2013 Volume 36 Issue 8 Pages 1241-1247
2013 Volume 36 Issue 8 Pages 1241-1247
Histamine H1-receptor blockers are used to treat all types of itch resulting from serious skin diseases such as atopic dermatitis, as well as from renal and liver diseases. However, they often lack efficacy in chronic itch, a profound clinical problem that decreases quality of life. The development of effective treatments requires a full understanding of the fundamental mechanisms of itch. Recent studies have indicated that the pathogenic mechanisms of itch also involve agonists other than histamine, including proteases, neuropeptides, cytokines, and opioids, as well as their cognate receptors. Release of these pruritogenic mediators and modulators into the periphery may directly activate itch-mediating C-fibers via specific receptors on the nerve terminals. Histological observations have shown increased epidermal nerve densities in patients with atopic dermatitis, suggesting that the higher density is at least partly responsible for itch sensitization. This hyperinnervation is likely induced by an imbalance between nerve elongation and repulsion factors produced by keratinocytes. Neuronal matrix metalloproteinases are also involved in the penetration of nerve fibers into the extracellular matrix. Moreover, itch-mediating fibers such as gastrin-releasing peptide+ (GRP+) and Mas-related G-protein coupled receptor A3+ (MrgprA3+) fibers are present in the skin. Clinically, emollients or UV-based therapies can partly control epidermal nerve density, but new substances and classes of antipruritic drugs are needed. This review highlights recent knowledge regarding epidermal nerve fibers that are partly involved in itch sensitization, and discuss peripheral mechanisms and treatments of itch, especially in atopic dermatitis.
Itch (or pruritus) has been defined as an unpleasant sensation that provokes the desire to scratch. Itch is also believed to signal danger from various environmental factors or physiological abnormalities. Clinically, chronic itch is a burdensome clinical problem that decreases quality of life,1) and it frequently accompanies a variety of inflammatory skin conditions and systemic diseases. Recent studies have indicated that chronic itch is associated with increases in insomnia2) and suicide3) and reductions in patient productivity at work and in the classroom.4) The development of anti-pruritic treatments therefore requires an understanding of the fundamental mechanisms of itch.
Itch and pain are two basic modalities that are initiated and mediated by primary sensory neurons with cell bodies in the dorsal root ganglia (DRG) and trigeminal ganglia. These neurons are highly diverse in somal sizes, expression of ion channels and receptors, innervation territories, and electrophysiological properties.5) Small-diameter DRG neurons with unmyelinated axons (C-fibers) are the major neuronal types that mediate itch and pain.5,6) The sensations of itch and pain are distinct, and each can elicit different behavioral responses, such as scratching to remove irritants and withdrawal to avoid tissue injury, respectively.
Recent studies have implicated histamine-dependent and histamine-independent pathways in transmitting itch. Other systems, including proteases, neuropeptides, cytokines, and opioids, and their cognate receptors, such as thermoreceptors, proteinase-activated receptors (PARs), Mas-related G-protein coupled receptor (Mrgprs) and opioid receptors, are involved in the histamine-independent itch pathway. These pruritogenic mediators and modulators, released in the periphery, may directly activate itch-mediating fibers, especially C-fibers, by binding to specific receptors on the nerve terminals.5,7) These systems also cross-sensitize each other in the enhancement of itch.8) In addition, cutaneous nerve fibers are activated by exogenous mechanical, chemical, and biological stimuli, resulting in itch responses.7,9) Therefore, they are regarded as anti-pruritic targets in patients with chronic itch, including those with atopic dermatitis.
Histological investigations have shown that the density of epidermal nerve fibers is higher in the skin of patients with atopic dermatitis (Fig. 1A) and xerosis than in healthy controls5,7) (Fig. 1B), although the increases of nerve density in patients with pruigo nodularis and psoriasis remain unclear.10–12) Similar findings have been observed in animal models, such as NC/Nga mice, a model of atopic dermatitis model,13,14) and in dry skin model mice.15,16) Such increases in nerve density are also found in the dermis of patients with atopic dermatitis and psoriasis.17,18) These findings are indicative of increases in sensory nerve fibers responsive to exogenous triggering factors and to various endogenous pruritogens from cutaneous cells, such as immune cells and keratinocytes, suggesting that hyperinnervation is partly responsible for itch sensitization.
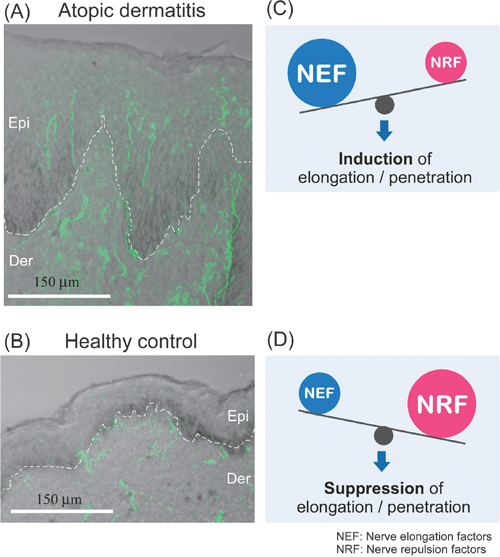
A, B; Immunohistochemical images of nerve fibers overlapped with differential interference microscopic images. PGP9.5-immunoreactive fibers (green) are observed at higher densities in the epidermis of atopic dermatitis (A). In contrast, PGP9.5-immunoreactive fibers are mainly distributed in the epidermal–dermal border of healthy controls (B). Scale bars=150 µm. epi, epidermis; der, dermis. C, D; Epidermal NEF levels are higher and epidermal NRF levels are lower in atopic than in healthy skin, suggesting the induction of penetration and/or elongation into the epidermis (C). In contrast, epidermal NEF levels are lower and epidermal NRF levels are higher in healthy than in atopic skin, suggesting the suppression of penetration and/or elongation into the epidermis (D). NEF, nerve elongation factors; NRF, nerve repulsion factors.
Epidermal hyperinnervation is probably caused by an imbalance in nerve elongation factors, such as nerve growth factor (NGF), and nerve repulsion factors, such as semaphorin 3A (Sema3A), produced by keratinocytes7) (Figs. 1C, D). These axonal guidance molecules may also act on keratinocytes, immune cells and vascular endothelial cells, and may be indirectly involved in the modulation of itching.
3.1. Nerve Elongation FactorsNerve Growth Factor (NGF)Keratinocyte-derived NGF is a major mediator of cutaneous innervation density, in that local NGF concentrations are higher in the lesional skin of patients with atopic dermatitis, psoriasis, prurigo nodularis, contact dermatitis and xerosis than in normal skin.5) In adult rat primary sensory neurons, NGF has been shown to upregulate neuropeptides, especially substance P and calcitonin-gene-related peptide (CGRP),19) both of which are involved in the hypersensitivity of itch sensation and neurogenic inflammation.20) Using a co-culture model of porcine DRG neurons and human skin cells, human atopic keratinocytes were found to produce elevated levels of NGF and to mediate an increased outgrowth of CGRP-immunoreactive fibers, whereas human atopic fibroblasts did not mediate this outgrowth.21) These findings indicate that keratinocytes are key factors in hyperinnervation in individuals with atopic dermatitis. Intradermal injection of NGF was also shown to sensitize nociceptors for cowhage- but not histamine-induced itch in human skin.22) Thus, increased NGF in the skin may sensitize primary afferents, thereby contributing to chronic itch such as atopic dermatitis. Interestingly, tumor necrosis factor (TNF)-α has been found to enhance NGF production in human keratinocytes.23) TNF-α is a pivotal proinflammatory cytokine in the innate immune response and a key molecule in skin inflammation. Mast cells have been identified as important potential sources of TNF-α.20) In addition, skin barrier disruption up-regulates TNF-α in the epidermis of acetone-treated mice, an acute dry skin model.24) These findings suggest that TNF-α is a positive regulator of NGF in the skin.
AmphiregulinAmphiregulin (AR), a protein belonging to the epidermal growth factor family, has been found to affect nerve outgrowth.25,26) AR expression was previously shown to be up-regulated in the epidermis of NC/Nga mice with atopic dermatitis-like symptoms,13) suggesting that AR may be involved in the modulation of epidermal nerve density. In addition, AR down-regulates expression of epithelial cell–cell junctional molecules such as E-cadherin and ZO-1 to construct adherens junctions and tight junctions, both of which are critical for skin barrier function.27) Indeed, the levels of expression of E-cadherin and ZO-1 were decreased in the epidermis of atopic NC/Nga mice, together with the increased expression of AR.13) Since AR affects the integrity of cell–cell junctions, these findings suggest the attenuation or abrogation of protective function against external mechanical, chemical and biological stimuli in inflammatory skin diseases.
ArteminArtemin is a glial cell line-derived neurotrophic factor. Artemin-expressing fibroblasts have been shown to accumulate in skin lesions of patients with atopic dermatitis.28) Moreover, dermal fibroblasts secrete artemin in response to substance P, released by cutaneous nerve fibers, inducing itch and/or neurogenic inflammation via the activation of neurokinin-1 (NK-1) receptor.29) Intradermal injection of artemin into mice resulted in peripheral nerve sprouting and thermal hyperalgesia.28) Therefore, artemin may be partly involved in hypersensitivity to warm sensations, mimicking warmth-provoked itch in atopic dermatitis.
3.2. Nerve Repulsion FactorsSemaphorin 3A (Sema3A)Sema3A is the first member of the semaphorin family shown to cause growth cone collapse in neurons, i.e., to function as an axonal repulsion factor, through its interaction with a neuropilin-1⁄plexin-A receptor complex.30) In addition, Sema3A inhibits NGF-induced sprouting of sensory afferents in adult rat spinal cord,31) whereas elevated levels of NGF reduce the Sema3A-induced collapse of sensory growth cones.32)
Several recent studies showed that Sema3A transcripts were expressed in cultured normal human epidermal keratinocytes.33,34) Sema3A proteins are mainly distributed in the suprabasal layer of normal human skin,33) consistent with findings showing that Sema3A is expressed in differentiated keratinocyte cultures.34) Moreover, epidermal Sema3A levels were lower in patients with atopic dermatitis than in healthy controls, while an increase in epidermal nerve density was found in the skin.33) Increased epidermal nerve density in acute dry skin mice was also associated with decreased levels of Sema3A expression,35,36) suggesting that decreasing the expression of Sema3A accelerates epidermal nerve growth in patients with dry skin condition such as atopic dermatitis and xerosis. Thus, epidermal innervation may be regulated by a fine balance between NGF and Sema3A.
Anosmin-1Anosmin-1 is an extracellular matrix glycoprotein encoded by the KAL1 (Kallmann syndrome 1 sequence) gene, the gene responsible for the X chromosome-linked recessive form of Kallmann syndrome.37) Anosmin-1 has been found to inhibit neurite outgrowth in cultured rat DRG neurons.38) KAL1 transcripts were also expressed in cultured keratinocytes and in normal human skin. Anosmin-1 was strongly expressed in the basal cell layer of normal skin, but its level of expression was lower in atopic skin, concomitant with an increase in epidermal nerve density. Thus, keratinocyte-derived anosmin-1 may be at least partly involved in modulating epidermal nerve density in patients with atopic dermatitis.38)
3.3. Roles of Matrix Metalloproteinase (MMP)-2 and MMP-8 in Cutaneous Nerve GrowthThe process of cutaneous nerve growth in pruritic skin such as atopic dermatitis requires several MMPs for growth cones to penetrate the three-dimensional extracellular matrix (ECM) barriers. Using in vitro models of ECM, such as Matrigel and type I collagen gel, MMP-2 localized on the growth cone was found to be involved in penetration into the basement membrane39) (Figs. 2A, B). In addition, MMP-8 secreted by nerve fibers was shown to be involved in nerve growth within the dermis, consisting mostly of types I and III collagens40) (Figs. 2A, C). The levels of expression of MMP-2 and MMP-8 were up-regulated by NGF and down-regulated by Sema3A, and both were induced by their enzymatic substrates, but not altered by non-substrate molecules. The selection and up-regulation of MMPs corresponding to the ECM components surrounding the growing nerve fibers may be required for efficient nerve fiber penetration, suggesting that the coordinated activation of neurotrophin and ECM-integrin signaling is necessary for efficient and long-distance axon extension.41,42) Since class 3 semaphorin signaling inhibits integrin-mediated adhesion signaling, Sema3A stimulation of growing nerve fibers may provide a reverse signaling pathway for these events.43)

(A) NGF and Sema3A, which are produced by skin cells such as epidermal keratinocytes and fibroblasts, modulate the outgrowth of nerve fibers into the extracellular matrix (ECM) through production of neuronal MMPs. (B) NGF promotes MMP-2 production in sensory nerve fibers and activates pro-MMP-2 on the growth cone. Sema3A produced by keratinocytes and fibroblasts may have opposite effects on NGF-dependent events. (B′) Activated MMP-2 on the growth cone may contribute to penetration of nerve fibers into the basement membrane. (C) In the dermis, NGF also promotes MMP-8 production by sensory nerve fibers and its secretion by nerve fibers, probably growth cones. Sema3A may have opposite effects on NGF-dependent events. (C′) Activated MMP-8 may be involved in sensory nerve growth within the interstitial collagen matrix. epi, epidermis; der, dermis; BM, basement membrane.
Since specific markers of itch-mediating fibers have not been identified to date, they could not be histologically identified in the periphery. Recent studies, however, have demonstrated that GRP receptor expressing cells mediate the itch sensation in the spinal cord.44) In addition, increases in cutaneous nerve fibers containing GRP have been observed in NC/Nga mice with atopic dermatitis-like symptoms.14) Therefore, GRP secreted from the central terminals of primary afferents may be involved in the transmission of itch signals in the spinal dorsal horn. Intradermal injections of GRP were found to elicit scratching behavior in mice, and the itch-related response was at least partly induced by the release of pruritogens through activation of bombesin receptors in mast cells.45) A more recent study showed that serum GRP levels correlate with pruritus in patients with atopic dermatitis.46) Thus, serum GRP level may be useful as a biomarker for itch and disease severity in patients with atopic dermatitis.
4.2. Mas-Related G-Protein Coupled Receptors (Mrgprs)Recent studies have demonstrated that the histamine-independent itch pathway involves members of the family of over 50 Mrgprs, especially MrgprAs, MrgprB4-5, MrgprC11 and MrgprD, which are restricted to small diameter DRG neurons in mice.47) Chloroquine and bovine adrenal medulla peptide 8-22 (BAM8-22) elicit itch-related scratching through MrgprA3 and MrgprC11, respectively, in mice.48) Both chloroquine and BAM8-22 also elicited itch in humans.49,50)
A more recent study using conditional transgenic mice revealed that ablation of MrgprA3+ DRG neurons led to substantial reductions in scratching evoked by multiple pruritogens and occurring spontaneously under chronic itch conditions.51) However, pain sensitivity remained intact in these mice. Moreover, mice in which transient receptor potential vanilloid 1 (TRPV1) was exclusively expressed in MrgprA3+ DRG neurons exhibited itch, but not pain, behavior in response to capsaicin. Although MrgprA3+ DRG neurons were sensitive to noxious heat, activation of TRPV1 in these neurons by noxious heat did not alter pain behavior. These findings suggest that MrgprA3 defines a specific subpopulation of DRG neurons that mediate itch. In mouse skin, MrgprA3+ fibers exclusively innervated the epidermis and responded to multiple pruritogens, suggesting that they are peripheral itch-specific fibers.
Anti-NGF approaches have been tried to treat itch of atopic dermatitis in NC/Nga mice. Intraperitoneal administration of anti-NGF neutralizing antibody to atopic NC/Nga mice significantly attenuated both the increased number of nerve fibers in the epidermis and scratching behavior, but did not ameliorate scratching that had already developed.52) Similarly, application of the TrkA antagonists AG879 and K252a to the nape of atopic NC/Nga mice significantly improved established dermatitis and scratching behavior and reduced the numbers of nerve fibers in the epidermis, suggesting the importance of NGF in the pathogenesis of atopic dermatitis-like skin lesions.53) Thus, NGF and its receptors may be among the antipruritic targets in pruritic skin diseases such as atopic dermatitis.
5.2. Sema3A Replacement TherapyRecombinant Sema3A replacement approaches (intradermal injection or ointment application) were found to significantly inhibit scratching behavior and to improve dermatitis in NC/Nga mice with atopic dermatitis-like symptoms compared with controls.54,55) The therapeutic efficacy of exogenous Sema3A on atopic dermatitis-like symptoms was greater than that of current agents, such as betamethasone and tacrolimus.55) Moreover, histological analyses showed decreases in (i) the numbers of epidermal nerve fibers; (ii) the numbers of inflammatory infiltrates; (iii) the production of cytokines; (iv) the density of dermal blood vessels; and (v) epidermal thickness in Sema3A-treated lesional skin.54,55) These findings suggest that exogenous Sema3A not only affects sensory nerve fibers, but other cells that express neuropilin-1, include immune system cells, endothelial cells and keratinocytes.56) Thus, Sema3A and its receptors are promising therapeutic targets for atopic dermatitis.
5.3. Effectiveness of Conventional Therapies on Axonal Guidance MoleculesSeveral existing therapies normalize abnormal levels of axonal guidance molecules such as NGF and Sema3A in pruritic skin, reducting epidermal nerve density.
5.4. Olopatadine (A Histamine H1-Receptor Antagonist)Oral administration of olopatadine hydrochloride, a histamine H1-receptor (H1R) antagonist, significantly suppressed scratching behavior, improved dermatitis, and inhibited neurite outgrowth in the lesional skin of mice with atopic dermatitis-like symptoms. Notably, olopatadine treatment increased Sema3A expression in the epidermis.57,58) Although it is unclear whether these effects are caused by specific blocking of H1R signaling, olopatadine may in part improve imbalances between NGF and Sema3A in the epidermis.
5.5. Neurotropin (NTP)NTP, a non-protein extract isolated from the inflamed skin of rabbits inoculated with vaccinia virus, is widely used in Japan and China to treat various chronic pain conditions.59) In clinical studies in Japan, NTP has been shown to have anti-pruritic effects in patients with eczema, dermatitis and urticaria,60) and in those undergoing hemodialysis.61) NTP was found to inhibit NGF-induced neurite outgrowth of rat DRG neurons in vitro62) and to significantly reduce intraepidermal nerve growth in acetone-treated mice, a model for acute dry skin.63) In the latter, NTP significantly up-regulated epidermal Sema3A mRNA, but had no effect on expression of epidermal NGF mRNA.63) Thus, although its mechanisms are as yet unknown, NTP may reduce epidermal nerve density by inducing expression of Sema3A in the epidermis, resulting in suppression of itch.
5.6. EmollientA recent study using dry skin mice showed that application of heparinoid cream resulted in greater improvements in epidermal nerve density and epidermal NGF levels than application of petrolatum, although heparinoid cream had no effect on epidermal Sema3A levels.35) In addition, the increase of epidermal nerve fibers was more reduced by the immediate than the delayed application of emollients to dry skin, suggesting that the prompt application of emollients is more effective in normalizing epidermal hyperinnervation and expression of axon guidance molecules.
5.7. UV-Based TherapyVarious types of UV-based therapy, including psoralen-UVA (PUVA) and narrow-band UVB, have been widely used to treat patients with skin inflammation such as atopic dermatitis and psoriasis.64) The UV-based therapies were shown to reduce the number of cutaneous nerve fibers, especially in the epidermis, in patients with atopic dermatitis and psoriasis, and to inhibit pruritus.65,66) Similar effects of UV-based therapy on epidermal nerve fibers were observed in dry skin mice.36) The imbalance between Sema3A and NGF levels in the epidermis was normalized by PUVA or narrow-band UVB treatment.36,66) A recent study showed that excimer lamp treatment was the most effective form of UV-based therapy for intraepidermal nerve fibers.36)
5.8. New Anti-pruritic DrugsNalfurafine Hydrochloride (A Selective Kappa-Opioid Receptor Agonist)Several studies have demonstrated that mu- and kappa-opioid systems play pivotal roles in modulation of itch at the central nervous system. It is generally thought that the mu-opioid system induces itch, whereas the kappa-opioid system suppresses itch at the central level.7,67) Recently, the effectiveness of nalfurafine hydrochloride (REMITCH®), a selective kappa-opioid receptor agonist, on hemodialysis-related uremic pruritus was validated in a Phase III, randomized double-blind placebo-controlled trial.68) This drug is also expected to make further contributions to the treatment of patients with intractable itch, such as those with cholestasis and atopic dermatitis.
Peripheral mu- and kappa-opioid systems may also play important roles in pruritus of atopic dermatitis.69–71) One study revealed that topical application of mu-opioid receptor antagonist (naltrexone) cream to the skin inhibited itch in patients with atopic dermatitis.70) A peripherally restricted kappa-opioid agonist, ICI 204,448, also antagonized chloroquine-evoked scratching in mice.69) Moreover, the kappa-opioid system was found to be down-regulated in the epidermis of patients with atopic dermatitis and psoriasis, whereas the mu-opioid system was at normal levels.11,71) Down-regulation of the mu-opioid system and restoration of the kappa-opioid system by PUVA treatment have been observed in patients with atopic dermatitis, concomitant with a reduction in itch.71) Thus, these findings suggest that mu-opioid receptor antagonists or kappa-opioid receptor agonists have antipruritic effects at the peripheral level.
5.9. Aprepitant (A Neurokinin-1 (NK-1) Receptor Antagonist)Substance P is a key mediator in pruritus.72) In the periphery, substance P released from cutaneous nerve fibers induces itch and/or neurogenic inflammation.29) In addition, a recent study supports a role for NK-1 receptor-expressing spinal neurons in itch.73) Current evidence suggests that substance P is partially involved in the spinal transmission of itch signals.74)
Aprepitant is an oral NK-1 receptor antagonist75) widely used as an antiemetic agent in patients with chemotherapy-induced nausea and vomiting.76) Aprepitant was also found effective against intractable pruritus associated with Sézary syndrome, a leukemic, cutaneous, epidermotropic T-cell lymphoma.77) In a recent clinical trial, 16 of 20 patients with chronic itch treated with aprepitant showed a significant reduction in itch.78) Thus, although some patients did not respond, these findings indicated that aprepitant is effective in patients with chronic itch, including atopic dermatitis.
5.10. Histamine H4 Receptor (H4R) AntagonistsH4R have been reported involved in histamine-evoked itch in animal models.79) While specific H4R agonists induced itch, pretreatment with the H4R antagonist JNJ7777120 inhibited this response, and histamine or H4R agonist-evoked itch was attenuated in H4R-deficient mice. Moreover, inhibiting both H1R and H4R almost completely eliminated histamine-evoked scratching.80) A more recent study showed that the H1R antagonist olopatadine and the H4R antagonist JNJ7777120 reduced scratching behavior and skin inflammation in the NC/Nga mouse model of chronic allergic dermatitis induced by repeated challenges with picryl chloride.58) Thus, H4R antagonists may be effective in patients with chronic itch, including those with atopic dermatitis.
This review presents recent knowledge regarding itch sensitization associated with epidermal nerve density controlled by nerve elongation factors (e.g., NGF) and nerve repulsion factors (e.g., Sema3A) through the regulation of expression of MMPs, especially in atopic dermatitis. In addition, treatment with anti-NGF agents, Sema3A replacement and other treatments such as UV-based therapies may normalize epidermal nerve fiber density. New substances and classes of antipruritic drugs are needed. The research on and development of anti-pruritic drugs may contribute to improvements in the quality of life of patients who suffer from intractable pruritus such as atopic dermatitis.
This work was supported by a Health Labor Sciences Research Grant for Research on Allergic Disease and Immunology from the Japanese Ministry of Health, Labour and Welfare, by a KAKENHI (20591354 and 2079081) and a “High-Tech Research Center” Project for Private Universities: matching fund subsidy from MEXT, and by a JSPS Research Fellow.