2014 Volume 37 Issue 11 Pages 1758-1765
2014 Volume 37 Issue 11 Pages 1758-1765
Cryptotanshinone (CT), isolated from the dried roots of Salvia militorrhiza, has been reported to have protective effects on myocardial and cerebral ischemia/reperfusion (I/R) injury both in vitro and in vivo. However, its effects and underlying mechanism on hepatic I/R injury remain unclear. To investigate its effects on hepatic I/R injury, thirty male Sprague-Dawley rats were randomized into 3 groups: a sham group, a vehicle-treated hepatic I/R group and a CT-treated (50 mg/kg) group. The hepatic I/R and CT-treated groups were subjected to 60 min of normothermic ischemia of the left lateral lobe of the liver, followed by 4 h of reperfusion. The animals were then sacrificed to collect the serum and the left liver lobe for assay. Hepatic function was protected, as evidenced by significantly reduced alanine aminotransferase (ALT), aspartate aminotransferase (AST) and malondialdehyde (MDA) levels in the CT-treated group as compared with I/R group. The terminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) demonstrated significantly decreased apoptosis in the CT-administration animals. Western blotting demonstrated upregulation of the proapoptotic protein Bcl-2, as well as decreased levels of the activated form of caspase-3 and the cleaved form of its substrate, poly(ADP-ribose) polymerase (PARP) in the CT-treated group compared with those of the I/R group. In addition, the phosphorylation of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (MAPKs) was inhibited by CT. Our data suggest that CT attenuates hepatic I/R injury by inhibiting the intrinsic pathway of apoptosis, mediated partly through the inhibition of JNK and p38 MAPK phosporylation.
Liver normothermic ischemia and reperfusion (I/R) injury is a common situation in patients with liver transplantation, resection, and trauma and remains a significant clinical problem.1–3) It is a major cause of morbidity and mortality after liver surgery. Currently, the treatments of hepatic I/R injury is merely supportive care. So there is urgent need of new therapeutic strategies to prevent patients with liver I/R from organ damage.
It has been demonstrated that I/R-induced hepatic injury is initially triggered by reactive oxygen species (ROS). ROS result in peroxidizing membrane lipids, chromosomal malformation, nucleic acid base changes, or DNA ruptures which can induce hepatocellular apoptosis, an important mechanism for cell death following hepatic I/R injury.4) Numerous key factors have been demonstrated to be involved in ROS-mediated cell apoptosis, including the Bcl-2 family, caspase-3 and cytochrome c.5) Caspase-3 is one of the executioner caspases responsible for the cleavage and inactivation of poly(ADP-ribose) polymerase (PARP), an enzyme thought to be involved in DNA repair and surveillance of genome integrity in response to environmental stress.6) Bcl-2 family is considered to have an important role in the intrinsic apoptotic pathway, also called mitochondrial pathway. The proapoptotic Bax promotes intrinsic apoptosis by forming oligomers in the mitochondrial outer membrane, participating in the release of apoptogenic molecules, such as cytochrome c; oppositely, the antiapoptotic Bcl-2 inhibits mitochondrial apoptosis by blocking the release and oligomerization of Bax. The balance between Bax and Bcl-2 proteins has been linked with the induction of apoptosis in cell death in kidney, heart, and brain after I/R.7,8)
Cryptotanshinone (CT) (Fig. 1), one of active ingredients of Salvia miltiorrhiza root, has been widely used in the treatment of cardiovascular diseases,9) hematological abnormalities,10) and hyperlipidemia.11) Recently, the effects of CT on I/R injury have also been demonstrated by scientists. In 2009, Jin et al. reported that CT reduces myocardial I/R injury by inhibiting tumor necrosis factor (TNF)-alpha-induced expression of adhesion molecules in rats.9) Then, Park et al. demonstrated that CT exerted neuroprotective effects in experimentally induced transient cerebral ischemic damage animals.12) CT has also been reported to protect against myocadial hypoxia-induced mitochondrial apoptosis in vitro.13) Moreover, hepatoprotective effect of CT in endotoxin-induced fulminant hepatic failure has been revealed.14) However, the effect of CT on hepatic I/R injury has not been investigated so far. Therefore, we set this experiment to explore the effect of CT on hepatic I/R injury and possible mechanisms.
Cryptotanshinone (purity ≥99%) was purchased from National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. Bicinchoninic acid (BCA) protein assay kit was obtained from Pierce Biolab (Pierce, Brebières, France). Antibodies specific for mitogen-activated protein kinase (MAPK) family proteins (phospho- extracellular signal-regulated kinase (ERK) 1/2, ERK1/2, phospho-p38, p38, phospho-c-Jun N terminal kinase (JNK), JNK), caspase-3, rabbit polyclonal anti-Bax antibody, and rabbit polyclonal anti-Bcl-2 antibody were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). Horseradish peroxidase conjugated anti-rabbit or anti-mouse immunoglobulin G (IgG) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The NE-PER™ Nuclear and Cytoplasmic Extraction kit was purchased from Pierce Biotechnology (Rockford, IL, U.S.A.). In Situ Cell Death Detection Kit for terminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) staining kit was from Roche Molecular Biochemicals (Shanghai, China). Superoxide dismutase (SOD) and malondialdehyde (MDA) assay kits were purchased from Jiancheng Institute of Biological Engineering (Nanjing, China). All other reagents were analytical grade.
AnimalsAge-matched male Sprague-Dawley rats weighting 250–300 g were supplied by the Animal Research Center of Sun Yat-Sun University. Rats were maintained under standard conditions and were fed rodent chow and water. The characteristics and food intake are shown in Table 1. The amount of food intake by each animal was 20–30 g every day during the study period, and the appetite was not affected by the treatment. All surgical procedures and care administered to the animals were in accordance with Institutional guidelines for the care and use of laboratory animals.
| Groups | Age (weeks) | Gender | Body weight (g) | Food intake (g intake/d/each animal) |
|---|---|---|---|---|
| Sham | 8 | Male | 280±15 | 26±3 |
| I/R | 8 | Male | 280±20 | 25±5 |
| CT+I/R | 8 | Male | 285±15 | 25±4 |
Animals were divided into 3 groups of 10 each. They were CT-treated group, hepatic I/R group and sham group. Animals in the CT-treated group received daily intraperitoneal injections of CT (50 mg/kg/d) dissolved in 1% Tween 80 saline for 7 d before the I/R procedure; animals in the hepatic I/R group received intraperitoneal injections of vehicle (2 mL saline containing 1% Tween 80) for 7 d before the procedure. The dose and timing of CT treatment in the present study were selected based on previous report14) and our preliminary experiments. After they were anesthetized with 10% chloral hydrate, the animals underwent laparotomy, and then livers were isolated. The hepatic artery and portal vein to the left and the median liver lobes were occluded with an atraumatic vascular clamp. The abdominal cavity was temporally closed with towel forceps, and covered with wet saline gauzes. The blood vessels supplying the median and left lobe was occluded by micro-vascular clamp to achieve 70% liver ischemia for 1 h. Blood flow was restored by removal of the clamps after 1-h occlusion. The abdominal cavity was closed with suture. Animals in the sham group were treated with vehicle for 7 d, and then laparotomized for 5 min without blocking liver inflow.15–17) Four hours later, animals were sacrificed and blood samples were collected from the inferior venacava for serum biochemical markers determination. The liver was retrieved and washed in phosphate-buffered saline (PBS). The left liver lobe was separated and samples snap frozen in liquid nitrogen for further analysis.
Measurement of Plasma Alanine Transaminase (ALT), Aspartate Aminotransferase (AST) Levels, and Tissue MDA, SOD LevelsBlood sample were centrifuged at 3000 rpm for 15 min at room temperature. Plasma was collected and frozen under −70°C until analyzed. Plasma ALT, and AST concentrations were measured with Hitachi 7180 Clinical Analyzer (Hitachi, Japan). The hepatic tissue samples were stored at liquid nitrogen before measurements of tissue MDA, and SOD levels. On the day of use, frozen tissue samples were quickly weighed and homogenized 1 : 10 in ice-cold PBS. The homogenates were then centrifuged at 14000×g at 4°C for 15 min. The supernatants were separated and used for enzyme activity assays and protein determination. The tissue MDA level, and SOD activity were determined by enzymatic colorimetric methods using the commercial kits. The assay was performed according to the manufacturer’s instructions.
HistopathologyFormalin-fixed hepatic tissue samples were embedded in paraffin according to the traditional method. Sections (3 µm thick) were cut and stained with hematoxylin–eosin (H&E) for observation under a light microscope.
TUNEL StainingTUNEL staining was employed to detect liver cell apoptosis. Serial sections of 6 µm thickness were prepared from livers. The sections were deparaffinized and rehydrated. After that, TUNEL staining was performed according to the manufacturer’s instruction for the TUNEL assay kit. Then the sections were counterstained with hematoxylin. Finally, total hepatocytes and TUNEL-positive cells were observed under light microscopy.18) In a blind manner, apoptotic cells were counted in 10 randomly chosen fields per section and expressed as the number of cells per field.
Western BlottingAfter reperfusion for 4 h, the liver samples (area at risk) were suspended in a buffer that contained 10 mM Tris (pH 7.5), 1.5 mM MgCl2, 10 mM KCl and 0.1% Triton X-100 and then lysed by homogenization. Protein concentration of each sample was measured with a BCA protein assay kit according to the manufacturer’s instructions.
Samples (20 µg protein) were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gels with electrophoresis (Bio-Rad, Hercules, CA, U.S.A.) and the gel was transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 10% skim milk for 1 h and then incubated overnight at 4°C with 1 : 2000 dilution of the corresponding primary antibody: anti-p-ERK 1/2, p38, p-JNK, cleaved caspase-3, cleaved poly(ADP-ribose) polymerase (PARP), Bcl-2 and Bax. After washing, the membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase. The membrane was immersed in the enhanced chemiluminescence solution for 60 s. The gel images were visualized using Chem-Doc (Bio-Rad), and densitometric analysis was performed with Quantity One 1-D Analysis software (Bio-Rad).
Statistical AnalysisResults were presented as means±S.E.M. The Student’s t-test was used to compare mean values. In all comparisons, p<0.05 was considered to be statistically significant.
To evaluate the effects of CT on hepatic I/R injury, the levels of ALT, AST in sera and MDA, SOD in tissue acquired from each group of rats were measured. As shown in Fig. 2(a), the levels of ALT, AST and MDA increased significantly by 27, 20 and 3 times (p<0.01) in the I/R group compared with the sham group, respectively. In contrast to MDA (a lipid peroxidation indice), as the free radical originated from the lipid peroxidation could consume considerable SOD, the activity of SOD was markedly decreased by 86% in I/R group when comparing with the sham group (p<0.01). Conversely, in CT pre-treated group, there were significant reductions by 53%, 37% and 45% in the levels of ALT, AST and MDA respectively after CT treatment (p<0.05). And SOD activity was increased by 4.78 times (p<0.01) compared with I/R group.

Rats were randomly assigned into Sham operation group as control (Sham), normothermic I/R and CT pretreatment group. I/R and CT groups were subjected to 60 min of normothermic ischemia and 4 h of reperfusion. The plasma levels of ALT, AST, tissue level of SOD and MDA were determined, shown in (a). Data are presented as mean±standard error of the mean (S.E.M.) (n=10). ** p<0.01 for I/R group versus Sham operation group, ## p<0.01 for CT group versus I/R group. Representative hematoxylin and eosin (H&E) stained sections of liver are shown in (b). Original magnifications: ×200.
The pathological features of the liver tissues from the three groups after H&E staining are also shown in Fig. 2(b). The structures of the liver tissues were completely maintained and remained ordered in the sham group, whereas a disordered lobular structure, marked swelling, vacuolization of hepatocytes around the central vein, and polymorphonuclear cell infiltration were observed in the I/R group. However, the administration of CT clearly reduced all the pathological features apparent in the I/R group.
CT Pretreatment Decreased I/R-Induced Apoptosis in LiverTo investigate whether CT can prevent apoptotic cell death, TUNEL staining of live tissue was performed. As shown in Fig. 3(a), a number of TUNEL positive cells were observed in the I/R group; however the amount in CT-treated group was markedly decreased.

(a) TUNEL staining showed the apoptotic cells in three groups. Original magnifications: ×200. The number of TUNEL positive apoptotic cells were counted directly under microscope and expressed as the number of apoptotic cells per high power view. (b) Western blotting showed the expression of cleaved-caspase-3 (17, 19 kD) and cleaved PARP in three groups. The band densitometry analysis was performed using Image J software. All data are presented as mean±S.E.M. (n=10). ** p<0.01 for I/R group versus Sham operation group, # p<0.05 for CT group versus I/R group, ## p<0.01 for CT group versus I/R group.
To further evaluate the antiapoptotic effect of CT, the cleavage of caspase-3 and its substrate PARP in I/R liver after CT treatment was measured by Western blotting. The activated form of caspase-3 (17, 19 kD) were expressed at a significantly lower level in CT-treated animals than that of I/R group. Accordingly, expression of PARP, an important regulator of apoptosis, was significantly decreased in the liver of CT-treated animals (p<0.05) (Fig. 3(b)).
CT Preconditioning Reduced Bax/Bcl-2 RatioAs previously mentioned, Bcl-2 family plays important role in apoptosis, the balance between Bax and Bcl-2 proteins determines the possibility of cells to survive or undergo apoptosis after a certain stimulus or injury.19,20) Therefore, to explore the potential protective mechanism of CT against hepatic I/R injury, we measured the changes in Bcl-2 and Bax protein expression in the three groups. As showed in Fig. 4, it is clear that CT pretreatment significantly reduced the expression of Bax and increased the expression of Bcl-2 (p<0.05).

Fresh liver tissues were harvested at the end of the reperfusion. The protein levels of Bax and Bcl-2 were determined by Western blotting. The band densitometry analysis was performed using Image J software, and the data are presented as mean±S.E.M. (n=10). * p<0.05 for I/R group versus Sham operation group, # p<0.05 for CT group versus I/R group.
The mitogen-activated protein kinases (MAPK) such as extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK are thought to play an important role in the regulation of apoptosis.21) Thus, the effects of CT on the regulation of MAPK family proteins were examined by western boltting. As shown in Fig. 5, the phosphorylated levels of JNK (p<0.01) and p38 (p<0.05) were significantly reduced through CT treatment, However, the phosphorylation of ERK was increased slightly, compared with I/R group.
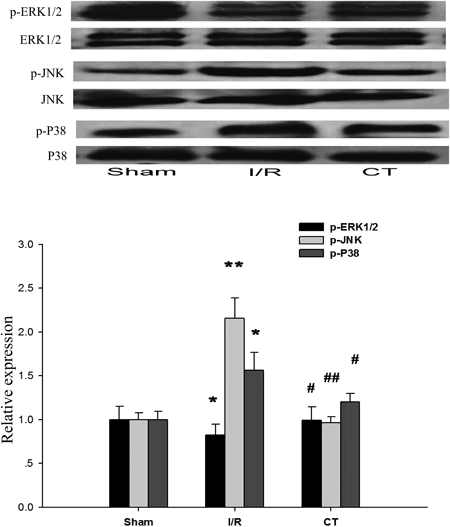
Fresh liver tissues were harvested at the end of the reperfusion. The protein levels of p-ERK1/2, p-JNK and p-p38 were determined by Western blotting. Total ERK1/2, JNK and p38 were used as a control of the protein amount in the same samples. The band densitometry analysis was performed using Image J software, and the data are presented as mean±S.E.M. (n=10). * p<0.05 for I/R group versus Sham operation group, ** p<0.01 for I/R group versus sham operation group, # p<0.05 for CT group versus I/R group, ## p<0.01 for CT group versus I/R group.
In the current study, the evidence that pre-treatment with CT had a protective effect against I/R induced hepatic apoptosis in rats was provided by us. We have shown that CT attenuates hepatic I/R injury and that the histopathological changes caused by I/R, such as cellular swelling, are clearly ameliorated by CT, which are consistent with changes of enzyme levels, such as ALT and AST. The anti-apoptotic effect of CT was evidenced by the facts, that with CT treatment, the number of TUNEL-positive hepatic cells had a significant decrease, accompanied by the reduction of caspase-3 activation (presented by cleaved caspase-3 expression) and PARP cleavage.
Many factors contribute to I/R injury with ROS accumulation, massive granulocyte infiltration, and hepatocyte apoptosis. ROS play central roles during hepatic I/R injury.22,23) Under physiological conditions, ROS are quickly detoxified by endogenous antioxidants such as SOD. Transgenic mice overexpressing SOD display significantly improved hepatic I/R injury compared with normal hosts.24) The MDA level, an indice of lipid peroxidation caused by ROS and a criterion to evaluate the severity of reperfusion injury, is increased during I/R. The level of aminotransferase, an important indicator of oxidative damage, is also increased in I/R injury.25) Many studies have reported that CT is an antioxidant being effective to cardiovascular cell damage11,26) and carbon tetrachloride-induced hepatic injury.27) Similarly, in our study, the SOD activities after applying CT, were dramatically higher compared with I/R group, but the MDA content and the aminotransferase activity significantly lower. The present results indicated that the actions of CT to alleviate hepatic I/R injury might be associated with its antioxidative activity.
Apoptosis of hepatocytes and sinusoidal endothelial cells is a critical mechanism of injury in the ischemic liver.28) Multiple factors activate apoptotic cascade proteins. As much evidence supports, caspase-3, an executioner of apoptosis, plays a dominant role after hepatic ischemia.29) In addition, Bcl-2 family plays important roles in hepatocyte intrinsic apoptosis, particularly Bcl-2 and Bax. The Bcl-2/Bax ratio determines the cells’ fate after an apoptotic stimulus.30,31) CT has previously been shown with reducing Bax/Bcl-2 ratio and inhibiting cytochrome c release in myocardial cell under hypoxia condition.13) Consistantly, our study showed that the caspase-3 activity in the group treated with CT was remarkably decreased compared with that of I/R group. Furthermore, CT treated group restored Bcl-2/Bax ratio. These suggested that CT alleviated hepatic I/R injury by suppressing apoptosis and the mitochondrial pathway associated members of Bcl-2 families were involved in CT-inhibited apoptosis.
Although CT possesses a variety of biological effects such as anti-inflammatory, antioxidative, antimetabolic, and anticancer effects, the precise molecular targets or pathways of CT to regulate apoptosis still remain unclear. MAPK cascade plays a central role in hepatocyte apoptosis, and it is considered as an attractive therapeutic target.32) There are three major groups of MAPKs: classic ERK, JNK, and p38 MAPK. Cursio et al. reported the activation of ERK1/2 pathway and the pro-apoptotic effects of JNK on I/R induced liver apoptosis in rats.21) ERK1/2 activation inhibits apoptosis by a conformational change in Bax required for its translocation to the mitochondria33) and the activation of caspase-3.34) In contrast, prolonged stimulation of p38 MAPK or JNK promotes apoptosis in a variety of other models. Kakizaki et al. identified the protective effect of inhibition of p38 on I/R-induced liver apoptosis in rats.35) In deoxycholic acid-induced hepatocyte apoptosis, inhibition of p38 MAPK is one antiapoptotic mechanism.36) It has also been reported that Licorice compounds attenuate JNK activation to reduce bile acid-induced apoptosis in rat hepatocytes.37) Taken together, these results suggested an important role of MAPKs in the regulation of apoptosis during liver I/R. Previous study has shown that CT protected primary cultured rat hepatocytes from bile acid-induced apoptosis by inhibiting JNK phosphorylation.38) Jin et al. also reported that CT significantly suppressed JNK, ERK and p38 phosphorylation in D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure rat model.14) Similarly, our results showed the inhibitory effects of CT on the expression of p-JNK and p-p38 MAPK, and slightly induction effect on p-ERK, which indicated that anti-apoptotic effect of CT was related to the decrease of JNK and p38 MAPK activation in hepatic I/R injury in rats. However, to elucidate their specific role, we have to carry out the specific inhibitor experiment to JNK and p38 MAPK either in vitro or in vivo.
Besides MAPKs pathway, lots of other signaling pathways were reported in I/R injury regulation, such as signal transducer and activator of transcription (STAT),39) Src homology 2 domain-containing phosphatases (SHPs)40) and AMP-activated protein kinase (AMPK)41) pathways. And there have been a series of reports suggesting effects of CT on such molecules. For instance, it is reported that CT inhibits human glioma cell proliferation by suppressing STAT3 signaling; an oncogenic protein constitutively activated in cancer cells and predicts a poor clinical outcome.42) Jung et al. also reported that CT induced apoptosis in chronic myeloid leukemia cells through Janus activated kinase 2 (JAK2)/STAT3 inhibition and SHP1 induction.43) Recently, CT has been veryfied to be an irreversible inhibitor of SHP2, a phosphatase also linked to STAT signaling, makes CT a potential to be developed to treat SHP-associated diseases.44) In addition, CT was found a novel AMPK pathway activator and enhanced apoptotic cell death in HepG2 hepatoma in a AMPK-dependent manner.45) Thus, whether CT exerts its anti-apoptotic effect in hepatic I/R injury via these pathways needs further studies.
In conclusion, our study has demonstrated that CT provides significant hepatoprotective effects against I/R induced apoptosis in rats via over expression of the antiapoptotic protein Bcl-2 and might related to the decrease of JNK and p38 MAPK phosphorylation.
This study was supported by Grants from the National Natural Science Foundation of China (30972917 and 31300774), the Fundamental Research Funds for the Central Universities (No. 2013ZM0067), the Hospital Pharmacy Foundation of Gongdong Province (2010A13) and the Medical Scientific Research Foundation of Guangdong Province (A2013193).