2014 Volume 37 Issue 1 Pages 169-173
2014 Volume 37 Issue 1 Pages 169-173
Increased spontaneous locomotive activity and oxygen consumption have been reported in transgenic mice overexpressing leptin in the liver. In the present study, we examined whether the overexpression of leptin altered glycolytic and oxidative metabolic enzymatic activities as well as the composition of myosin heavy chain (MHC) isoforms in skeletal muscle. Enzymatic activities of lactate dehydrogenase (LDH) and citrate synthase (CS) were quantified in gastrocnemius muscle (GAS) and the red portion of tibialis anterior muscle (TA) from leptin transgenic (Tg) mice and non-Tg mice. The composition of MHC isoforms was measured in soleus muscle (SOL) and extensor digitorum longus muscle (EDL) from the two groups. In red TA, LDH-to-CS ratio was significantly lower in Tg than in non-Tg (p=0.014), whereas no significant change was observed in GAS. The composition of MHC isoforms was not significantly different in SOL or EDL between Tg and non-Tg groups. Our data indicate that chronic overexpression of leptin reduces the ratio of glycolytic to oxidative capacity without changing muscle fiber types particularly in red muscles. This metabolic change may contribute to the increased spontaneous locomotive activity and oxygen consumption in Tg mice reported previously.
Leptin is an adipocyte-derived hormone that plays a role in regulating body weight by enhancing energy metabolism and reducing appetite. Transgenic mice that overexpress leptin in the liver1) and mice chronically treated with leptin2) showed lean phenotype and improved glucose homeostasis. Skeletal muscle is one of the targets of leptin and accounts for about 70–80% of insulin-induced glucose uptake,3) thus skeletal muscle is one of the key organs for glucose homeostasis. Chronic and acute treatment of leptin has been shown to facilitate glucose uptake and fatty acid oxidation via activation of AMP-activated protein kinase (AMPK) in skeletal muscle.4,5) These findings support the ongoing efforts for the therapeutic application of leptin for the treatment of insulin resistance and type 2 diabetes,6,7) warranting further research to overcome leptin resistance in diet-induced obesity.4,8,9)
Our group previously showed increased spontaneous locomotive activity and greater oxygen consumption in leptin transgenic mice chronically overexpressing leptin in the liver compared to normal mice.4) This finding allows us to postulate that chronic treatment of leptin may enhance the fatigue resistance of skeletal muscle by improving oxidative capacity. Because AMPK plays a pivotal role in mitochondrial biogenesis in muscle cell,10) upregulated AMPK activity induced by chronic overexpression of leptin may enhance oxidative capacity more strongly compared to glycolytic capacity in skeletal muscle. In fact, a previous study11) has shown that chronic treatment with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), a cell permeable activator of AMPK, increased metabolic enzymatic activities for mitochondrial respiration and β-oxidation with a decrease in glycolytic enzyme activity. High oxidative capacity relative to glycolytic capacity, a typical metabolic property observed in slow-type muscle fibers12) and in endurance-trained skeletal muscles,13) confers muscle fibers fatigue-resistant. Enhanced fatigue resistance in skeletal muscle may improve the effectiveness of exercise programs for obesity and metabolic disorders.
In addition to their metabolic properties, muscle fiber types classified by myosin heavy chain (MHC) isoforms are also involved in the fatigue resistance of skeletal muscle. In mammalian limb skeletal muscles, MHC isoforms consist of type I, IIa, IIx, and IIb, and muscle fiber types classified by the four MHC isoforms possess different ATPase activity and tension cost as well as shortening velocity.14,15) Endurance training16) and chronic muscle contraction17) change the composition of MHC isoforms, and this change is considered as one of the functional adaptations to improve fatigue resistance in skeletal muscle. However, it is unknown if chronic overexpression of leptin alters muscle fiber types represented by MHC isoforms.
In the present study, we examined the long-term effect of the overexpression of leptin on MHC isoforms and metabolic enzymatic activities in skeletal muscle. For this purpose we used leptin transgenic skinny mice where serum leptin level is five times higher than that of wild-type mice.4) The data presented here indicate that chronic overexpression of leptin enhance the metabolic capacity for oxidation as opposed to lactate fermentation without changing muscle fiber types in red skeletal muscle.
All experimental procedures were undertaken in accordance with the guidelines for animal experiments of Kyoto University and were approved by the Animal Research Committee of Graduate School of Human and Environmental Studies, Kyoto University and by the Animal Research Committee of Graduate School of Medicine, Kyoto University. Leptin transgenic (Tg) mice were prepared as reported previously.1) In this mouse model, a fusion gene comprising the human serum amyloid P component promoter and mouse leptin cDNA coding sequences was inserted to the genomic DNA so that the transgene expression was targeted to the liver. Compared to the leptin transgenic mice to which an adipocyte-specific promoter was applied,18) our mouse model exhibited higher plasma leptin concentration and showed a skinny phenotype. Male Tg mice (n=5) and non-Tg mice (n=7) aged 13 or 15 weeks old were housed in temperature-controlled room and fed ad libitum. After overnight fasting, the soleus (SOL), extensor digitorum longus (EDL), tibialis anterior (TA), and gastrocnemius (GAS) muscles were dissected from the mice under anesthesia. TA was cut into white and red portions. The dissected muscles were immediately frozen and stored at −80°C. Blood samples were obtained from the abdominal aorta, and blood lactate concentration was immediately measured using Lactate Pro (Arkray, Kyoto, Japan).
MHC IsoformsThe composition of MHC isoforms of SOL and EDL was measured as described previously.19–21) Briefly, the samples were separated on an SDS-polyacrylamide (7%) gel, and then the gel was stained with coomassie brilliant blue. The bands representing MHCs were classified as type I, IIa/x or IIb isoforms, and the intensity of the bands was quantified using MultiAnalyst software (Bio-Rad, CA, U.S.A.). The composition of MHC isoforms was calculated so that the total percentage of the three isoforms reached 100% in each lane. The percentage of each MHC isoform in Tg groups was statistically compared with the percentage of the corresponding MHC isoform in non-Tg group.
Metabolic Enzyme ActivitiesActivities of lactate dehydrogenase (LDH) and citrate synthase (CS), representative enzymes for glycolytic capacity and mitochondrial respiratory capacity, respectively,22) were measured as described previously.19) GAS and the red portion of TA were homogenized in homogenate buffer (1 : 20 w/v). The homogenates were frozen and thawed three times and the supernatants were used for the measurement. Absorbance of the samples was measured for 5 min at 30°C.
Statistical AnalysisData are shown as mean±standard error (S.E.). Student’s t-test was performed to compare between Tg and non-Tg groups. A p value of <0.05 was considered statistically significant.
Body weight in Tg and non-Tg groups is shown in Fig. 1. Although the difference in average body weight was not statistically significant, four of five Tg mice showed lower body weight than that of all seven non-Tg mice, indicating that the constitutive expression of leptin contributed to the reduction in body weight. Blood lactate concentration was not significantly different between Tg and non-Tg groups (Table 1).
Enzymatic activities are shown in Fig. 2. LDH activity was not significantly different between Tg and non-Tg groups in red TA or GAS. CS activity was not significantly different between the two groups in either muscle. LDH-to-CS ratio in the red TA was significantly lower in the Tg group than in the non-Tg group (p=0.014). Composition of MHC isoforms is shown in Fig. 3. Composition of MHC isoforms was not significantly different between Tg and non-Tg in SOL or EDL.

Dots indicate body weight of individual mice in Tg (n=5) and non-Tg (n=7) groups. Horizontal bars indicate mean value in each group.
| Tg | Non-Tg | |
|---|---|---|
| Lactate (mmol/L) | 7.9±2.2 | 8.6±1.8 |
Values are expressed as mean±S.E. (n=5 for Tg and n=7 for non-Tg).

(a) LDH and (c) CS activities and (e) LDH-to-CS ratio in red TA. (b) LDH and (d) CS activities and (f) LDH-to-CS ratio in GAS. Values are expressed as mean+S.E. (n=5 for Tg and n=7 for non-Tg).
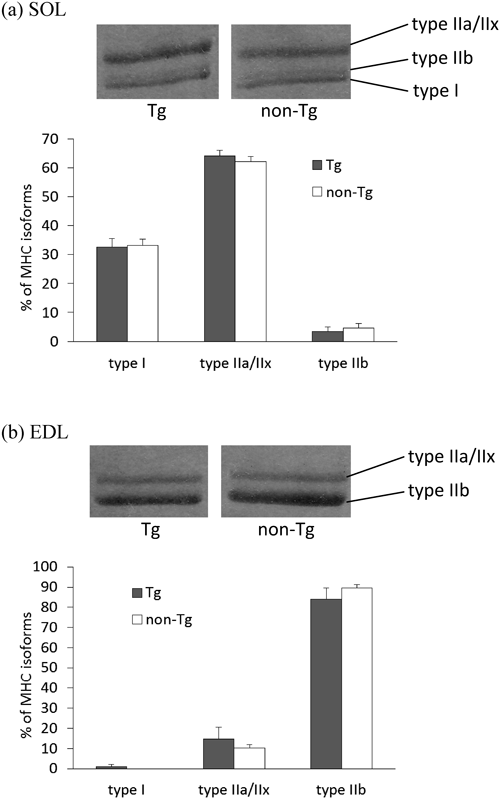
Values are expressed as mean+S.E. (n=5 for Tg and n=7 for non-Tg). Representative MHC bands are shown above the graphs.
The main finding of the present study is that constitutive overexpression of leptin decreased LDH-to-CS ratio in red TA, but not in GAS. SOL and EDL, representative oxidative red muscle and glycolytic white muscle respectively, were examined for MHC isoform pattern, and there was no statistically significant difference between Tg and non-Tg in those muscles. The ratio of metabolic enzyme activities, e.g. LDH-to-CS (lactate fermentation/citric acid cycle), is taken as relative measures of metabolic capacities in skeletal muscle.23) These results suggest that chronic leptin treatment can enhance oxidative capacity relative to glycolytic capacity in the red skeletal muscle without fiber-type change.
Decreased LDH-to-CS ratio in the Tg group could contribute to the increase in aerobic exercise capacity. In voluntary daily activities, average forces exerted by limb muscles are less than 30% of maximal voluntary force,24,25) and oxidative fibers are preferentially recruited at this intensity of exercise.26) Blood lactate concentration was not significantly different between Tg and non-Tg groups, suggesting that neither duration nor intensity of anaerobic exercise differed between the two groups. Taken together, low LDH-to-CS ratio in red muscle from Tg mice could contribute to a higher amount of spontaneous locomotive activity at aerobic exercise intensity and thus greater oxygen consumption as reported previously.4)
Considering our previous study showing elevated phosphorylation level of AMPKα and a high AMP-to-ATP ratio in SOL, a typical red skeletal muscle, from Tg mice,4) lower LDH-to-CS ratio observed in red TA from Tg mice could be mediated by increased AMPK activity. Apart from the fact that sympathetic denervation interferes with muscle AMPK activation by leptin,5) the precise mechanism for the increased AMPK activity and AMP-to-ATP ratio in red muscle from Tg mice has not been approached. In light of our present data, however, this phenomenon can perhaps be explained by the increased recruitment of oxidative fibers in Tg mice, implied by the increased spontaneous locomotive activity in Tg mice as stated above. A previous study27) showed that the mitochondrial metabolic enzymes such as CS and carnitine palmitoyltransferase II had higher activities in skeletal muscles from cp/cp rats, which possess an autosomal recessive mutation in the leptin receptor gene, than in skeletal muscles from homozygous wild-type and heterozygotes. The findings of McClelland et al. may argue against our results showing leptin-dependent decrease in LDH-to-CS ratio. Chronic exposure of these rats to leptin may give answers to the question of whether the leptin receptor is necessary for the changes in metabolic enzyme activities in skeletal muscle.
Our results on MHC isoforms suggest that a higher amount of spontaneous locomotive activity or greater oxygen consumption in Tg mice was not attributable to the change in muscle fiber types. Putman et al.11) showed that chronic AICAR administration increased CS activity and decreased LDH activity without significant changes in the composition of MHC isoforms or fiber types in rat skeletal muscle, which corresponded to the present study. The effect of leptin on muscle fiber types via AMPK may not be as evident as the effect of leptin on metabolic enzyme activities.
In the present study, Tg mice group showed a tendency for lower body weight compared to non-Tg. Our previous study4) showed significant reduction in body weight in Tg mice with a similar level of food intake. Therefore, the weight-losing effect by the chronic overexpression of leptin can be attributed primarily to high oxygen consumption and increased spontaneous locomotive activity. Together with our previous data, the present study underscores the importance of chronic leptin treatment as a metabolic modifier that increases oxidative capacity relative to glycolytic capacity in red skeletal muscle.
Some pathological conditions could be observed as a result of constitutively high leptin concentration. For instance, Tg mice exhibited significant elevation in systolic blood pressure and urinary norepinephrine excretion.28) Obesity-induced hyperleptinemia played a crucial role in the progression of nonalcoholic steatohepatitis via enhanced responsivity to endotoxin in the liver.29) Hyperleptinemia in obese people leads to “leptin resistance” as well, under which the anti-obesity effect of leptin is attenuated.30) Therefore, in order to prevent those side effects, the dose and frequency of leptin administration should be properly controlled in clinical applications.
We are grateful to Miwa Iida, Shin Nohara and Radiance Lim for assistance. We appreciate cooperation by Dr. Tatsuya Hayashi in the present study. This work was supported by MEXT Grants-in-Aid B2:16390267, S2:16109007, B:18790634, B:25282201, and adipomics:15081101; a MHLW Health and Labor Science Research Grant; and Grants from JST, AstraZeneca, the Takeda Medical Research Foundation, the Smoking Research Foundation, Metabolic Syndrome Foundation, the Japan Foundation for Applied Enzymology.