2014 Volume 37 Issue 1 Pages 96-104
2014 Volume 37 Issue 1 Pages 96-104
Tranilast (TL), an antiallergic agent, has been clinically used in the treatment of bronchial asthma, although its clinical use has been limited by its poor solubility in water, photodegradation and systemic side effects. In this study, we prepared a gel ointment containing TL nanoparticles (TLnano gel ointment), and investigated its usefulness. In addition, we demonstrated the preventive effects of the TLnano gel ointment on inflammation in adjuvant-induced arthritis (AA) rats. The TLnano gel ointment was prepared using Bead Smash 12 (a bead mill) and additives including sodium docusate, 2-hydroxypropyl-β-cyclodextrin, methylcellulose and Carbopol 934; the mean particle diameter of the TL nanoparticles was 71.0±25.4 nm. In in vitro skin penetration experiments, the amount of penetrated TL, the penetration rate (Jc) and the penetration coefficient through the skin (Kp) of the TLnano gel ointment were significantly higher than those of a gel ointment containing TL microparticles (TLmicro gel ointment; particle diameter 50.5±26.3 µm). The TL concentrations in the skin tissue and plasma of rats receiving the TLnano gel ointment were also higher than in rats receiving the TLmicro gel ointment. In addition, the application of the TLnano gel ointment attenuated the increase in paw edema of the hind feet of AA rats in comparison with AA rats treated with the TLmicro gel ointment. These results suggest that TL nanoparticles can be applied to the formulation of a transdermal system, and that a transdermal formulation using TL nanoparticles might be a delivery option for the clinical treatment of RA.
Arthritis is a chronic disease that affects several parts of joints including the cartilage, synovium, tendons and muscles. Rheumatoid arthritis (RA) is a specific type of arthritis, and a complex chronic inflammatory disease dependent on multiple interacting environmental and genetic factors, including T cells,1) neutrophils,2) activated T lymphocytes,3) macrophages,4) eicosanoids5) and cytokines,6,7) making it difficult to understand its pathogenesis, and, so, to find effective therapies.8) In treatments for RA, the focus is on the reduction of pain, inflammation and joint damage. The principal pharmacological agents are nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, glucocorticoids and specific inhibitors of the mediator response.9)
Tranilast (TL), N-(3′,4′-dimethoxycinnamoyl) anthranilic acid, is a synthetic, multi-potential, anti-allergic, and anti-fibrotic drug reported to have various effects both in vitro as well as in vivo.10) The variety of TL functions published to date include the suppression of collagen synthesis by fibroblasts via down-regulation of cytokine release from monocytes/macrophages,11) the transcriptional and translational inhibition of matrix metallo-proteases in lipopolysaccharide-stimulated neutrophils,12) and the suppression of monocyte/macrophage infiltration and associated myocardial fibrosis in the deoxycorticosterone acetate/salt hypertensive rat.13) Recently, it was reported that TL suppresses the development of RA in multiple models, and TL is expected to become a novel therapeutic agent for human RA.14) Despite its positive pharmacological effects on inflammatory diseases, TL exhibits poor dissolution behavior and poor solubility (14.5 µg/mL in water), particularly under acidic conditions (0.7 µg/mL in buffer solution of pH 1.2),15) so that the daily dose of TL must be relatively high (300 mg/d). Therefore, the oral administration of TL is often limited due to systemic side effects that include liver dysfunction, abdominal discomfort, skin rash, and allergic cystitis.16) In addition, liquid TL has been found to be photodegradable. To overcome these drawbacks, the development of an efficacious drug delivery system is a key consideration.
Transdermal administration offers many advantages over conventional oral delivery for medications, including smooth and continuous drug delivery, reduced Cmax, and steadier systemic drug levels. This system improves the tolerability profile and permits the achievement of relatively high local drug concentrations without systemic side effects. However, the amount that can be administered transdermally is quite low since transdermal delivery is severely limited by the inability of the large majority of drugs to cross the skin at therapeutic rates due to the barrier imposed by the skin’s outer stratum corneum layer. Accordingly, considerable research efforts are focused on drug permeation, and various enhancing methods have been tried: iontophoresis and electroporation,17,18) phonophoresis,19,20) chemical methods and absorption enhancers.21,22) Recently, a transdermal delivery system using nanoparticles has been reported. Poly(lactic-co-glycolic acid) (PLGA) has been used for the preparation of nanoparticles with mean particle diameters of 50–200 nm.23–25) The pathways available for a drug to penetrate the stratum corneum are the transcellular pathway, intracellular pathway, and transaccessory pathway. The transcellular pathway seems to be the most promising route. It is reported that the allowable space of this pathway is 50–70 nm.26) Therefore, a combination of PLGA nanoparticles and iontophoresis has been used for the transdermal delivery of therapeutic agents. However, this method does not remove the problem of the photodegradability of the TL. On the other hand, solid TL nanoparticles (100–200 nm) show high photochemical stability.27) Based on these reports, we expected to be able to prepare a gel ointment containing solid TL nanoparticles <100 nm in size. In this study, we prepared this gel ointment containing solid TL nanoparticles, and investigated its usefulness. In addition, we demonstrated the preventive effects of this gel ointment on inflammation in adjuvant-induced arthritis (AA) rats.
TL microparticles were kindly donated by Kissei Pharmaceutical Co., Ltd. (Nagano, Japan). 2-Hydroxypropyl-β-cyclodextrin (HPβCD, average molar substitution, 0.6; average molecular weight (MW), 1380) was purchased from Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan). Low-substituted methylcellulose (MC, METOLOSE SM-4, average viscosity, 4 Pa·s in 20°C) was provided by Shin-Etsu Chemical Co., Ltd. (Tokyo, Japan). Sodium docusate (SD) was obtained from Sigma-Aldrich Japan (Tokyo, Japan). Carboxypolymethylene (Carbopol® 934) was purchased from Serva (Heidelberg, Germany). All other chemicals used were of the highest purity commercially available.
AnimalsMale Wistar rats, aged 7 weeks, and Dark Agouti (DA) rats, aged 6 to 13 weeks, were provided by Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan), housed under standard conditions (12 h/d fluorescent light (07 : 00–19 : 00), 25°C room temperature), and fed a commercial diet (CE-2, Clea Japan Inc., Osaka, Japan) and water. All animal experiments were performed in accordance with the Kinki University School of Pharmacy Committee Guideline for the Care and Use of Laboratory Animals.
Preparation of Gel Ointment Containing TL NanoparticlesCarbopol® 934 was added to distilled water, allowed to swell for 1 h at room temperature, and neutralized with 5% ammonia water (gel base). TL nanoparticles were prepared using zirconia beads and Bead Smash 12 (a bead mill, Wakenyaku Co., Ltd., Kyoto, Japan). The zirconia beads (diameter: 2 mm) were added to solid TL microparticles (particle size 50.5±26.3 µm, means±S.D.), and the mixture was crushed with the Bead Smash 12 (4200 rpm, 60 s×10 times, 4°C). The particles size of milled TL was 0.071±0.025 µm (means±S.D., Fig. 1). After milling, the TL nanoparticles were added to the Carbopol® 934 gel base containing SD, HPβCD or MC, and stirred until the mixture became uniform. The gel ointment containing TL microparticles was prepared by adding TL microparticles, SD, HPβCD or MC into the Carbopol® 934 gel base (TLmicro gel ointment). The dispersity of the nanoparticles in the ointment base was confirmed as follows: 0.75% TLmicro and TLnano gel ointments were divided into 10 parts, and the TL concentration in each part was measured by HPLC (TLmicro, 0.76±0.08; TLnano, 0.74±0.03, %, means±S.E., n=10). The particle size was measured using a nanoparticle size analyzer SALD-7100 (Shimadzu Corp., Kyoto, Japan; refractive index 1.60–0.10i). The compositions of the gel ointments containing TL are shown in Table 1. The TLmicro and TLnano gel ointments were stable for one month after preparation (mean particle diameters: TLmicro, 52.1±0.9; TLnano, 0.079±0.152, µm, means±S.D.), and no decrease in TL concentration in the TLmicro and TLnano gel ointments was observed during the one month.

(A) Cumulative distribution and frequency of TLmicro gel ointment, (B) cumulative distribution and frequency of TLnano gel ointment. Gel ointments were dispersed in water, and the particle sizes were determined using a nanoparticle size analyzer SALD-7100 (refractive index 1.60–0.10i).
| Formulation | Content (g) | Treatment | |||||
|---|---|---|---|---|---|---|---|
| TL | SD | HPβCD | MC | Carbopol® | Distilled water ad. | ||
| TLmicro gel | 0.35, 0.5 or 0.75 | 0.005 | 0.5 | 0.5 | 3.0 | 100.0 | — |
| Milled-TLwithout additive gel | 0.75 | — | — | — | 3.0 | 100.0 | Bead mill |
| Milled-TLwithout SD gel | 0.75 | — | 0.5 | 0.5 | 3.0 | 100.0 | Bead mill |
| Milled-TLwithout HPβCD gel | 0.75 | 0.005 | — | 0.5 | 3.0 | 100.0 | Bead mill |
| Milled-TLwithout MC gel | 0.75 | 0.005 | 0.5 | — | 3.0 | 100.0 | Bead mill |
| TLnano gel | 0.35, 0.5 or 0.75 | 0.005 | 0.5 | 0.5 | 3.0 | 100.0 | Bead mill |
An experiment was carried out using 25, 220 and 450 nm pore size membrane filters (MF™-MEMBRANE FILRER, Merck Millipore, Tokyo, Japan) and a Franz diffusion cell.28) The donor side of the membrane filter was soaked in buffer (0.85% NaCl–10 mM phosphate buffer, pH 7.4) for 12 h at 4°C to equilibrate the membrane. Then 0.3 g TL gel ointment, as shown in Table 1, was spread uniformly over the membrane, which was then mounted in the Franz diffusion cell (reservoir volume 12.2 mL, 1.6 cm i.d. O-ring flange), and occluded with aluminium foil. The diffusion cells were thermoregulated in a water bath at 37°C for 48 h. One hundred microliter aliquots of sample solution were withdrawn from the reservoir chamber at the indicated time intervals and replaced with the same volume of buffer. Ten microliters of filtrate was added to 100 µL methanol containing 0.3 µg ethyl p-hydroxybenzoate (internal standard), and the mixture was filtered through a Chromatodisk 4A (pore size 0.45 µm, Kurabo Industries Ltd., Osaka, Japan). The solution (10 µL) was injected into an Inertsil® ODS-3 (3 µm, column size: 2.1 mm×50 mm) column (GL Science Co., Inc., Tokyo, Japan) on a Shimadzu LC-20AT system equipped with a column oven CTO-20A (Shimadzu Corp., Kyoto, Japan). The mobile phase consisted of acetonitrile–50 mM ammonium acetate (20 : 80) at a flow rate of 0.25 mL/min, the column temperature was 35°C, and the wavelength for detection was 230 nm.
In Vitro Skin Penetration of TLmicro and TLnano Gel OintmentsThe in vitro skin penetration experiment was carried out using the Franz diffusion cell.28) On the day before the experiment, the hair on the abdominal area of 7 week-old Wistar rats was carefully removed with an electric clipper and electric razor. The following day, pieces (3×3 cm area) of full-thickness abdominal skin were excised from the rats, and the adherent fat and other visceral debris were removed from the undersurface. The dermal side of the full-thickness skin was soaked in buffer (0.85% NaCl-10 mM phosphate buffer, pH 7.4) for 12 h at 4°C to equilibrate the skin. Then 0.3 g TL gel ointment was uniformly spread over the stratum corneum of the skin, which was then mounted in a Franz diffusion cell (reservoir volume 12.2 mL, 1.6 cm i.d. O-ring flange), and occluded with aluminium foil. The diffusion cells were thermoregulated in a water bath at 37°C for 30 h. The TL concentrations in the samples were determined by the HPLC method described above. The data obtained were analyzed using the following formula29) (Eqs. 1–3):
 | (1) |
 | (2) |
 | (3) |
where Jc is the TL penetration rate, Km is the skin/preparation partition coefficient, D is the diffusion constant within the skin, tlag is the lag time, δ is thickness of the skin (0.071 cm, average for 5 rats), Qt is the total amount of TL appearing in the reservoir solution at time t, and A is the effective area of skin (2 cm2); Jc and τ were estimated by fitting each penetration profile to Eq. 3. The penetration coefficient through the skin, Kp, is given by Jc/CTL. A nonlinear least-squares computer program (MULTI) was employed for the calculation.30)
Accumulation of TLmicro and TLnano Gel Ointments in Skin TissueOn the day before the experiment, the hair on the abdominal area of 7 week-old Wistar rats was carefully removed with an electric clipper and electric razor. The following day, 0.3 g of TL gel ointment was fixed on the shaved abdominal skin with an adhesive and immediately occluded with adhesive tape. At 24 h after the start of the experiment, the skin surface to which the gel ointment was applied was washed with saline, and the TL gel ointments were reapplied. The gel ointment on the skin surface was wiped off with a Kimwipe (NIPPON PAPER CRECIA, Tokyo, Japan) soaked in saline, and pieces (2 cm2) of abdominal skin to which the TL gel ointments were applied were excised at 0–48 h after application. The adherent fat and other visceral debris were removed from the undersurface, and the skin samples obtained were stored at −80°C until TL analysis. The frozen skin was homogenized in dimethyl sulfoxide using a Physcotron homogenizer (MICROTEC Co., Ltd., Chiba, Japan). The homogenates were centrifuged at 15000 rpm for 20 min at 4°C, and the supernatants were used for the measurement of TL. The TL concentrations in the samples were determined by the HPLC method described above.
In Vivo Percutaneous Absorption of TLmicro and TLnano Gel OintmentsOn the day before the experiment, the hair on the abdominal area of 7 week-old Wistar rats was carefully removed with an electric clipper and electric razor, and a cannula filled with 30 µg/mL heparin (silicone tubing; i.d., 0.5 mm, o.d., 1.0 mm) was inserted into the right jugular vein of the rats under pentobarbital anesthesia (40 mg/kg, intraperitoneally (i.p.)). 0.3 g of TL gel ointment was fixed on the shaved abdominal skin with an adhesive and immediately occluded with adhesive tape. At 24 h after the start of the experiment, the skin surface to which the gel ointment was applied was washed with saline, and the TL gel ointments were reapplied. Venous blood (200 µL) was collected at 0–96 h after the application of TL gel ointment from the jugular vein through the cannula. The blood was centrifuged at 3000 rpm for 20 min at 4°C, and the plasma obtained was stored at −80°C until TL analysis. The TL concentrations in the samples were determined by the HPLC method described above. The TL concentration in the plasma after a single injection of 0.3 mL of TL solution (50 µg/kg) into the femoral vein was analyzed according to Eq. 4:
 | (4) |
where CTL is TL concentration in the plasma, C0 is the initial concentration of TL in the plasma (2.51±0.09 µg/mL), ke is the elimination rate constant for TL from the plasma. The ke and distribution volume (Vd) data obtained from 5 experiments were 1.33±0.40 h−1 and 19.9±0.93 mL/kg, respectively.
The absorption of TL after a single administration of TL gel ointment was calculated as the apparent absorption rate constant (ka, h−1) according to Eq. 5:
 | (5) |
where CTL is the TL concentration in the plasma, D is the dose of TL administered (0.35–0.75% gel ointments, 0.3 g), ka is the absorption rate constant, t is time (0–24 h) after TL administration, tlag is lag time (h), Vd is the distribution volume, F is the fraction of TL absorbed. In addition, the plasma TL concentration data after repeated administration of TL gel ointments (0.3 g/d, interval 24 h) were estimated by Eq. 6
 | (6) |
where τ is the interval after TL administration (24 h), N is the frequency of TL administration. A nonlinear least-squares computer program (MULTI) was employed for these calculation (Eq. 4–6).
The area under the TL concentration–time curve (AUC0–24h) was analyzed according to the following equation (Eq. 7):
 | (7) |
Briefly, AUC0–24h was determined according to the trapezoidal rule up to 24 h, which was the time of the final TL concentration measurement.
Application of TL Gel Ointments to AA RatsArthritis was induced by the injection of 50 µL of adjuvant, a suspension of 10 mg/mL heat-killed Mycobacterium butyricum (Difco, Detroit, MI, U.S.A.) in Bayol F oil, into the plantar region of the right hind foot and tail of DA rats. The control group received 50 µL of Bayol F oil. The rats were hooded to prevent them from licking, and 0.3 g of TL gel ointment was applied to right foot daily (9 : 00). When reapplying the gel ointment, the old gel ointment on the skin surface was first wiped off with saline on a Kimwipe. The application of TL gel ointments was started 14 d after adjuvant injection. In this study, inflammation during the development of AA was assessed by measuring paw volume (paw edema), a parameter of inflammation, by plethysmometry. In this paper, the inflammatory scores are represented as AUC (the area under the paw volume–time curve, AUC14–42d). The values of AUC14–42d were calculated according to the following equation (Eq. 8):
 | (8) |
where V and t are the paw volume and the number of days after adjuvant injection, respectively.
Statistical AnalysisAll values are represented as mean±standard error of the mean (S.E.). Unpaired Student’s or Aspin–Welch’s t-tests were used to determine statistical difference, and multiple groups were evaluated by one-way ANOVA followed by Dunnett’s multiple comparison. The p values less than 0.05 were considered significant.
Figure 2 shows the effects of additives on the release of TL from gel ointments containing TL particles. The release of TL from gel ointments containing TL nanoparticles was increased by a combination of additives (SD, HPβCD and MC). The releases from Milled-TLwithout SD, Milled-TLwithout HPβCD, Milled-TLwithout MC gel ointments were approximately 62.0, 52.1 and 37.6% in comparison with the release from the TLnano gel ointment at 48 h after the application of 0.75% TL gel ointment. Figure 3 shows the penetration profiles of TL through the membrane filter after the application of TLmicro and TLnano gel ointments. The TL release from the TLnano gel ointment was significantly higher than that from the TLmicro gel ointment. Although the TL release from the TLnano gel ointment was similar in experiments using 220 and 450 nm pore size membranes, the TL penetration profile of the TLnano gel ointment through a 25 nm pore size membrane was significantly lower than that through the 220 and 450 nm pore size membranes. Figure 4 shows the penetration profiles of TL through rat skin after the application of TLmicro and TLnano gel ointments, and Table 2 summarizes the pharmacokinetic parameters calculated from the in vitro skin penetration data. The amounts of TL that penetrated increased linearly after the application of either TL gel ointment into the donor chambers, but the penetration rate (Jc) and penetration coefficient through the skin (Kp) values of the TLnano gel ointment were significantly higher than those of the TLmicro gel ointment. For the TLmicro gel ointment, a decrease in the skin/preparation partition coefficient (Km) and an increase in the diffusion constant within the skin (D) were observed as the TL concentration in the gel ointment increased. On the other hand, the Km and D values of the TLnano gel ointment were similar for 0.35–0.7% TL concentrations. The lag times (tlag) for the TLmicro and TLnano gel ointments showed no significant difference. Figure 5 shows the amount of TL in rat skin tissue after the application of TLmicro and TLnano gel ointments. The amounts of TL in the skin tissues of rats receiving the TLnano gel ointment were higher than those of rats receiving the TLmicro gel ointment. Figure 6 shows the absorption profiles of TL through rat skin following the applications of TLmicro and TLnano gel ointments, and Table 3 summarizes the pharmacokinetic parameters calculated from the in vivo percutaneous absorption data. The plasma concentration of TL increased following the application of both the TLmicro and TLnano gel ointments, but the apparent absorption rate constant (ka) and AUC0–24h values in the skin of rats receiving the TLnano gel ointment were significantly higher than those of rats receiving thes TLmicro gel ointment (Fig. 6A, Table 3). Figure 6B shows the plasma TL concentration data for the repetitive administration of TLmicro or TLnano gel ointments. The TL plasma concentration following the application of the 0.75% TLnano gel ointment reached a plateau at 48–96 h with a value of 1.7–3.3 µg/mL (Css).
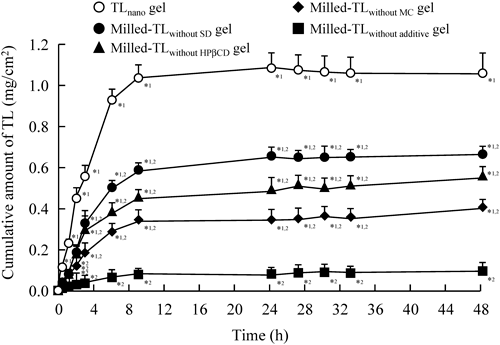
0.3 g of the TL gel ointment described in Table 1 was applied to a 450 nm pore size membrane for 0–48 h. The data represent the means±S.E. of 6 independent experiments. *1 p<0.05 vs. Milled-TLwithout additive gel. *2 p<0.05 vs. TLnano gel.

0.3 g of 0.75% TL gel ointment containing micro- (open symbols) or nanoparticles (closed symbols) was applied to 25 (circles), 220 (triangles) and 450 nm (squares) pore size membranes for 0–48 h. The data represent the means±S.E. of 6 independent experiments. *1 p<0.05 vs. TLmicro gel for each category. *2 p<0.05 vs. TLnano gel for 450 nm pore size membrane.

0.3 g of TL gel ointment containing micro- (open symbols) or nanoparticles (closed symbols) was applied to abdominal skin pieces for 0–30 h. The data represent the means±S.E. of 3–6 rat skins. * p<0.05 vs. TLmicro gel for each category.
| Ointment | Jc (µg/cm2/h) | Kp (×10−4 cm/h) | Km | tlag (h) | D (×10−4 cm2/h) | |
|---|---|---|---|---|---|---|
| 0.35% | TLmicro gel | 0.60±0.05 | 5.72±0.46 | 0.68±0.05 | 11.26±1.27 | 0.60±0.14 |
| TLnano gel | 0.84±0.07* | 7.98±0.24* | 0.64±0.03 | 9.41±0.47 | 0.89±0.05 | |
| 0.50% | TLmicro gel | 0.82±0.03** | 5.50±0.20 | 0.50±0.04** | 10.84±0.78 | 0.78±0.08** |
| TLnano gel | 1.30±0.10*,*** | 8.06±0.54* | 0.68±0.07* | 10.34±0.75 | 0.85±0.13 | |
| 0.75% | TLmicro gel | 1.06±0.07** | 4.80±0.29 | 0.38±0.16** | 9.23±0.47 | 0.92±0.05** |
| TLnano gel | 1.73±0.23*,*** | 7.67±0.90* | 0.66±0.10* | 10.17±0.24 | 0.83±0.06 | |
The experiments were carried out using a Franz diffusion cell; the pharmacokinetic parameters were calculated according to Eqs. 1–3. TLmicro gel: gel ointment containing TL micro, TLnano gel: gel ointment containing TL nanoparticles. The data represent the means±S.E. of 3–6 rat skins. * p<0.05 vs. TLmicro gel ointment for each category; ** p<0.05 vs. 0.35% TLmicro gel ointment for each category; *** p<0.05 vs. 0.35% TLnano gel ointment for each category.

0.3 g of TL gel ointment containing micro- (open columns) or nanoparticles (closed columns) was applied to the abdominal skin of rats for 0–48 h. The data represent the means±S.E. of 3–6 rats. * p<0.05 vs. TLmicro gel for each category.

0.3 g of 0.75% TL gel ointment containing micro- (open circles) or nanoparticles (closed circles) was applied to the abdominal skin of rats for 0–96 h. (A) Plasma concentration of TL after a single application of TL gel ointment for 0–24 h, (B) plasma concentration of TL after repetitive application of TL gel ointment for 0–96 h. The plasma TL concentration data after the repetitive application of TL gel ointments (0.3 g/day, interval 24 h) were estimated according to Eqs. 4–6. Solid lines represent the fitted curves for multiple applications of 0.75% TL gel ointment containing micro- or nanoparticles. The data represent the means±S.E. of 3–6 rats.
| Ointment | ka (×10−2 h−1) | tlag (h) | F | AUC0–24h (µg·h/mL) |
|---|---|---|---|---|
| TLmicro gel | 2.6±0.5 | 0.76±0.06 | 0.13±0.05 | 4.6±0.8 |
| TLnano gel | 4.6±0.8* | 0.50±0.03* | 0.78±0.10* | 37.4±4.2* |
0.3 g of 0.75% TL gel ointment was applied to the abdominal skin; the pharmacokinetic parameters were calculated according to Eqs. 4, 5 and 7. TLmicro gel: gel ointment containing TL microparticles, TLnano gel: gel ointment containing TL nanoparticles. The data represent the means±S.E. of 3–6 rats. * p<0.05 vs. TLmicro gel ointment for each category.
Figure 7 shows the changes in the paw edema volume in the right (A) and left (B) hind feet of AA rats. Paw edema in the right hind foot injected with adjuvant appeared on the day following injection, and reached a maximum after 14 d. On the other hand, paw edema of the left hind foot, which was not injected with adjuvant, was not observed during the first 7 d after adjuvant injection, but clearly increased from 10 d (1.62±0.19, means±S.E. of 10 independent rat), and reached a maximum at 14 d. Figure 8 and Table 4 show the preventive effects of TLmicro and TLnano gel ointments on paw edema in AA rats. Paw edema of the right hind feet of AA rats to which the TLmicro gel ointment was applied was less in comparison with AA rats treated with gel ointment without TL (control gel ointment) in the days following adjuvant injection. However, paw edema of the left hind feet of AA rats to which the TLmicro gel ointment was applied did not differ significantly from that of rats receiving the control gel ointment. In contrast to the results in AA rats treated with the TLmicro gel ointment, the application of the TLnano gel ointment attenuated the increases in paw edema of both the right and left hind feet, and the AUC14–42d values of the right and left hind feet of AA rats treated with the TLnano gel ointment were significantly lower than those of AA rats treated with the TLmicro or control gel ointments (Table 4).

Arthritis was induced by injecting 50 µL of adjuvant, a suspension of 10 mg/mL heat-killed Mycobacterium butyricum in Bayol F oil, into the plantar region of the right hind foot and tail of DA rats. The data are presented as means±S.E. of 10 independent rats.

The applications of TL gel ointment were started 14 d after adjuvant injection. 0.3 g of TL gel ointment was applied to the right foot once a day (9 : 00). The data are presented as means±S.E. of 5–10 independent rats. * p<0.05 vs. TLmicro gel ointment for each category.
| Ointment | AUC14–42d (mL·d) | ||
|---|---|---|---|
| Right | Left | ||
| Control | 80.9±0.6 | 64.6±0.8 | |
| 0.35% | TLmicro gel | 77.3±0.7* | 66.3±1.7 |
| TLnano gel | 69.5±1.7*,** | 62.6±1.1*,** | |
| 0.75% | TLmicro gel | 75.2±0.9* | 64.2±1.0 |
| TLnano gel | 65.3±1.3*,** | 59.4±0.8*,** | |
The values for AUC14–42d were calculated according to Eq. 8. Control: gel ointment without TL applied AA rat, TLmicro gel: gel ointment containing TL microparticles applied AA rat, TLnano gel: gel ointment containing TL nanoparticles applied AA rat. The data represent the means±S.E. of 5–10 independent rats. * p<0.05 vs. Control for each category; ** p<0.05 vs. TLmicro gel for each category.
Nanoparticles are defined as any material with at least one dimension that is smaller than 100 nm in size.31) Nanoparticles can be engineered to carry drug payloads, image contrast agents, or gene therapeutics for diagnosing and treating disease, with cancer being a primary focus, and research into nanomedicines has increased enormously during the past years.31–39) However, there are many interesting unanswered questions concerning the therapeutic applications of nanotechnology, and it is important that further studies to develop drug delivery systems using nanoparticles be carried out.40) In this study, we prepared a gel ointment using solid TL nanoparticles, and investigated its usefulness. In addition, we demonstrated the preventive effect of the gel ointment containing TL nanoparticles on inflammation in AA rats.
TL nanoparticles were prepared using zirconia beads and Bead Smash 12, which resulted in the preparation of high quality TL nanoparticles (particle size, 0.071±0.025 µm, means±S.D.) (Fig. 1). The data indicated that the milled TL nanoparticles were homogeneous with a narrow particle size distribution. Clinically, ointments, creams and gels are used in formulations for transdermal therapeutic systems, and gels are particularly used pharmaceutically as lubricants and also as carriers for many drugs to provide a local effect and percutaneous absorption.41,42) Therefore, a gel ointment containing TL nanoparticles was prepared using Carbopol 934, which has excellent thickening, emulsifying and gelling properties, allowing the uniform incorporation of the TL nanoparticles. However, the release of TL from gel ointments containing TL nanoparticles was lower in the in vitro experiment using 450 nm pore size membranes (Fig. 2). Therefore, we used additives to enhance the release of TL from the gel ointments. Additives are very important in the preparation of high quality nanomedicines, since they affect the environment around the surface of the particles. In a previous study, we reported that the addition of HPβCD, MC and a surface-active agent was suitable for the preparation of nanoparticles using mill methods.43) Cyclodextrins are cyclic oligosaccharides comprising R-D-glucose linked by R-(1–4) glucosidic bonds. The potential use of natural cyclodextrins and their synthetic derivatives has been studied extensively in efforts to improve certain properties such as solubility, stability, and/or bioavailability.44) HPβCD is a cyclic oligosaccharide with a hydrophilic outer surface and a lipophilic cavity at its center. It is capable of forming inclusion complexes with many lipophilic drugs by taking up the drug molecule, or part of it, into the lipophilic cavity.45,46) MC is a water-soluble substance with a high degree of purity, uniformity and transparency. MC solutions are neutral, odorless, and tasteless. MC solutions are also stable over a wide pH range. In the preparation of gel ointments containing TL nanoparticles, we used the HPβCD, MC and SD, which is a surface-active agent, as additives. The addition of SD, HPβCD or MC increased TL release from gel ointment, and the TL amount of release from the TLnano gel ointment was 11-fold that a Milled TLnano without additive gel ointment (Fig. 2). Moreover, the 25 nm pore size membrane, which is smaller than the TL nanoparticles in this study, suppressed the TL release from the TLnano gel ointment (Fig. 3). This result suggests that the TLnano gel ointment emits in the state of TL nanoparticles. We also investigated the photochemical stability of the TLnano gel ointment under fluorescent light (wave length: 400–700 nm). Liquid TL in dimethyl sulfoxide was found to be photodegradable when exposed to 58 W/m2 for 12 h (remaining content 67.8±4.0% vs. before photo illumination, mean±S.E., n=3); however, a high degree of photochemical stability was observed for the TLnano gel ointment (remaining content 98.6±2.7% vs. before photo illumination, mean±S.E., n=3). In addition, the TLmicro and TLnano gel ointments were stable for 1 month after preparation (mean particle diameters: TLmicro, 52.1±0.9; TLnano, 0.079±0.152, µm, means±S.D.), with no decrease in TL concentration the ointments observed. These results show that the formula developed in this study is suitable for the preparation of TL gel ointments containing nanoparticles.
In in vitro skin penetration experiments using rats, the amounts of TL that penetrated the skin increased linearly in the case of either TL gel ointment up to 30 h after the application of the gel ointment, but the penetration became greater beyond 30 h. It is known that the skin surface can be damaged by the application of some drugs, and that the damaged surface leads to an increase in the penetration of the drug.47) Therefore, in the in vitro skin penetration experiment, we used data collected in 0–30 h range to calculate the pharmacokinetic parameters. The amount of penetrated TL, the penetration rate (Jc), and the penetration coefficient through the skin (Kp) for the TLnano gel ointment were all significantly higher than those of the TLmicro gel ointment (Fig. 4, Table 2). A decrease in the skin/preparation partition coefficient (Km) and an increase in the diffusion constant within the skin (D) were observed with increasing TL concentration in the TLmicro gel ointment. On the other hand, the Km and D values for the TLnano gel ointment were similar in the 0.35–0.75% TL concentration range. In the in vivo study, the TL concentrations in the skin tissue and plasma of rats receiving the TLnano gel ointment were also significantly higher than those of rats receiving the TLmicro gel ointment (Table 3, Figs. 5, 6). We show that the supply of TL from the TLnano gel ointment was higher than that from TLmicro gel ointment in this study (Figs. 3, 4). Therefore, the high supply of TL from the TLnano gel ointment may be related to the TL concentrations in the skin tissue and plasma. These results show that the characteristics of the TLmicro and TLnano gel ointments in skin differ, and suggest that the effects for local and systemic therapy are greater following the application of the TLnano gel ointment than the TLmicro gel ointment.
In studies to develop transdermal therapeutic systems for treating RA, the selection of the experimental animal is very important. The AA rat is an animal model in which arthritis is induced by the injection of an adjuvant. Inflammatory pain during the development of AA is assessed by measuring paw edema.48,49) Paw edema in AA rats is known to involve two inflammatory processes, primary and secondary inflammation. Primary inflammation starts from the day following the injection of adjuvant into the right hind foot. Secondary inflammation is observed from 7 d after adjuvant injection, and reaches a maximum in both feet 14 d after adjuvant injection into the right and/or left hind foot.50,51) It is noteworthy that changes in the biological characteristics of AA rats correspond to those that occur in human RA.48–51) Therefore, AA rats provide a useful model for use in studies to evaluate transdermal therapeutic systems in RA.
Recently, it was reported that TL suppresses the development of RA in multiple models, and that the therapeutic effects of TL are partly mediated by the inhibition of mast cell activation and osteoclastogenesis.14,52) In this study, paw edema of the right hind feet of AA rats treated with the TLmicro gel ointment was significantly decreased (Table 4, Fig. 8). In contrast to the results in the right hind feet of AA rats treated with the TLmicro gel ointment, paw edema of the left hind feet of AA rats treated with the TLmicro gel ointment was similar to that of rats treated with gel ointment containing no TL (control gel ointment). In the right hind feet, the preventive effects of the TLnano gel ointment were significantly greater than in the case of the TLmicro gel ointment (Fig. 8A). It is suggested that the achievement of relatively high local TL concentrations in the case of the TLnano gel ointment resulted in an effective therapy. On the other hand, the application of the TLnano gel ointment also attenuated the increases in paw edema of the left hind feet of AA rats, and the AUC14–42d values of both the right and left hind feet were significantly decreased in comparison with AA rats treated with control or TLmicro gel ointments (Table 4). We show that TL penetration from the TLnano gel ointment into the blood is greater than that from the TLmicro gel ointment (Table 3, Figs. 5, 6). Therefore, the data suggest that high TL concentrations in the plasma following the application of the TLnano gel ointment causes the decrease in paw edema of the left hind feet of AA rats and the positive therapeutic effects.
Further studies are needed to elucidate the precise mechanisms for the skin penetration route of gel ointments containing TL nanoparticles. In addition, it is important to clarify a suitable formulation for a transdermal therapeutic system for RA using TL nanoparticles. Therefore, we are now investigating the therapeutic effects of transdermal systems using TL nanoparticles and various additives on inflammation in AA rats.
In conclusion, we have developed a new gel ointment system that includes TL nanoparticles using Bead Smash 12 and additives including SD, HPβCD, MC and Carbopol 934. The percutaneous penetration of TL from the gel ointment and the therapeutic effect on inflammation for the TLnano gel ointment were significantly higher than those for the TLmicro gel ointment, and our findings suggest that a transdermal therapeutic system using nanoparticles may enable a medication to be applied without high-systemic levels, providing efficient and effective therapy that spares patients from unwanted side effects. A transdermal formulation using TL nanoparticles may provide a delivery option for the clinical treatment of RA.