cultured with Fura-2 AM (2 µmol/mL) for 45 min. Analysis was performed by flow cytometry.
Western Blot AnalysisCells were treated with telekin (2.5–10 µmol/L) for 48 h. Both adherent and floating cells were collected and lysed by lysis buffer (comprising: Tris–Cl, sodium dodecyl sulfate (SDS), 10% Glycerol, 100 mmol/L dithiothreitol (DTT)) at ice about 20 min for getting the total protein. The protein of total cells was separated by 12% SDS-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. The membrane was blocked with 5% non-fat milk in TBS-T buffer (10 mmol/L Tris–HCl, 150 mmol/L NaCl, and 0.05% Tween-20, pH 7.8) for at least 1 h at room temperature. After a short washing in TBS-T buffer, the membrane was incubated overnight in a solution of monoclonal anti-bodies specific for Bcl-2, Bax, caspase-9, caspase-3, Apaf-1, and GAPDH (diluted 1 : 800) at 4°C. The membrane was then incubated with secondary horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) or anti-rabbit IgG (diluted 1 : 5000; Santa Cruz Biotechnology). Proteins on the membrane were visualized using an enhanced chemiluminescence detection system (ECL®, Amersham Biosciences). Protein expression was quantified by densitometry using the Image J and Java software.
In detecting cytochrome c experiment, cells were separated into two fractions consisting of mitochondrial and cytosol using the mitochondria/cytosol isolation kit (Boster Inst. Biotech, Wuhan, China). 2×107 cells were washed with ice-cold PBS and suspended in 500 µL of isolation buffer containing protease inhibitors. After incubating the cells on ice for 10 min, they were homogenized in a Dounce homogenizer (20 strokes using Pestle B). Unbroken cells and nuclei were pelleted by centrifugation at 800×g for 10 min at 4°C. Supernatants were collected and centrifuged at 12000×g for 15 min at 4°C and the pellet were washed to repeat centrifugation. Finally, cytosolic (supernatant) and mitochondrial fractions (pellet) were collected for Western blot.18)
StatisticsAll experiments were repeated at least in triplicate. Statistical analysis was conducted by using an analysis of variance (ANOVA) followed by Turkey’s t-test. p-values less than 0.05 were considered to be statistically significant.
We first performed MTT colorimetric assays to test the cytotoxicity of these compounds (Fig. 1) isolated from C. divaricatum on several cancer lines and normal hepatocellular cells. As shown in Table 1, telekin displayed the strongest potency required to inhibit tumor cells growth. Telekin also exhibited low cytotoxicity toward normal cells HL-7702, as indicated by its higher IC50 values compared with those of the cancer cells. The IC50 values (48 h) of telekin for A549, HepG-2, U87, and HL-7702 were 17.8, 7.1, 9.0, and 54.7 µmol/L, respectively.

1 telekin, 2 11α,13-dihydrotelekin, 3 graveolide, 4 carabrone, 5 carabrol.
| Extract compounds | A549 | HepG-2 | U87 | HL-7702 |
|---|---|---|---|---|
| 1 (Telekin) | 17.8±2.6 | 7.1±1.2 | 9.0±1.1 | 54.7±2.1 |
| 2 (11α,13-Dihydrotelekin) | >40 | >40 | >40 | >60 |
| 3 (Graveolide) | 26.4±2.5 | 18.2±3.2 | 21.3±2.4 | 59.2±3.6 |
| 4 (Carabrone) | >40 | >40 | >40 | >60 |
| 5 (Carabrol) | 32.5±2.8 | 32.8±3.0 | 36.3±3.1 | >60 |
| Adriamycin | 1.4±0.9 | 0.8±0.5 | 1.2±0.3 | 6.8±1.1 |
Effects of compounds isolated from C. divaricatum on the tumor and normal cells were examined by MTT method. Cells were treated with various concentrations of compounds. IC50 values (µmol/L) of compounds for 48 h were calculated. Data were expressed as means±S.D. of three independent experiments. HL-7702 is a normal human liver cell line. Each data are expressed as the mean±S.D. obtained from triplicate experiments.
Given that telekin exhibited excellent anti-proliferation activity on HepG-2 cells, we used hepatocellular carcinoma cells (HepG-2, Smmc-7721, and LX-2) to further investigate the function of telekin and the mechanisms that underlie such function. Figure 2 shows that telekin treatment resulted in significant inhibitory effects on the cells growth of all the tested hepatocellular carcinoma cell lines. These effects occurred in a dose- and time-dependent manner. Our results also indicated that about 50% of the hepatocarcinoma cells died during treatment with 4 µmol/L to 5 µmol/L telekin for 72 h. However, little cytotoxicity for normal cells HL-7702 was observed (cytotoxicity (72 h) of HL-7702 not shown here).

(A–C) Dose- and time-dependent cytotoxicity of telekin in HepG-2, Smmc-7721, and LX-2 cells. Cells were treated with 1.9–30 µmol/L telekin, respectively, for 24–72 h. Cells viability was determined relative to the population of untreated control cells at the same time point. Each data are expressed as the mean±S.D. obtained from triplicate experiments.
Telekin was further tested to evaluate whether this cytotoxic effect is related to apoptosis. The quantitative analysis of hepatocarcinoma cell apoptosis was monitored by using FITC-Annexin V and propidium iodide (PI) double staining through flow cytometry. Flow cytometric analysis revealed that the proportion of cells stained with Annexin V increased in telekin-treated cells in a dose-dependent manner. The percentages of Annexin V-positive cells were 9.8%, 22.8%, 45.9%, 72.7% (HepG-2), 10.4%, 26.8%, 51.7%, 59.0% (Smmc-7721), 15.9%, 24.6%, 32.7%, and 66.3% (LX-2) for cells treated with 0 to 10 µmol/L telekin for 48 h (Fig. 3A). The representative patterns of cell apoptosis from flow cytometric analysis are shown in Fig. 3B.

(A) Quantification of telekin induced-apoptosis using flow cytometric analysis. Histograms were obtained from triplicate experiments. * p<0.05 and ** p<0.01 vs. telekin 0 µmol/L group. (B) Representative apoptotic profile of hepatocellular carcinoma cells treated with or without telekin 10 µmol/L by flow cytometric analysis. (C) Fluorescence micrographs (DAPI staining) of hepatoma carcinoma cells treated or untreated with telekin at magnification of 20×.
The morphological changes in the cell lines were observed by DAPI staining in Smmc-7721 and HepG-2 cells. Telekin treatment induced marked apoptotic morphological alterations, including cell shrinkage and granular apoptotic body formation (Fig. 3C).
Telekin Induced the Loss of ΔΨm and the Release of Cytochrome cWe also examined the effects of telekin on mitochondrial membrane potential (ΔΨm) in hepatocarcinoma cells. The examination was conducted by JC-1 staining performed with a flow cytometric procedure. Results showed that telekin caused a loss of ΔΨm in a dose-dependent manner for Smmc-7721 and HepG-2 cells (Fig. 4A). Meanwhile, increased mitochondrial membrane permeability was confirmed by the translocation of cytochrome c from the mitochondria to the cytosol. Figure 4B illustrates that an obvious release of cytochrome c from the mitochondria was observed in telekin-treated cells. These results suggest that the mitochondria-mediated pathway is involved in telekin-induced apoptosis.

(A) After cells were treated with 2.5–10 µmol/L telekin for 24 h, ΔΨm was measured by flow cytometry. * p<0.05 and ** p<0.01 vs. telekin 0 µmol/L group. (B) Western blot analysis of the cytochrome c release in HepG-2 cells. Telekin reduced the release of cytochrome c from mitochondria into the cytosol as shown in Western blot analysis and the corresponding histograms quantified by densitometry were expressed on the under side. * p<0.05 and ** p<0.01 vs. telekin 0 µmol/L group.
ROS generation was involved in the mechanism of mitochondrial damage. We measured the DCF-derived fluorescence, which is an index of ROS accumulation with or without telekin-treated cells, by flow cytometric assay. A greater accumulation of ROS was observed in telekin-treated (2.5 to 10 µmol/L for 24 h) HepG-2 and Smmc-7721 cells than in the control (telekin 0 µmol/L) cells (Fig. 5).
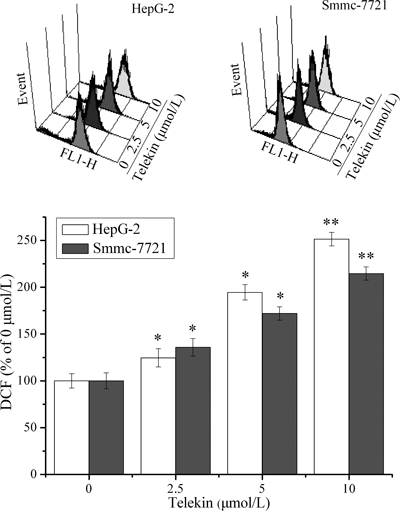
After cells were treated with 2.5–10 µmol/L telekin for 24 h, ROS accumulation was quantified by measuring DCF-derived fluorescence. The fluorescence intensity of cells without telekin treatment was set at 100% (telekin 0 µmol/L group). Representative ROS patterns (up side) and the corresponding histograms (below side, statistical analysis was obtained from triplicate experiments) of HepG-2 and Smmc-7721 cells were displayed using flow cytometric analysis. * p<0.05 and ** p<0.01 vs. telekin 0 µmol/L group.
The disturbance in mitochondrial function, which involves Ca2+ overload, a decrease in membrane potential, and breakage in mitochondrial structure, is an important event in apoptosis via the mitochondria-mediated pathway. We used HepG-2 cells to detect intracellular free Ca2+ levels. Telekin induced a gradual increase in intracellular free Ca2+ levels in a time-dependent manner (Fig. 6). After a 24 h incubation with 10 µmol/L telekin, HepG-2 cells exhibited a maximum three-fold increase in Ca2+ levels compared with the control (telekin 0 h) cells.

HepG-2 cells were treated with telekin (10 µmol/L) for 0–24 h. The concentration of Ca2+ was evaluated as described in Materials and Methods. The fluorescence intensity of cells without telekin treatment (telekin 0 h group) was set at 100%. * p<0.05 and ** p<0.01 vs. telekin 0 h group.
Bcl-2 family proteins play an important role in apoptosis controlled by mitochondria. We analyzed the expression levels of Bcl-2 and Bax proteins by Western blot after telekin treatment. A significant change in Bax and Bcl-2 expression (an increase in Bax and a decrease in Bcl-2) was observed in telekin-treated HepG-2 cells in a dose-dependent manner (Fig. 7).

After HepG-2 cells were treated with telekin for 48 h, Bax and Bcl-2 expression were analyzed by Western blot and the corresponding histograms quantified by densitometry were expressed on the right side. * p<0.05 and ** p<0.01 vs. telekin 0 µmol/L group.
Western blot was used to analyze whether caspase activation is involved in the mitochondria-mediated pathway in the telekin-induced apoptosis of HepG-2 cells. After the cells were treated with 2.5 to 10 µmol/L telekin for 48 h, Apaf-1, pro-caspase-9, and pro-caspase-3 in HepG-2 cells highly decreased. Moreover, cleaved caspase-9 and caspase-3 notably increased in a time- and dose-dependent manner (Figs. 8A, B).

(A) Telekin induced the decrease of Apaf-1 levels and the activation of caspases-9 and caspases-3 in dose-dependent manner. The corresponding histograms quantified by densitometry were expressed on the right side. * p<0.05 and ** p<0.01 vs. the 0 µmol/L telekin group. (B) Telekin induced the activation of caspases-9 and caspases-3 in time-dependent manner. The corresponding histograms quantified by densitometry were expressed on the right side. * p<0.05 and ** p<0.01 vs. telekin 0 h group.
With increasing applications of well-known natural anti-cancer drugs extracted from plants in cancer chemotherapy, exploring anti-cancer agents from natural plants has become an effective strategy for anticancer drug development and chemotherapy studies. Firstly, effects of telekin and its analogs isolated from C. divaricatum on anti-proliferation in cancer and normal cell lines were investigated. Our MTT assay results indicated that compounds 1 (telekin), 3 (graveolide), and 5 (carabrol) exhibited excellent anti-proliferation in three cancer cells lines and lower cytotoxicity in normal liver cell lines. Especially, telekin showed the largest potency to anti-cancer activity in vitro with the IC50 of 7.1–17.8 µmol/L, while compounds 3 and 5 have the IC50 of 18.2–26.4 µmol/L and 32.8–36.3 µmol/L, respectively. Compound 2 has almost non cytotoxicity effect compared with telekin and compounds 3 and 5, which indicated that α-methylene-γ-lactone moiety is very necessary to cytotoxicity activity. Many investigations have demonstrated that the cytotoxicity of sesquiterpene lactones was mainly caused by α-methylene-γ-lactone moiety. The α-methylene-γ-lactone moiety can react by Michael-type addition with biological nucleophiles (the most reactive of which are the thiol containing cysteine residues in proteins).19) Although compounds 4 and 5 have the very similar structures, the former possessing α-methylene-γ-lactone moiety in structure as well did not show any cytotoxic activity at 40 µmol/L. This result can be explained by the different molecular geometry and electronic features of them. The most active compound telekin was employed as the tested drug. Furthermore, we investigated the molecular mechanisms underlying anti-proliferative effect of telekin on hepatocarcinoma cells with a focus on a mitochondria-mediated apoptosis.
In the intrinsic pathway, cellular stress increases mitochondrial membrane permeability, followed by cytochrome c release, apoptosome formation with Apaf-1, and caspase activation.20,21) Many studies have demonstrated that ROS plays a central role in the induction of apoptosis via a variety of mechanism, such as increased mitochondrial permeability, opening of transition pores, release of pro-apoptotic factors, and activation of caspase-9 by various anti-cancer agents.22–25) However, the molecular mechanism of eudesmane-type sesquiterpene lactone-induced ROS production is not well understood. It clarify that mitochondria is considered to be a major source of ROS, and ROS are kept at harmless levels through the antioxidant systems. The balance between oxidative stress and endogenous thiol buffers present is precisely kept by intracellular redox status in the cell.26) This delicate equilibrium breaks down when apoptosis is induced, following distinct but sometimes overlapping mechanisms.27) Enhanced production of intracellular ROS possibly attribute to the depletion of the cellular thiol system. In fact, a many of cancer therapeutic drugs can induce apoptosis via perturbing this equilibrium.28) In terms of chemical structure, telekin belongs to sesquiterpene lactone. A variety of sesquiterpene lactone compounds possess diverse biological activities associated with their α-methylene-γ-lactone structures. It is noteworthy that sesquiterpene lactone potentially combines with thiol groups by a Michael type reaction resulting the depletion of GSH reservoir.29) Thus, telekin might induce ROS production through impairing the antioxidative defense system via depleting the thiol buffers.
When the increase of ROS reaches a threshold level, it triggers the opening of transition pores, which would lead to the decrease of ΔΨm and induce the side leakage of electrons from complex I, II, and III of the electron transport chain leading to rapid reaction with molecular oxygen to yield superoxide anion.30,31) Moreover, mitochondrial ROS regulate Ca2+ channels and stimulate a specific Ca2+ release, thereby contributing to the maintenance of cellular Ca2+ homeostasis.32,33) Excessive Ca2+ load to the mitochondria may also induce ROS production and apoptosis by stimulating the release of apoptosis-promoting factors from the mitochondrial intermembrane space to the cytoplasm.34–36) In the current work, telekin-treated HepG-2 cells showed an early increase in cellular ROS production and Ca2+ overload before apoptosis, indicating that mitochondrial damage is possibly related to Ca2+ release caused by oxidative stress.
In addition, the increased expression of Bax (a pro-apoptotic protein) and decreased expression of Bcl-2 (an anti-apoptotic protein) were also observed in telekin-treated HepG-2 cells. Some studies have recently reported that ROS could regulate Bcl-2 expression levels and play a pro-apoptotic role by causing the down-regulation and degradation of Bcl-2 proteins through the ubiquitin-proteasomal pathway.37)
Conversely, lower ROS levels were also observed in Bcl-2-expressing cells.38) Telekin caused mitochondria damage possibly by increasing ROS production and affecting the balance between Bcl-2 and Bax expression levels, thereby activating the classic apoptosis signaling pathway. The initiator caspase-9 is activated upon binding with Apaf-1 in a complex with cytochrome c, subsequently activating the effector caspase-3.39)
In conclusion, telekin-induced apoptosis in hepatocarcinoma cells is associated with the mitochondria-mediated pathway. Telekin induces ROS accumulation and Ca2+ overload. Additionally, the imbalance between Bax and Bcl-2 contributes to mitochondrial membrane permeability, cytochrome c release into the cytosol, and caspase-9 and caspase-3 activation. However, the detailed mechanism by which telekin affects the molecular connections between ROS production and mitochondrial apoptotic pathways remains unclear. Further studies regarding this issue are ongoing.
This work was supported by Grants from the National Natural Science Foundation of China (No. 81273532) and the Shandong Provincial Natural Science Foundation (No. ZR2011HQ028).