2014 Volume 37 Issue 11 Pages 1776-1781
2014 Volume 37 Issue 11 Pages 1776-1781
Dissolving microneedles (DMs) were applied to glucose monitoring in the dermal interstitial fluid (ISF) of rats and their potential as an alternative blood glucose monitoring device was evaluated. Sodium chondroitin sulfate was used to prepare DM array chips, which consisted of 300 DMs/cm2. The mean length of the DMs was 475±18 µm and the mean diameter of the basement was 278±8 µm. After DMs were inserted into the skin of the hair-removed rat abdomen, a wet unwoven cloth containing 10–30 µL of water was placed on the skin and ISF was extracted. By increasing the absorbed amount of water on the unwoven cloth from 10 to 30 µL, the extracted amount of glucose increased from 1.66±0.35 µg to 2.75±0.61 µg. Increasing the adhesion time of the wet unwoven cloth to the skin from 0.1 to 5.0 min, increased the amount of ISF glucose from 1.99±0.13 µg to 5.04±0.38 µg. The relation between the amount of glucose in ISF and blood glucose concentrations was examined. With increase in the adhesion time, the coefficient of determination, r2, increased from 0.501 to 0.750. The number of DMs also affected the relationship and values of the coefficient of determinations, r2 were: 0.340 (25 DMs), 0.758 (50 DMs), 0.763 (100 DMs), 0.774 (200 DMs), and 0.762 (300 DMs). These results indicate the usefulness of DMs as an alternative blood glucose monitoring device.
Approximately 250 million people in Japan have diabetes, which is associated with damage to the heart, kidneys, eyes and central nervous system.1) Diabetic patients should frequently monitor their blood glucose levels, about six times a day, in order to maintain appropriate blood glucose levels. Patients use needles to obtain blood samples from their fingers and blood glucose levels are measured with an enzyme assay method. However, this method is invasive and causes pain. In addition, concerns regarding infectious diseases often reduce the frequency by which patients take blood samples. Therefore, the development of a non-invasive method for blood glucose monitoring is desired. Transcutaneous spectroscopic methods have been reported previously,2,3) however, electromagnetic energy is almost entirely absorbed by skin tissue. Therefore, the reliability of these electronic technologies is insufficient. Although ultrasound,4) reverse iontophoresis,5) and electroporation6) have also been examined as non-invasive methods to monitor glucose levels, they have not yet been clinically introduced because of skin damage, pain, and low accuracy.
Microneedle technology is attracting attention as an alternative method for non-invasive glucose monitoring.7,8) Microneedles (MNs) were originally designed to percutaneously deliver drugs into the systemic circulation and have been classified into four categories9,10): (i) hollow type MNs, extremely small needles through which drug solutions are injected into the skin, (ii) coating type MNs, made of metallic and/or silastic substances, on which surface drugs are coated, (iii) pierce type MNs, made of metallic and/or silastic microneedles, through which microconduits are made in the skin followed by the application of drug solutions and/or cream after removal of the MNs, and (iv) dissolving microneedles (DMs), made of soluble polymers such as sodium chondroitin sulfate, dextran, and sodium hyaluronic acid, on which drug molecules are formulated as a solid dispersion.11,12) Of these, pierce type MNs, made of glass and plastic, have been used to monitor blood glucose levels. An MN array was stamped on the skin and interstitial fluid (ISF) was obtained by applying negative pressure, 200–500 mmHg.13) Thus, ISF could be collected without pain using a microneedle array, and glucose monitoring was performed with the ISF obtained. However, since these MNs were made of glass and plastic, the risks associated with these non-biological materials must be considered. We are currently investigating DMs in which biopolymers are used as the base material for the systemic delivery of peptide/proteins. The high physiological availabilities (PA) of insulin, 91.3–97.7%,14) and low molecular weight heparin (LMWH), 81.5–102.3%, were previously reported in rats.15) In addition, high bioavailabilities (BA) were obtained for recombinant human growth hormone (rhGH), 87.5%, in rats16) and for erythropoietin (EPO), 82.1–99.4%, in mice.17) The relative BA of interferon (IFN) against a subcutaneous injection of IFN solution was 79.9–117.8% in rats.18) The DMs used in those studies were prepared with sodium chondroitin sulfate and dextran. Sodium chondroitin sulfate has been used in the treatment of arthritis. Dextran is also used to treated peripheral circulatory disturbances associated with head injuries. Therefore, DMs made of these biopolymers are more advantageous than metallic and plastic MNs from the standpoint of safety. In the present study, DMs made of sodium chondroitin sulfate were used to collect ISF from rat skin, and their potential as an alternative glucose monitoring device was evaluated in rats.
Insulin from the bovine pancreas, 28 IU/mg, was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Glucose was obtained from Nacalai Tesque Inc. (Kyoto, Japan). Hydroxypropyl cellulose was obtained from Nippon Soda Co., Ltd. (Tokyo, Japan). Sodium chondroitin sulfate, carboxyvinylpolymer (Hiviswako 103®), and glucose CII test Wako were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Unwoven cloth was obtained from Creat Co., Ltd. (Osaka, Japan). Male Wistar rats were used in the present study and standard solid-meal commercial food was obtained from Japan SLC Inc. (Hamamatsu, Japan). All other materials were of reagent grade and were used as received.
Preparation of DM Array ChipsOne hundred microliters of distilled water was added to 40 mg of sodium chondroitin sulfate, and chondroitin glue was obtained by mixing well. After the glue was degassed under reduced pressure, it was dispensed into a mold containing 300 inverted cone-shaped wells with an area of 1.0 cm2. Each well had a depth of 500 µm and diameter of 300 µm at its top. The mold was covered with a 300-g steel plate, and the glue was added to the wells and dried. A chip was made of the mixture of cellulose acetate and hydroxypropyl cellulose (10 : 1) using the tableting machine, Handtab-100 (Ichihashi Seiki, Kyoto, Japan). The width of the chip was 2.0 mm and its diameter was 17 mm. After the plate was removed, glue consisting of 15 mg of chondroitin sulfate and 25 mL of distilled water was painted over the chip and covered on the mold. After being dried under the pressure of a stainless steel plate for 3 h, the chip was removed and DMs were obtained as arrays on the chip.
Glucose Solution Administered to RatsGlucose, 1.0 g, and carboxyvinylpolymer, 40 mg, were dissolved in 1.0 mL of phosphate buffered saline (PBS).
Microscopic Observation of DMsA DM array chip was observed using a digital videomicroscope (VH-5500; Keyence Co., Ltd., Osaka, Japan) under normal light.
Collection Method of ISF by DMsMale Wistar rats, 341±23 g, were anesthetized with sodium pentobarbital, 50 mg/kg. Body temperature was maintained at 37°C during the experiment by warming with a lamp over the body. Hair on the abdominal region of each rat was removed with a shaver (BRAUN Contour 5866; De’Longhi Japan Corp., Tokyo, Japan). In the case of glucose-loaded rats, glucose solution was administered by an abdominal injection of 0.5–7.0 g/rat. The dose of glucose was designed for wide rage patients from severe diabetic patients whose glucose level was about 200 mg/dL to healthy subjects. At the predetermined time, a DM array chip was applied to the rat skin using an applicator with a collision speed of 2.0 m/s following a second pressure of 2.5 N for 1–3 min.19) After the DM array chip was removed from the skin, wet unwoven cloth, 15.0 mm×15.0 mm, to which 10, 20, and/or 30 µL of distilled water had been absorbed, was attached to the skin for 0.1, 1.0, 2.0, 3.0, and 5.0 min to collect ISF. ISF samples were obtained by centrifuging the unwoven cloth at 12000 rpm for 5 min at 4°C using the Kokusan H-201FR centrifuge (Kokusan Co., Ltd., Tokyo, Japan). In addition, 0.1 mL blood samples were collected from the left femoral vein. Plasma samples were obtained by centrifuging blood samples at 12000 rpm for 15 min at 4°C using the same centrifuge. The obtained ISF and plasma samples were stored at –80°C until the assay.
Microscopic Observation of Rat Skin PhysiologyMale Wistar rats, 315±13 g, were anesthetized with sodium pentobarbital, 50 mg/kg. Body temperature was controlled during the experiment at 37°C by warming. Hair on the abdominal region of each rat was removed with a shaver and the image of the skin was recorded by a camera, Nikon D-200 (Nikon, Tokyo, Japan) under normal light. Thereafter, DM array chip was administered to the rat skin. Just after the DM array chip was removed from the abdominal skin, the image of the skin was also recorded by a camera. In addition, at 1 h after the removal of DM array chip, the images of the skin were recorded.
All animal protocols were approved by the Institutional Animal Care and Use Committee. Experiments were conducted in accordance with the Guidelines for Animal Experimentation, Kyoto Pharmaceutical University.
StatisticsAll values are expressed as the mean±S.E. Significant differences were assumed to be significant when p<.05 (Student’s unpaired t-test). The coefficient of determination, r2, was evaluated using linear regression analysis.
The upper panel in Fig. 1 shows DMs made of chondroitin sulfate before being applied to the rat skin. Twenty-five DMs were formed on a chip. The mean length of the DMs was 475±18 µm and the mean diameter of the basement was 278±8 µm. After being inserted into the rat skin, DM array chips were recovered and their shapes were also shown in the lower panel in Fig. 1. The mean length of the recovered DMs was 192±13 µm. By subtracting this value from that obtained before their application, the mean inserted length of DMs was estimated to be 283 µm.
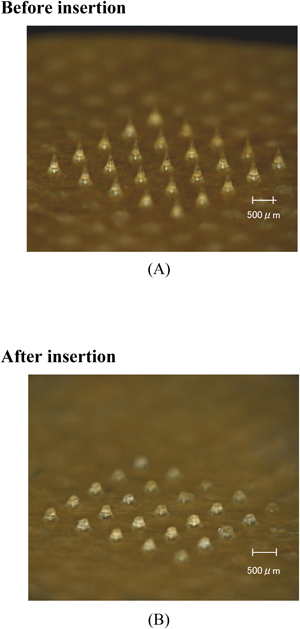
(A) Each DM was made of chondroitin sulfate and was 475±18 µm in length with a mean diameter at the basement of 278±8 µm. (B) DMs recovered after being applied to rat skin. The mean length of the recovered DMs was 192±13 µm.
The effects of the amount of water absorbed into the unwoven cloth on the amount of glucose extracted from ISF were examined under the conditions of a 1.0-min adhesion time and the use of a chip with 100 DMs. The results are shown in Fig. 2. As the amount of absorbed water increased from 10 to 20 and 30 µL, the extracted amount of glucose linearly increased from 1.66±0.35 µg to 2.23±0.23 µg and 3.25±0.61 µg, respectively. The maximum loading amount of water in the unwoven cloth, 15.0 mm×15.0 mm, was 30 µL; therefore, further increases in the water load were not performed in this study.
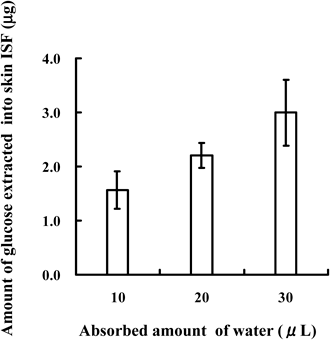
Each point represents the mean±S.D. of 4–6 experiments.
Next, the effect of the adhesion time of unwoven cloth to the rat skin on the amount of glucose extracted from ISF were evaluated and the results are shown in Fig. 3. As the adhesion time increased from 0.1 min to 5.0 min, the amount of glucose extracted from ISF increased from 1.99±0.13 µg (0.1 min) to 2.45±0.23 µg (1.0 min), 2.94±0.27 µg (2.0 min), 3.65±0.36 µg (3.0 min), and 5.04±0.38 µg (5.0 min), respectively. The effects of the adhesion time on the relationship between plasma glucose concentrations and the amount of glucose extracted from ISF were examined with 0.1 min, 1.0 min, 2.0 min, and 3.0 min adhesion times in a group of rats in which ISF was extracted with 30 µL water-loaded unwoven cloth. The results are shown in Fig. 4. The relation between plasma glucose concentrations and the amount of glucose extracted from ISF was compared under the individual conditions. By increasing the adhesion time from 0.1 min to 3.0 min, r2 increased from 0.501 (0.1 min) to 0.681 (1.0 min), 0.694 (2.0 min), and 0.748 (3.0 min) and a strong correlation was observed between plasma glucose concentrations and the amount of glucose extracted from ISF. If the adhesion time was increased, the correlation seemed to also increase since the collected amount of ISF also increases. However, the adhesion time over 3 min was not carried out, in view of the practicability. From the stand point of clinical practice, the sampling time becomes shorter, the QOL of the patients increases. Therefore, the adhesion time was fixed to 3.0 min and the effects of the number of DMs formed on a chip: 25, 50, 100, 200, and 300, on the relation between plasma glucose concentrations and the amount of glucose extracted from ISF were assessed. Figure 5 shows the relation between plasma glucose concentrations and the amount of glucose extracted from ISF with 30 µL water-loaded unwoven cloth. A strong correlation was not observed when a chip with 25 DMs was used to collect ISF from rat skin; r2 was 0.340, as shown in Fig. 5(A). When the number of DMs was increased to 50, the correlation became stronger, r2=0.758. However, no further improvements were observed as the number of DMs was increased from 50 to 100, 200, and 300, because the r2 values were 0.763, 0.774, and 0.762, respectively. When the number of DM is increased, the values of r2 were not changed. This phenomenon suggested that lower number of DM is better device for the patient since the physical stress is decreased. On the other hand, although the number of DM was increased, the density of microconduit formed on the rat skin did not change. Therefore, the coefficient of determination was not changed. If the number of DM in the fixed area was increase, the extracted amount of ISF would be increased and the coefficient of determination would also increase. The skin was examined pathologically to ascertain the safety of DMs and the results are shown in Fig. 6. The lower left panel shows the rat skin condition just after the application of DMs. By the application of DMs, microconduits were made on the skin. The lower right panel in Fig. 6 shows the skin condition at 1 h after the application of DMs. Microconduits were not detected and the skin structure recovered a normal physiology.

ISF was collected with unwoven cloth attached for 0.1, 1.0, 2.0, 3.0, and 5.0 min. Each point represents the mean±S.D. of 4–6 experiments.
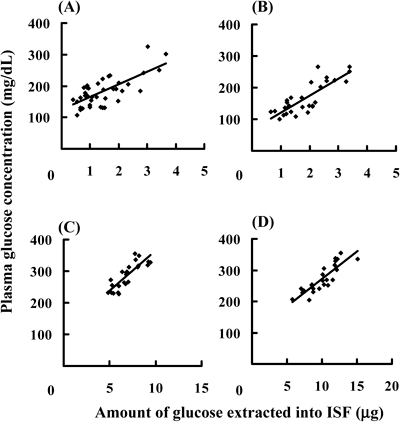
ISF was collected with unwoven cloth attached for (A) 0.1 min, (B) 1.0 min, (C) 2.0 min, and (D) 3.0 min. (A) y=40.4x+124.6, r2=0.501, (B) y=36.7x+145.7, r2=0.681, (C) y=25.4x+110.7, r2=0.694, (D) y=17.6x+95.8, r2=0.748.

(A) y=312.3x+127.3, r2=0.340 (n=37), (B) y=247.6x+106.3, r2=0.758 (n=41), (C) y=121.1x+116.3, r2=0.763 (n=45), (D) y=59.5x+118.2, r2=0.774 (n=47), (E) y=28.9x+108.8, r2=0.762 (n=48).
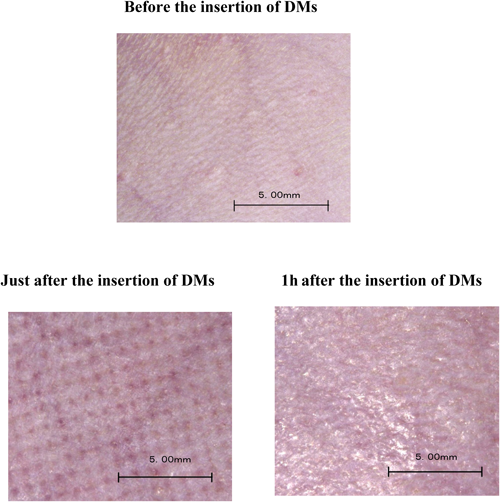
The upper panel: Before the insertion of DMs. The lower left panel: Just after the insertion of DMs. The lower right panel: 1 h after the insertion of DMs.
Stout et al. performed a comparative study on glucose levels in dermal interstitial fluid and finger capillary blood samples and obtained a coefficient of correlation of 0.923–0.951.20) They compared the ISF collection quantity between under the normal pressure and under the decompression condition. There was no significant difference in the ISF collection quantity. Reduced pressure did not significantly improve the relation between plasma glucose concentrations and the extracted amount of glucose from ISF; the sensitivity of the glucose assay was not increased. Therefore, our study used the normal pressure sampling method from the report of Stout et al.20) On the other hand, the coefficient of determination was affected by inter-individual variation. Because all the data in this study were collected from multiple points on multiple individuals, rats. If it is made to be multiple samples from 1 individual in the large animal, this coefficient determination would be improved.
Wang et al. reported the usefulness of glass microneedles for the extraction of dermal ISF for glucose monitoring.21) However, their microneedles were made of glass. Sato et al. also examined the relationship between blood glucose concentrations and ISF in the skin by collecting microneedles.22) Their microneedles, which were in 300 µm length, were made of polycarbonate; therefore, there was a risk of injury when these microneedles broke in the skin of patients. On the other hand, our DMs were made of the biopolymer, chondroitin sulfate, which has been used as a pharmaceutical in the treatment of arthritis. The clinical dose of chondroitin sulfate is 20–300 mg. The length of our conical DM was 475±18 µm and the mean diameter of the basement was 278±8 µm. As the shape of our DMs was conical, the weight of one DM was approximately 10 µg. When 300 DMs are formed on a chip, the total amount of chondroitin sulfate was approximately 3.0 mg, which is markedly lower than the clinical dose of chondroitin sulfate. Therefore, no side effects were expected even if the formulated chondroitin sulfate was completely absorbed into the systemic circulation. Furthermore, we previously investigated the local toxicity of DMs, and confirmed the safety of chondroitin sulfate DMs.23) Pathological and biochemical studies showed that skin physiology recovered to normal conditions within 1 h of the removal of DMs from rat skin to which DMs were attached for 3 min and/or 6 h. We also examined the physiology of the rat skin visually using a videomicroscope. Rat skin recovered to normal conditions within 1 h of the removal of DMs. Taken together, these results indicate that DMs can be used as a non-invasive device for alternative glucose monitoring in ISF.
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan—Supported Program for the Strategic Research Foundation at Private Universities, 2008–2013. This study was also supported by a Grant-in-Aid for Scientific Research provided by MEXT, 2010–2013.