2014 Volume 37 Issue 11 Pages 1727-1735
2014 Volume 37 Issue 11 Pages 1727-1735
Delamanid is a new drug for the treatment of multidrug-resistant tuberculosis. Individuals who are co-infected with human immunodeficiency virus and Mycobacterium tuberculosis may require treatment with a number of medications that might interact significantly with the CYP enzyme system as inhibitors or inducers. It is therefore important to understand how drugs in development for the treatment of tuberculosis will affect CYP enzyme metabolism. The ability of delamanid to inhibit or induce CYP enzymes was investigated in vitro using human liver microsomes or human hepatocytes. Delamanid (100 µM) had little potential for mechanism-based inactivation on eight CYP isoforms (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4). Delamanid’s metabolites were noted to inhibit the metabolism of some CYP isoforms, but these effects were observed only at metabolite concentrations that were well above those observed in human plasma during clinical trials. Delamanid (≤10 µM) did not induce CYP1A2, CYP2C9, and CYP3A4 activities in human hepatocytes, and there were no increases in CYP1A2, CYP2B6, CYP2C9, and CYP3A4 mRNA levels. Taken together, these data suggest that delamanid is unlikely to cause clinically relevant drug–drug interactions when co-administered with products that are metabolized by the CYP enzyme system.
Tuberculosis (TB) has become a global health emergency with 8.8 million incident cases, including an estimated 1.1 million individuals who are co-infected with human immunodeficiency virus (HIV).1,2) These infections act synergistically in co-infected individuals to increase the risk of the development of active TB following latent infection.3) The emergence of multidrug-resistant TB (MDR-TB) [i.e., TB caused by strains of Mycobacterium (M.) tuberculosis resistant to the first-line drugs isoniazid and rifampin] has hampered the global response to the TB epidemic.1) Current treatment regimens for MDR-TB are more toxic, last longer, and are less effective than treatment regimens for drug-sensitive TB.4) Further, the mortality of patients co-infected with HIV and MDR-TB can exceed 60% following diagnosis.5)
It is estimated that CYP enzymes are responsible for the metabolism of approximately three-quarters of the most prescribed drugs that are cleared by metabolism, including antiretroviral agents used to treat HIV.6–8) Unwanted drug–drug interactions often result from the inhibition or induction of one or more CYP isoforms by a first drug, resulting in increased or decreased exposure, respectively, of a second drug that is metabolized by the same isoforms.9,10) Unintended alterations in the exposure to drugs or their metabolites may have significant effects including increased toxicities or therapeutic failure.9,10) It is therefore critical to understand the potential of new anti-TB drugs to inhibit or induce CYP enzymes.
Delamanid (OPC-67683, nitro-dihydro-imidazooxazole derivative), (R)-2-methyl-6-nitro-2-[(4-{4-[4-(trifluoromethoxy)phenoxy]piperidin-1-yl}phenoxy)methyl]-2,3-dihydroimidazo[2,1-b]oxazole, was synthesized and screened by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan) as a novel anti-TB agent.11) Delamanid inhibits mycolic acid synthesis and has demonstrated potent pre-clinical in vitro and in vivo activity against both drug-susceptible and drug-resistant strains of M. tuberculosis.12) Further, delamanid has shown anti-TB activity in patients with drug-sensitive TB13) and in patients with MDR-TB, and is being specifically developed to treat MDR infections.14,15) Delamanid has been approved as a new drug for the treatment of MDR-TB and has been launched onto the market in Europe and Japan.
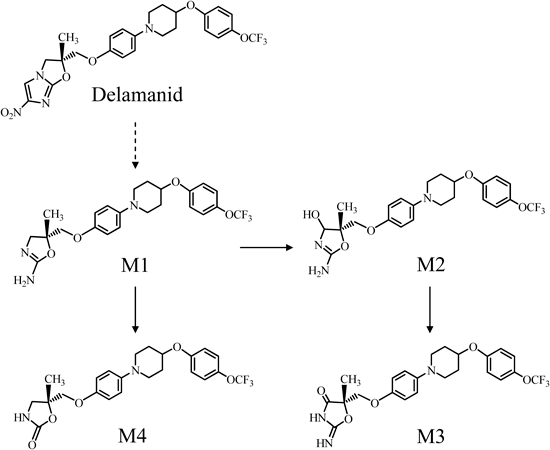
The in vitro metabolism of delamanid using human and animal liver microsomes has been evaluated.12) Since reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent metabolites were hardly detected in the incubation mixture, the results suggested that delamanid was not metabolized by CYP enzymes. However, following oral administration of delamanid to humans and animals, at least four metabolites, M1, M2, M3, and M4, have been detected and identified in the plasma. From the chemical structures of metabolites, the proposed metabolic pathways of delamanid are shown in Fig. 1. Delamanid is considered to be primarily metabolized to (R)-2-amino-4,5-dihydrooxazole derivative (M1). The primary metabolite, M1, is proposed to undergo subsequent biotransformation reactions, including oxidations to (4RS,5S)-2-amino-4,5-dihydro-4-hydroxyoxazole derivative (M2) and M2 to (S)-2-imino-oxazolidin-4-one derivative (M3). M1 is further proposed to be metabolized to (R)-4,5-dihydro-2-oxooxazole derivative (M4).
CYP1A2, CYP2A6, CYP2B6, CYP2C, CYP2D6, CYP2E1, and CYP3A are principally involved in the oxidation of a large number of xenobiotic chemicals as well as endogenous compounds and these expressions have been reported at the protein level in the human liver microsomes.16) CYP1A2, CYP2B6, CYP2C9, and CYP3A4 are known as the inducible isoforms by the nuclear receptors, aryl hydrocarbon receptor, constitutive androstane receptor, and pregnane X receptor, and these nuclear receptors have been recognized as important roles in drug-related inductions of the CYP enzymes in the human liver.17) It is therefore significant to evaluate the inhibitory and inductive potential of these CYP isoforms in the drug development process. The inhibitory effects of delamanid have been already investigated on CYP1A2, CYP2A6, CYP2B6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 activities using human liver microsomes in vitro, and delamanid has been reported to have no inhibitory effects on these activities at concentrations up to 100 µM.12) In the present study, we evaluated mechanism-based inactivation (MBI) of delamanid and the inhibitory effects of the metabolites of delamanid on eight CYP enzymes using human liver microsomes in vitro. Further, we investigated the inductive effects of delamanid on CYPs including CYP1A2, CYP2B6, CYP2C9, and CYP3A4 in human hepatocytes.
Delamanid and its metabolites (M1, M2, M3, and M4) were synthesized by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). 7-Ethoxyresorufin, resorufin, acetaminophen coumarin, bupropion hydrochloride, tolbutamide, hydroxytolbutamide, paclitaxel, diclofenac sodium salt, (±)-bufuralol hydrochloride salt, (±)-hydoxybufuralol maleate salt, chlorzoxazone, nifedipine, testosterone, and 6β-hydroxytestosterone were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). Other chemicals were obtained from the following sources: phenacetin, 7-hydroxycoumarin, and midazolam from Wako Pure Chemical Industries, Ltd. (Osaka, Japan); hydroxybupropion, 6α-hydroxypaclitaxel, and 4′-hydroxydiclofenac from Becton, Dickinson and Company (Franklin Lakes, NJ, U.S.A.); S-(+)-mephenytoin, (±)-4′-hydroxymephenytoin, 6-hydroxychlorzoxazone and 1′-hydroxymidazolam from Toronto Research Chemicals, Inc. (Toronto, Canada); oxidized nifedipine from Oxford Biomedical Research (Rochester Hills, MI, U.S.A.). All reagents and solvents were of analytical grade or higher. Human liver microsomes were supplied by Becton, Dickinson and Company (50 donors and 150 donors) and Xenotech, LLC (Lenexa, KS, U.S.A.) (50 donors). Human fresh individual hepatocytes prepared by Xenotech, LLC were used. Cryopreserved human individual hepatocytes were purchased from Celsis IVT (Baltimore, MD, U.S.A.).
Inhibitory Effects on Cytochrome P450sThe inhibitory potential of delamanid’s metabolites (M1, M2, M3, and M4) was evaluated and determined with the modified methods in previous reports.12,18) The experimental conditions used in the inhibitory study are described in Tables 1 and 2. Substrates were dissolved in appropriate solvents. The final concentration of these solvents in each reaction mixture was less than 1% (v/v). Metabolites (test products) and known CYP inhibitors (positive controls) were freshly prepared in dimethyl sulfoxide (DMSO), and the DMSO concentration was 0.5% (v/v) in incubation mixtures. Human liver microsomes supplied by Xenotech, LLC were used for metabolites, M1 and M4, and those supplied by Becton, Dickinson and Company (150 donors) were used for metabolites, M2 and M3. Incubation mixtures were prepared on ice, without NADPH generating system [or NADPH/reduced nicotinamide adenine dinucleotide (NADH)] or without substrate, in the test product (1, 3, 10, 30, or 100 µM), positive control or vehicle control with pH 7.4 phosphate buffer (100 mM) using human liver microsomes (0.02–1 mg/mL). Incubation mixtures were then pre-incubated at 37°C. Each reaction was initiated by the addition of the NADPH-generating system (or NADPH/NADH) or the substrate solution, and the incubation mixtures were then incubated at 37°C. Each reaction was terminated with an appropriate stop reagent (Tables 1, 2). Test reactions were conducted in duplicate for each metabolite. Calibration curves were constructed to accurately quantitate the activity of each CYP enzyme under evaluation (CYP1A2, CYP2A6, CYP2B6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4). Analysis of inhibition of enzymatic activity for each test product, positive control or vehicle control was conducted by HPLC or LC-MS/MS. No marked difference was observed between vehicle control activities, even though the studies were performed under different conditions (Tables 1, 2). The inhibitory activity of each concentration of each test product or positive control was calculated as the percent difference of remaining activity with respect to the vehicle control. The IC50 values for each test product were obtained using WinNonlin (Pharsight, St. Louis, MO, U.S.A.). The I/Ki values, where I is inhibitor concentration from the maximal circulating plasma concentration following twice daily administration of 100 mg delamanid in humans and Ki is 0.5×IC50 [as the substrate concentration is investigated by approximately Km (Tables 1, 2)], were calculated for test products with IC50 values <100 µM.
| CYP isoform | Substrate | Solvent | (µM) | Protein (mg/mL) | Incubation time (min) | Stop solution | Positive control | (µM) | Remaining activity (%) |
|---|---|---|---|---|---|---|---|---|---|
| CYP1A2 | 7-Ethoxyresorufin | a | 0.5 | 0.2 | 10 | a | α-Naphthoflavone | 0.1 | 6.0 |
| CYP2A6 | Coumarin | b | 1 | 0.2 | 2 | a | 8-Methoxypsoralen | 0.2 | 7.5 |
| CYP2B6 | Bupropion | b | 100 | 0.2 | 30 | a | Ticlopidine | 0.2 | 15.0 |
| CYP2C8/9 | Tolbutamide | b | 250 | 0.2 | 30 | a | Sulfaphenazole | 50 | 11.2 |
| CYP2C19 | S-Mephenytoin | b | 20 | 0.2 | 30 | a | Tranylcypromine | 25 | 15.8 |
| CYP2D6 | Bufuralol | b | 10 | 0.2 | 20 | a | Quinidine | 1 | 17.9 |
| CYP2E1 | Chlorzoxazone | b | 40 | 0.2 | 30 | a | Diethyldithiocarbamate | 200 | 20.3 |
| CYP3A4 | Nifedipine | a | 30 | 0.2 | 10 | a | Ketoconazole | 0.5 | 8.9 |
| CYP3A4 | Testosterone | b | 50 | 0.2 | 10 | a | Ketoconazole | 0.5 | 4.3 |
a: Acetonitrile; b: Methanol. Vehicle control activity was 18.9, 465.0, 148.0, 81.8, 13.0, 38.5, 97.3, 1730.0, and 1430.0 pmol/min/mg for CYP1A2, CYP2A6, CYP2B6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 (nifedipine), and CYP3A4 (testosterone), respectively. Each activity represents the mean of the data in duplicate.
| CYP isoform | Substrate | Solvent | (µM) | Protein (mg/mL) | Incubation time (min) | Stop solution | Positive control | (µM) | Remaining activity (%) |
|---|---|---|---|---|---|---|---|---|---|
| CYP1A2 | 7-Ethoxyresorufin | a | 0.5 | 0.4 | 10 | b | α-Naphthoflavone | 0.1 | 11.0 |
| CYP2A6 | Coumarin | b | 2 | 0.05 | 30 | e | 8-Methoxypsoralen | 1 | 11.7 |
| CYP2B6 | Bupropion | c | 100 | 0.2 | 30 | e | Ticlopidine | 0.2 | 48.6 |
| CYP2C8/9 | Tolbutamide | c | 400 | 0.5 | 60 | f | Sulfaphenazole | 100 | 16.1 |
| CYP2C19 | S-Mephenytoin | c | 100 | 0.5 | 60 | e | Tranylcypromine | 100 | 11.1 |
| CYP2D6 | Bufuralol | b | 20 | 1 | 30 | e | Quinidine | 100 | 5.7 |
| CYP2E1 | Chlorzoxazone | d | 100 | 0.5 | 15 | g | Diethyldithiocarbamate | 300 | 42.9 |
| CYP3A4 | Nifedipine | b | 50 | 0.5 | 10 | g | Ketoconazole | 100 | 0.0 |
| CYP3A4 | Testosterone | c | 100 | 0.02 | 10 | e | Ketoconazole | 100 | 0.0 |
a: Acetonitrile; b: Ethanol; c: Methanol; d: 1% (w/v) Sodium carbonate aqueous solution; e: Chlorpropamide/acetonitrile; f: Hydrochloric acid; g: Ethyl acetate. Vehicle control activity was 11.7, 270.5, 107.2, 67.3, 19.6, 24.1, 392.4, 1961.2, and 3838.0 pmol/min/mg for CYP1A2, CYP2A6, CYP2B6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 (nifedipine), and CYP3A4 (testosterone), respectively. Each activity represents the mean of the data in duplicate.
The potential for MBI of delamanid in each CYP isoform was further investigated. Human liver microsomes [Becton, Dickinson and Company (50 donors), 0.2–5 mg/mL] were incubated at 37°C for 30 min in pH 7.4 phosphate buffer (100 mM) for five conditions (first incubation), #1 (enzyme), #2 {enzyme+test product [delamanid (100 µM)]}, #3 [enzyme+NADPH (2.5 mM)], #4 {enzyme+test product [delamanid (100 µM)]+NADPH (2.5 mM)}, and #5 [enzyme+positive control+NADPH (2.5 mM)]. Following the first incubation, an aliquot of each mixture was added to a second mixture containing NADPH/NADH in pH 7.4 phosphate buffer (100 mM). The experimental conditions used in the inhibitory study for the second incubation are described in Table 3. Substrates and positive controls were dissolved in DMSO. CYP substrates were then added and these reaction mixtures were incubated at 37°C until termination with chlorpropamide/acetonitrile. Incubations were performed in duplicate. Calibration curves were constructed to accurately quantitate the activity of each CYP enzyme under evaluation. Following the second incubation, analysis of each enzymatic activity for conditions #1 to #5 was conducted by LC-MS/MS. The activity of each enzymatic reaction (condition #2 or #4 and #5) was compared with that of the corresponding control (condition #1 or #3) and expressed as a percentage of that control. The difference of the remaining activity was then calculated between samples with and without NADPH. It is reported that a decrease in activity of 15.0% is identified as the cutoff value for identifying inactivators for CYP enzymes.19) It was prospectively determined that % difference of remaining activity, <15.0% would have little potential for MBI for the corresponding isoform.
| CYP isoform | Substrate | (µM) | Protein (mg/mL) | Incubation time (min) | Positive control | (µM) |
|---|---|---|---|---|---|---|
| CYP1A2 | Phenacetin | 50 | 0.2 | 20 | Furafylline | 10a) |
| CYP2A6 | Coumarin | 5 | 0.1 | 10 | 8-Methoxypsoralen | 10a) |
| CYP2B6 | Bupropion | 50 | 0.2 | 10 | Ticlopidine | 10a) |
| CYP2C8 | Paclitaxel | 10 | 0.2 | 20 | Quercetin | 50 |
| CYP2C9 | Diclofenac | 5 | 0.05 | 20 | Sulfaphenazole | 10 |
| CYP2C19 | S-Mephenytoin | 100 | 0.5 | 30 | Tranylcypromine | 50 |
| CYP2D6 | Bufuralol | 20 | 0.2 | 20 | Quinidine | 10 |
| CYP3A4 | Testosterone | 100 | 0.02 | 10 | Ketoconazole | 10 |
| CYP3A4 | Midazolam | 5 | 0.05 | 20 | Ketoconazole | 10 |
a) Each value represents the concentration of the first incubation. Reaction mixture for the second incubation contained pH 7.4 phosphate buffer (100 mM), NADPH/NADH (2.5 mM), first incubation mixture diluted by 10-fold, and each substrate.
Hepatocytes were isolated and cultured according to previously described methods.20–25) Three lots of human fresh hepatocytes were used for the assessment of CYP1A2, CYP2C9, and CYP3A4 activities and the relative mRNA levels. Hepatocytes were seeded at approximately 1.3×106 viable cells/mL on collagen-coated 60 mm culture dishes and were placed in a humidified culture chamber (37°C, at 95% relative humidity, 95/5% air/carbon dioxide). After two to three hours, the media was replaced with modified Chee’s medium (MCM) containing ITS+ (6.13 µg/mL insulin, 6.13 µg/mL transferrin and 6.13 ng/mL selenous acid), linoleic acid (5.25 µg/mL), bovine serum albumin (1.23 mg/mL), penicillin (49 U/mL), streptomycin (49 µg/mL), dexamethasone (0.098 µM), and Matrigel (250 µg/mL). Cultures were allowed to adapt to the culture environment for three days, with daily replacement of supplemented MCM (without Matrigel). After the adaptation period, hepatocyte cultures were then treated daily for three consecutive days with supplemented MCM containing 0.1% (v/v) DMSO (vehicle control), one of three concentrations of delamanid (0.1, 1, or 10 µM), 100 µM omeprazole [CYP1A2 inducer (positive control)], or 10 µM rifampin [CYP2C9 and CYP3A4 inducer (positive controls)]. Following the exposure period, microsomal samples were prepared from the human hepatocytes based on the method described by Madan et al.26) and were stored at −80°C.
Microsomal incubations were conducted at 37°C in incubation mixtures containing potassium phosphate buffer (50 mM, pH 7.4), MgCl2 (3 mM), ethylenediaminetetraacetic acid (EDTA) (1 mM), nicotinamide adenine dinucleotide phosphate (NADP) (1 mM), glucose-6-phosphate (5 mM), glucose-6-phosphate dehydrogenase (1 Unit/mL), and a substrate. The substrates were phenacetin (80 µM) for CYP1A2, diclofenac (100 µM) for CYP2C9, and testosterone (250 µM) for CYP3A4. Reactions were started by the addition of the NADPH-generating system and were stopped after 30 min (for CYP1A2) or 10 min (for CYP2C9 and CYP3A4) by the addition of acetonitrile containing the internal standard (d4-acetaminophen for CYP1A2, d4-4′-hydroxydiclofenac for CYP2C9, or d3-6β-hydroxytestosterone for CYP3A4). Precipitated protein was removed by centrifugation and supernatant fractions were analyzed by LC-MS/MS to determine CYP enzyme activities.
Approximately 24 h after the last treatment, total RNA was extracted using the TRIzol (Invitrogen, Carlsbad, CA, U.S.A.) and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA, U.S.A.). The RNA integrity was analyzed with the RNA 6000 Nano Assay Kit (Agilent Technologies, Santa Clara, CA, U.S.A.). Single-stranded cDNA was prepared from total mRNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, U.S.A.). The amplification was carried out in a total volume of 20 µL using TaqMan Gene Expression Master Mix (Applied Biosystems) with an Applied Biosystems 7300 Real Time polymerase chain reaction (PCR) sequence detection system. CYP1A2, CYP2C9, CYP3A4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression levels were measured with real-time reverse transcription (RT)-PCR using TaqMan gene expression assays (Applied Biosystems), including CYP1A2 (assay ID: Hs00167927_m1), CYP2C9 (assay ID: Hs00426397_m1), CYP3A4 (assay ID: Hs00604506_m1), and GAPDH (assay ID: Hs99999905_m1) primer sets. The relative mRNA levels of target genes were determined by normalizing the raw data to the GAPDH mRNA level. The relative gene expression was determined by the comparative Ct method (ΔΔCT method).
Three lots of cryopreserved human hepatocytes were used for the effects of delamanid on CYP2B6 mRNA levels. Hepatocytes were thawed in InVitroGRO CP Medium (Celsis IVT) supplemented with Torpedo Antibiotic Mix (Celsis IVT) and seeded at 0.7×106 viable cells/mL in collagen-coated 24-well plates. After 4 h, media was changed to the supplemented CP Medium, and hepatocytes were cultured for 2 d with daily replacement with the supplemented CP Medium. Following a 2-d adaptation period, hepatocytes were then treated daily for two consecutive days with InVitroGRO HI Medium containing 0.1% (v/v) DMSO (vehicle control), one of three concentrations of delamanid (0.1, 1, or 10 µM), or 750 µM phenobarbital [CYP2B6 inducer (positive control)].
Approximately 24 h after the last treatment, total RNA was extracted from the additional culture of hepatocytes and purified using RNeasy Micro Kit (Qiagen). The RNA integrity was analyzed by the same method as described above. Single-stranded cDNA was prepared from total mRNA using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany). The amplification was carried out using LightCycler 480 Probes Master (Roche Diagnostics) with LightCycler 480 II sequence detection system (Roche diagnostic). CYP2B6 and hypoxanthine phosphoribosyltransferase 1 (HPRT1) mRNA expression levels were measured with real-time RT-PCR using TaqMan gene expression assays (Applied Biosystems), including CYP2B6 (assay ID: Hs04183483_g1) and HPRT1 (assay ID: Hs02800695_m1) primer sets. The mRNA levels were determined by normalizing the raw data to the HPRT1 mRNA level. The relative gene expression was according to the same method as other CYP isoforms.
Data ProcessingAll inhibition data were calculated using the mean of the data in duplicate. All induction data about CYP enzyme activities and mRNA levels were shown as the mean±S.D. of the data from three individual hepatocyte preparations. Fold increase was expressed as the ratio of the CYP enzyme activities and mRNA levels of compound-treated groups to that of vehicle-treated group. Percent positive control was calculated as follows. Percent positive control (%)={[(fold change in treated sample)−1]/[(fold change in positive control)−1]}×100
The inhibitory potential of delamanid’s metabolites (M1, M2, M3, and M4) (1–100 µM) on the catalytic activities of eight CYP isoforms in human liver microsomes is presented in Table 4. The positive control inhibitors used in each experiment produced the expected inhibitory effects on the activities of the corresponding CYP isoforms (Tables 1, 2). Delamanid’s metabolites at concentrations up to 100 µM inhibited the activities of multiple CYP isoforms, though to varying extents. M1 inhibited the activities of all CYP isoforms except for CYP2E1-mediated activity with the IC50 values between 18.3 and 87.5 µM. M2 inhibited CYP2A6 and CYP2B6 activities with the IC50 values of 42.3 and 32.7 µM, respectively. This metabolite also inhibited CYP3A4 activity with the IC50 value of 10.9 µM when testosterone 6β-hydroxylation was used as the metabolic test reaction, but was much less effective at inhibiting the CYP3A4 activity when nifedipine oxidation was examined (IC50>100 µM). M3 slightly inhibited CYP2B6, CYP2C8/9, and CYP2C19 activities, but no IC50 values fell below 100 µM (IC50>100 µM). M4 inhibited CYP2B6, CYP2C8/9, and CYP2C19 activities with the IC50 values between 25.2 and 89.4 µM.
| CYP isoform | Metabolic reaction | IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| Delamanida) | M1 | M2 | M3 | M4 | ||
| CYP1A2 | 7-Ethoxyresorufin O-deethylation | >100 | 41.0 | >100 | >100 | >100 |
| CYP2A6 | Coumarin 7-hydroxylation | >100 | 87.5 | 42.3 | >100 | >100 |
| CYP2B6 | Bupropion hydroxylation | >100 | 24.3 | 32.7 | >100 | 25.2 |
| CYP2C8/9 | Tolbutamide methylhydroxylation | >100 | 30.7 | >100 | >100 | 42.9 |
| CYP2C19 | S-Mephenytoin 4′-hydroxylation | >100 | 18.3 | >100 | >100 | 89.4 |
| CYP2D6 | Bufuralol 1′-hydroxylation | >100 | 28.9 | >100 | >100 | >100 |
| CYP2E1 | Chlorzoxazone 6-hydroxylation | >100 | >100 | >100 | >100 | >100 |
| CYP3A4 | Nifedipine oxidation | >100 | 53.6 | >100 | >100 | >100 |
| CYP3A4 | Testosterone 6β-hydroxylation | >100 | 35.8 | 10.9 | >100 | >100 |
a) IC50 values of delamanid were calculated from previous report in ref. 12. Each value was calculated using the mean of the data in duplicate for each metabolite. IC50 values were calculated when the mean remaining activity at 100 µM was less than 50% of control.
Our previous paper indicates that delamanid does not inhibit any activities of the CYP isoforms (IC50>100 µM).12) The I/Ki values for delamanid and each of its metabolites that had IC50 values less than 100 µM are presented in Table 5. The maximal plasma concentration (I value) for delamanid based on twice daily oral administration of 100 mg delamanid for 2 months (56 d) in combination with a background drug regimen in humans has been reported to be 0.78 µM.14) Maximal concentrations for its metabolites were determined to be 0.32 µM for M1, 0.12 µM for M2, 0.22 µM for M3, and 0.13 µM for M4 using the plasma samples. The IC50 values of metabolites from the in vitro study were at least 50 times higher than the plasma concentrations of metabolites in humans. The I/Ki values calculated for the metabolites M1, M2, and M4 were between 0.003 and 0.035.
| CYP isoform | I/Ki | ||||
|---|---|---|---|---|---|
| Delamanid | M1 | M2 | M3 | M4 | |
| CYP1A2 | ND | 0.016 | ND | ND | ND |
| CYP2A6 | ND | 0.007 | 0.006 | ND | ND |
| CYP2B6 | ND | 0.026 | 0.007 | ND | 0.010 |
| CYP2C8/9 | ND | 0.021 | ND | ND | 0.006 |
| CYP2C19 | ND | 0.035 | ND | ND | 0.003 |
| CYP2D6 | ND | 0.022 | ND | ND | ND |
| CYP2E1 | ND | ND | ND | ND | ND |
| CYP3A4 | ND | 0.012 | ND | ND | ND |
| CYP3A4 | ND | 0.018 | 0.022 | ND | ND |
I=inhibitor concentration from the maximal circulating plasma concentration; Ki=inhibition constant. ND=not determined, as IC50 was above 100 µM. Km=Michaelis constant. Ki was calculated by the following equation, Ki=0.5×IC50, as the substrate concentration was investigated by approximately Km.
The potential for MBI of delamanid in each CYP isoform was investigated using human liver microsomes. These results are shown in Table 6. The positive control inhibitors used in each experiment produced the expected inhibitory effects on the CYP activities. The difference of the remaining activity between samples with and without NADPH (condition #2 and #4, respectively) for delamanid (100 µM) during the first incubation was less than 13.8%. Delamanid was therefore considered to have little potential for MBI.
| CYP isoform | Metabolic reaction | Remaining activity (%) | % Difference of remaining activity | Potential of MBI | Remaining activity (%) condition #5 | |
|---|---|---|---|---|---|---|
| Condition #2 | Condition #4 | |||||
| CYP1A2 | Phenacetin O-deethylation | 97.5 | 111.3 | 13.8 | Little potential | 4.9 |
| CYP2A6 | Coumarin 7-hydroxylation | 97.1 | 92.6 | 4.5 | Little potential | 2.5 |
| CYP2B6 | Bupropion hydroxylation | 92.5 | 94.3 | 1.8 | Little potential | 2.7 |
| CYP2C8 | Paclitaxel 6α-hydroxylation | 102.7 | 106.1 | 3.4 | Little potential | 5.2 |
| CYP2C9 | Diclofenac 4′-hydroxylation | 95.9 | 87.7 | 8.2 | Little potential | 2.7 |
| CYP2C19 | S-Mephenytoin 4′-hydroxylation | 109.1 | 96.0 | 13.1 | Little potential | 10.1 |
| CYP2D6 | Bufuralol 1′-hydroxylation | 96.9 | 96.1 | 0.8 | Little potential | 8.6 |
| CYP3A4 | Testosterone 6β-hydroxylation | 92.4 | 88.2 | 4.2 | Little potential | 28.9 |
| CYP3A4 | Midazolam 1′-hydroxylation | 106.6 | 95.6 | 11.0 | Little potential | 0.1 |
Each value was calculated using the mean of the data in duplicate. Remaining activity of condition #2 (enzyme+test product) or #4 (enzyme+test product+NADPH) and #5 (enzyme+positive control+NADPH) was compared with that of the corresponding control [condition #1 (enzyme) or #3 (enzyme+NADPH)] and expressed as a percentage of control. Percent (%) difference of remaining activity, <15.0% would have little potential for mechanism-based inactivation for the corresponding isoform.
The effects of delamanid on the enzymatic activities of CYP1A2, CYP2C9, and CYP3A4 and on mRNA levels for these enzymes were examined using freshly isolated human hepatocytes. In addition, its effects on CYP2B6 were investigated at mRNA levels using cryopreserved human hepatocytes. These results are shown in Tables 7 and 8. The human hepatocytes from three different donors were independently used, and the results were expressed as the mean±S.D. of the data from three individual hepatocyte preparations. Treatment of cultured hepatocytes with the positive controls omeprazole (CYP1A2), phenobarbital (CYP2B6), or rifampin (CYP2C9 and CYP3A4) caused the anticipated increases in isoform-specific activities and corresponding mRNA levels. Hepatocyte cultures that were similarly treated with delamanid at concentrations up to 10 µM showed no changes in CYP1A2 and CYP2C9 activities and relative mRNA levels. No effects on CYP2B6 mRNA levels were observed at 0.1 and 1 µM delamanid concentrations, and only a marginal increase of 1.48-fold was observed in hepatocyte culture treated with delamanid at 10 µM. Three days of treatment with delamanid at concentrations up to 1 µM had no effects on CYP3A4 activities and CYP3A4 mRNA levels. Treatment with delamanid at a concentration of 10 µM had a minimal overall effect on CYP3A4 mRNA levels with an increase of 1.49-fold relative to the vehicle control (DMSO) (Table 8). However, identically treated hepatocyte cultures showed an overall decrease in CYP3A4 activities to 0.672-fold, compared with the vehicle control (Table 7).
| Treatment | Concentration (µM) | Enzyme activities (fold increase) | ||
|---|---|---|---|---|
| Phenacetin O-dealkylation | Diclofenac 4ʹ-hydroxylation | Testosterone 6β-hydroxylation | ||
| CYP1A2 | CYP2C9 | CYP3A4 | ||
| DMSO (Vehicle) | 0.1% (v/v) | 1.00±0.62 | 1.00±0.47 | 1.00±0.52 |
| Delamanid | 0.1 | 1.01±0.19 | 1.04±0.12 | 1.17±0.22 |
| Delamanid | 1 | 1.01±0.05 | 1.08±0.02 | 0.945±0.153 |
| Delamanid | 10 | 1.05±0.21 | 1.05±0.07 | 0.672±0.217 |
| Omeprazole | 100 | 15.0±6.5 | 1.69±0.31 | 2.16±0.64 |
| Rifampin | 10 | 1.66±0.83 | 2.42±0.33 | 5.86±2.51 |
DMSO=dimethyl sulfoxide. Vehicle control activities in 0.1% (v/v) DMSO were 61.1±38.1, 1120±520, and 2570±1340 pmol/min/mg for CYP1A2, CYP2C9, and CYP3A4 catalyzed reactions, respectively. Each value represents the mean±S.D. of the data from three individual hepatocyte preparations.
| Treatment | Concentration (µM) | CYP mRNA levels (fold increase) | |||
|---|---|---|---|---|---|
| CYP1A2 | CYP2B6 | CYP2C9 | CYP3A4 | ||
| DMSO (Vehicle) | 0.1% (v/v) | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| Delamanid | 0.1 | 0.908±0.146 | 1.12±0.04 | 0.911±0.105 | 1.09±0.21 |
| Delamanid | 1 | 1.07±0.22 | 1.24±0.18 | 0.892±0.061 | 0.953±0.234 |
| Delamanid | 10 | 1.22±0.61 | 1.48±0.19 | 1.04±0.24 | 1.49±0.61 |
| Omeprazole | 100 | 277±208 | NT | 1.74±0.73 | 2.78±1.09 |
| Phenobarbital | 750 | NT | 10.4±2.1 | NT | NT |
| Rifampin | 10 | 1.85±1.53 | NT | 3.28±1.09 | 9.84±5.96 |
DMSO=dimethyl sulfoxide; NT=not tested. Each value represents the mean±S.D. of the data from three individual hepatocyte preparations. Experiments were conducted using freshly isolated human hepatocytes for CYP1A2, CYP2C9, and CYP3A4 and cryopreserved human hepatocytes for CYP2B6.
When used in combination with a background treatment regimen for 56 d, delamanid has been shown to improve sputum culture conversion (i.e., from culture positive to culture negative for M. tuberculosis) in MDR-TB patients.14) Examination of delamanid’s effects on CYP inhibition and induction is an important first step in the planning of clinical trials examining delamanid’s interactions with other concomitantly administered medications. This report describes the in vitro effects of delamanid and its metabolites on CYP isoform activities or CYP mRNA levels. It is critical to understand how new anti-TB medications will affect the metabolism of drugs via CYP isoforms, given the number of antiretrovirals that are metabolized by these isoforms,6,9) the large number of patients who require treatment for HIV and TB co-infection,2) and the synergy between the disease processes of these infectious agents.3) This is particularly important for anti-TB drugs like delamanid in countries where MDR-TB is prevalent because of the widespread use of antiretrovirals that can cause significant CYP-mediated interactions with other concomitant medications. For example, low plasma concentrations of the antiretroviral efavirenz may lead to virologic failure, while high concentrations may result in central nervous system toxicity. Maintenance of therapeutically appropriate plasma concentrations of efavirenz in patients who require treatment for TB is of concern due to the CYP-mediated interactions caused by anti-TB drugs such as rifampin.27)
The usual dose in adults is 100 mg of delamanid twice daily administered orally. Delamanid should be used for long-term administration as an add-on therapy to 3 or more other anti-TB drugs to prevent the development of resistance to delamanid. It is thus considered to be important to clarify whether delamanid has the potential to inhibit or induce CYP enzymes. We have already reported that delamanid had no competitive inhibition on the activities of eight CYP isoforms in a previous study.12) In the present study, we investigated first MBI by delamanid on CYP enzymes. The results of the in vitro experiments indicated that delamanid (100 µM) appeared to have little potential for MBI (Table 6).
Following oral administration of delamanid to humans and animals, at least four metabolites, M1, M2, M3, and M4, have been detected and identified in the plasma. Further, the maximal plasma concentration for delamanid has been reported to be 0.78 µM following twice daily administration of 100 mg delamanid for 56 d in clinical trial.14) The maximal plasma concentrations for its metabolites were determined to be 0.32 µM for M1, 0.12 µM for M2, 0.22 µM for M3, and 0.13 µM for M4, suggesting that M1 is the most abundant metabolite in humans. The inhibitory effects of delamanid and its metabolites are considered to be minimal based on these plasma concentrations in humans. However, it is more important to evaluate this based on hepatic concentrations where CYP enzymes exist. In an in vivo study, the concentrations of delamanid and metabolites in liver were investigated in rats following repeated oral administration of delamanid. The results indicated that delamanid was observed in the liver at a high concentration of approximately 6 times that of the maximal plasma concentration (not reported). If the liver/plasma concentration ratio in humans is the same as that in rats, delamanid is presumed to be also no inhibitory effects on CYP enzymes based on human hepatic concentrations.
With the background of the metabolism of delamanid, we investigated additionally the inhibition potential of four metabolites. The inhibitory effects of delamanid’s metabolites were observed only at metabolite concentrations exceeding those observed in human plasma during clinical trials. While the metabolites M2, M3, and M4 in rat liver were not detected, the metabolite M1 was observed at a concentration of approximately 30% of delamanid (not reported). If the distribution of M1 in liver is the same as that of delamanid, the maximal liver concentration for M1 in humans is presumed to be approximately 2 µM. The I/Ki values from liver concentration are calculated to be <0.22. Considering that the plasma protein binding of M1 is extremely high, approximately 99.7% (0.3% as free form, not reported), free concentration of M1 around an enzyme site in the liver is presumed to be lower, suggesting that M1 has a low potential to induce drug–drug interactions due to the inhibition of CYP enzymes. The potential for MBI of delamanid’s metabolites was not investigated in the present study. Additional work may be necessary to study MBI of the principal metabolites in the future.
In the inhibitory study, one metabolite, M2, inhibited testosterone 6β-hydroxylase (IC50 of 10.9 µM), but not nifedipine oxidase (IC50>100 µM). Nifedipine has been reported to have freedom of movement and can bind to multiple CYP3A4 active sites, including the testosterone binding site, whereas testosterone is fixed to a certain part of the active site.28) The inhibition results suggest that M2 inhibits only a certain part of the active site for testosterone, not multiple sites.
According to the European Medicines Agency guideline,17) the in vitro study should be considered as a positive for the enzyme induction when the incubations with the test drug give rise to a more than a 2-fold increase in mRNA and the increases are seen in a concentration-dependent manner. In addition, an observed concentration-dependent increase in mRNA of <100% can be considered as a negative only when the increase in mRNA is less than 20% of the response of the positive control (percent positive control). In the present study, the extent of the increase in mRNA levels of CYP1A2, CYP2B6, CYP2C9, and CYP3A4 was less than 1.49-fold in all delamanid-treated groups (Table 8). The maximal value of percent positive control was 6% in CYP3A4 mRNA levels for 10 µM delamanid. In the same manner, the enzymatic activities of CYP1A2, CYP2C9, and CYP3A4 for delamanid were less than 1.17-fold increase of control (Table 7). Consequently, the delamanid (≤10 µM) showed no inductive effects on the enzymatic activities and mRNA levels of four CYP enzymes in cultured human hepatocytes.
Delamanid was found to be metabolized to M1 by human liver microsomes without NADPH/NADH (not reported). In the present induction study, the human hepatocytes were cultured and treated with delamanid for 2 or 3 d. Therefore, at least the most abundant metabolite, M1, must be present in the human hepatocytes. Delamanid and its metabolites including at least M1 were considered to be evaluated in this test system.
Treatment with up to 1 µM delamanid had also no effect on CYP3A4 activities and CYP3A4 mRNA levels. However, treatment with 10 µM delamanid resulted in an anomalous result, i.e., a small decrease in CYP3A4 activities and a slight increase (1.49-fold) in CYP3A4 mRNA levels. The reason for this discordant result is unclear. It may be possible that one or more metabolite(s) having CYP3A4 inhibitory activity was produced during culturing human hepatocytes with delamanid. Further work will be necessary to resolve this apparent discordant result.
In conclusion, delamanid (≤100 µM) showed no inhibitory effects on eight CYP isoforms and had little potential for MBI. Delamanid’s metabolites were noted to inhibit some CYP isoforms, but these effects were observed only at metabolite concentrations that were well above those observed in human plasma. Delamanid (≤10 µM) did not induce CYP1A2, CYP2B6, CYP2C9, and CYP3A4 in human hepatocytes. These data suggest that delamanid is unlikely to cause clinically relevant CYP-mediated drug interactions.
We are grateful to Dr. Hiroyuki Sasabe and Dr. Masayuki Furukawa of Tokushima Research Institute, Otsuka Pharmaceutical Co., Ltd. for helpful advice with scientific discussion. We also thank the many TB staff members of Otsuka Pharmaceutical Co., Ltd. for reviewing the manuscript.