2014 Volume 37 Issue 12 Pages 1919-1925
2014 Volume 37 Issue 12 Pages 1919-1925
We investigated the effect of puerarin on bone mass and marrow adiposity in ovariectomy (OVX)-induced osteoporosis. The rats were divided into four groups: control; OVX; OVX+estradiol (OVX-E); and OVX+puerarin treatment (OVX-GE). In vivo, bone mineral density (BMD) and histomorphometry were measured under microCT. The mechanical properties of tibia were obtained in 3-point bending test. Plasma osteocalcin and adiponectin were determined using enzyme-linked immunosorbent assay (ELISA). Alkaline phosphatase (ALP) were measured using biochemical methods. In vitro, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Oil Red O staining were used to compare osteoblast proliferation and adipocyte differentiation, respectively. Osteocalcin and adiponectin in culture supernatants were determined using ELISA. The results showed that puerarin significantly enhanced bone volume density and trabecular number compared with OVX and OVX-E groups (p<0.05, p<0.05, respectively). Puerarin increased energy to ultimate load, plasma osteocalcin and ALP (p<0.01). However, BMD in OVX-GE group was less than that in control (p<0.01) and OVX-E groups (p<0.05). The culture supernatants from OVX-GE group showed increased osteocalcin compared with those from OVX (p<0.01) and OVX-E groups (p<0.05). Puerarin lowered adiponectin in culture supernatant compared with supernatant from OVX group and inhibited the increase in adipocytes caused by OVX (p<0.01). However, the amount of lipids did not differ between OVX-GE and OVX groups. These findings suggest that puerarin likely enhances bone formation by stimulating the proliferation and differentiation of osteoblasts while slightly inhibiting the adipotic differentiation.
Puerariae radix, the root of Pueraria lobata (WILD.) OTRWI, a wild creeper leguminous plant, is one of the earliest and most important crude herbs used in Chinese medicine for various medicinal purposes. For the near decade, it has been reported using osteoporosis animal model that total isoflavones of puerariae isoflavone could protect osteoporosis induced by ovariectomized rats1,2) and could inhibit the secondary osteoporosis induced by dexamethasone in rats.3) As a kind of phytoestrogen, puerarin is regarded as one of isoflavone extract which exerting preventive effect on bone loss in ovariectomized rats.4) However, all the related animal experiment used total isoflavones of Pueraria lobata (WILD.) OTRWI. Their results were the comprehensive effects of daidzein, genistein and puerarin. We couldn’t judge the independent effect of puerarin on bone gain.
Puerarin is an active component extracted from the Chinese traditional medicine Pueraria lobata (WILD.) OTRWI. Structurely, puerarin is similar to estrogen. It has three –OH, where they combine with estradiol receptors and then exhibit estrogenic action. Therefore, it’s believed to exhibit beneficial effect on bone tissue by mimicking the biological responses of estrogens.5,6) Previously, we reported that Puerarin could suppress bone resorption and promote bone formation in vitro, but the former effect was stronger than the latter.7) However, Zhang et al.8) and Tiyasatkulkovit et al.9) observed strong promotion effects of puerarin on bone formation. These inconsistent results made it necessary to revaluate its effect on bone formation using multiple parameters. Therefore, this study investigated puerarin’s effect on bone foramtion for the first time by collectively evaluating bone structure, bone mineral density (BMD), bone biomechanical features, and biochemical markers.
As osteoblasts and adipocytes share bone marrow stromal stem cells (MSCs) as a precursor, a reciprocal association between bone mass and bone marrow adiposity has been noted frequently in human and animal models.10) We speculated that there were a balance between bone formation and bone marrow adiposity. However, less is known about how puerarin affects marrow adiposity in ovariectomized rats.
Taken together, we tested the effect of puerarin on bone mass collectively by BMD, bone micro-architecture, biomechanical and biochemical properties, and on marrow adipogenesis in a rat model of postmenopausal osteoporosis.
Puerarin freeze dried powder injection was purchased from Beijing Four Rings Biopharmaceutical Co., Ltd. The raw material comes from the Shaanxi Hanzhoung area of Pueraria lobata Good Agricultural Practice of Medicinal Plants and Animals bases. The impurity content is less than 0.5% (pH=5.5). The hydroxyl-puerarin content is less than 0.02%. This injection doesn’t contain propylene glycol in order to avoid hemolytic reaction.
α-Modified Eagle’s medium (αMEM), fetal bovine serum (FBS) and penicillin–streptomycin (5000 U/mL penicillin; 5000 µg/mL streptomycin) were obtained from GIBCO Laboratories (Grand Island, NY, U.S.A.). Estradiol, 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), oil O, β-sodium glycerophosphate, ascorbic acid, dexamethasone, insulin, and dexamethasone were bought from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Animals and Administration ProcedureSixty adult female Sprague-Dawley (SD) rats (weighing 205–225 g) from the Animal Center of National Birth Control Board were kept at constant temperature and humidity during the experiment period (temperature, 22±3°C; humidity, 50% ±20%; 12 h light: 12 h dark cycles, light on 07 : 15 a.m.). This animal experiment was performed under the guidelines and permission of Chinese Animal Care Committee. The study was approved by Ethics Committee of Peking University Health Science Center. They were randomly divided into 4 groups with 15 rats each: ①Sham: sham surgery; ②OVX: bilaterally ovariectomized under isoflurane anesthesia; ③OVX-E: treated with 10 µg/kg estradiol intraperitoneally (i.p.) once every 2 d after ovariectomized; ④OVX-P: treated with 50 mg/kg puerarin i.p. after ovariectomized once every 2 d. Sham and OVX group were treated with vehicle once every 2 d. General conditions were observed every day and the body weight of rats was weighed weekly. At 12th week, rats were anesthetized with pentobarbital and exsanguinated to death. In each group, 10 rats were for microCT, biomechanical and biochemical examination; 5 rats were for ex vivo cell culture.
Microtomographic Histomorphometry and BMD by MicroCTAs previously described,11) the proximal tibia thoroughly disssected free from soft tissue was fixed with 4% paraformaldehyde for 24 h, and subsequently washed with 10% saccharose solution. Twelve hours later, microtomography the proximal tibia was performed by SIEMENS MM Gantry LG CT camera scanner (Germany). Three-dimensional images of each proximal tibia were acquired with a voxel size of 10.34 μm in all spatial directions. The samples were scanned at a voltage of 60 kV and a current of 400 μA. A low-pass filter was used for removing noise, and the resulting gray-scale images were segmented. Additionally, an appropriate threshold was adjusted to extract the mineralized bone phase. The trebecular parts of the tibia were separated with semi-automatically drawn contours.
The volume of interest (VOI) was drawn using a slice-based method. The trabecular bone was carefully contoured on every five slices from the first slice. Meanwhile, the intermediate slices were interpolated by morphing. Each slice was subsequently visually inspected, and the contours were modified where it was deemed necessary. The microstructural indices were calculated directly from the VOI. Total volume (TV) was the volume of the whole sample scanned. Bone volume (BV) and the surface (BS) were determined using tetrahedrons corresponding to the enclosed volume of the triangulated surface. BV/TV and BS/BV were used in order to normalize the sizes of the samples. Mean trabecular thickness (Tb.Th.) was calculated from the local thickness at each voxel representing the trabecular meshwork. Trabecular number (Tb.N.) was determined by taking the inverse of the mean distance between the middle axes of the structure. Trabecular separation (Tb.Sp.) was calculated from the direct thickness of the nonbone parts. Using the microCT scanning data, three-dimensional (3D) images were reconstructed.
For determining BMD, the motif which contains six cylinders of different density was scanned in the same condition of the samples. The calculated VOI of the tibia was same as the VOI from the microtomography. The resulting linear equation was BMD (g/cm2)=9.1313 (VOI)−8518 (R2=1). When the VOI was determined, the mean was automatically calculated by microCT.
Biomechanical TestingThe tibia, which had previously been measured by microCT, was used for the three-point bend testing performed at the plunger speed of 1.0 mm/min using a computer-controlled mechanical testing machine (Instron 4302; Instron, Norwood, MA, U.S.A.). The load-deformation curves were recorded during the bending process. The sample space was 3 mm. The tested area was the secondary spongiosa of the proximal tibia.
Based on the data recorded by a connected computer, maximum load, energy to ultimate load, linear stiffness, and Young’s modulus were calculated. The maximum load (N) is the value of the load when the bone fractured. Stiffness (N/mm) was calculated as the slope of the linear portion of the load-deformation curve. Energy to ultimate load (mJ) was computed as the areas under the load-deforming curves. Young’s modulus (MPa) was computed from the initial slope of the stress–strain curve.
Plasma BiochemistryBlood samples (2 mL) from model rats were collected into plastic ethylene diamine tetraacetic acid (EDTA)–Na2 tubes and centrifuged for 10 min at 5000 rpm at 4°C. Then plasma samples were removed and transferred to new tubes. All plasma samples were frozen at −20°C until assay. Osteocalcin and adiponectin (R&D, U.S.A.) were tested in a two-site assay by enzyme-linked immunosorbent assay (ELISA). The intra- and interassay variations were less than 5%, and the detection limit was 0.1 ng/mL. Levels of alkaline phosphatase (ALP) were all tested by use of the automatic analyzer HITACHI 7180 (Japan).
Primary Osteoblasts and Adipocytes Isolation and CultureBone marrow cells were isolated from above rats. Generally, two ends of the femur were cut off, and the marrow caving was flushed with 1 mL αMEM and cultured in selective medium containing 10% heat-inactivated FBS at 1.0×107 cells/mL in 24-well plates (0.5 mL/well) in a water-saturated atmosphere containing 5% CO2 at 37°C. Two days after the proliferation and intiation of differentiation, the culture medium was replaced with new αMEM supplemented separately with 10 mM β-sodium glycerophosphate +0.05 M ascorbic acid +10 nM dexamethasone (for osteoblast differentiation), or 10 µg/mL insulin +10−7 M dexamethasone (for adipocyte differentiation).
MTT and Oil-Red O StainingCells proliferation was examined by MTT assay. When cells were cultured to the log phase, they were seeded on a 96-well plate (1×105 cells per well) for 24 h. Absorbance (A) was detected with an enzyme calibrator at 570 nm. There were 16 wells for each group.
The adipocyte differentiation was determined by Oil-Red O staining. The cells were divided into two experiments. Because the Oil-Red O staining is a special method to distinguish mature adipocyte from other cells, the cells were seeded on slices (1×1 cm2) and stained by Oil-Red O for counting adipocytes. The cells were fixed in 10% formalin in PBS for at least 10 min and washed with distilled water. Cells were completely dried before staining with 0.6% Oil-Red O solution in 60 : 40 (v/v) isopropyl alcohols: H2O for 30 min at room temperature. There were 3 slices for each group. In addition, the other cells were seeded in 96-well micro-plates and stained by Oil-Red O for quantitation of lipid accumulation. The droplets in the cytoplasm became red by staining with Oil-Red O. The staining procedure was same with above. After removing the staining solution, the dye retained in the cells was eluted into isopropanol just before the measurement of an optical density at 490 nm. There were 8 wells for each group.
Culture Supernatant BiochemistryAfter induced bone marrow cells were cultured for 7 d, the culture supernatant were collected and examined as the method above mentioned in plasma biochemsitry.
Statistical AnalysisStatistical analysis was performed using the SPSS Statistical Analysis System, Version 10.0. Statistical significance was determined by one-way ANOVA followed by post-hoc test. All results are expressed as means±S.D. Significance was considered at p<0.05.
We chose rats weighing 205–225 g and put them in each group using the random table. There was no significant difference in body weight at the beginning of the experiment. After 12-week treatment, all the rats increased their weights. The increase ratio of body weight in OVX group (the increase rate=36.37%±7.03%) was much more than that of Sham and OVX-E group (p<0.01, p<0.01, respectively). Estradiol sharply reduced ovariectomy-induced weight gain (p<0.01). Puerarin had more increase ratio in body weight compared with those of Sham (p<0.01) and OVX-E group (p<0.05) (the increase rate=31.33±5.26%), and had no difference with that of OVX group. These data suggested that puerarin couldn’t inhibit weight increase induced by ovariectomy. It was not as good as estradiol in this aspect (Fig. 1).

Rats were divided into 4 groups: control (Sham), ovariectomy (OVX), ovariectomy+estradiol (OVX-E), ovariectomy+puerarin treatment (OVX-GE). The values from each treatment were expressed as a percentage (last weight−first weight)/first weight (100%). Each datum represents the mean ±S.D. of 10 rats. ** p<0.01, significantly different from Sham group; $$ p<0.01, significantly different from OVX group; # p<0.05, significantly different from OVX-E group.
OVX group had a lower BMD compared with Sham group (p<0.05), which showed the success of modeling. Puerarin couldn’t improve the low BMD induced by ovariectomy (p<0.01, compared with Sham group; p<0.05, compared with OVX-E group), but estradiol could. Puerarin was weaker in the BMD elevation than estradiol (Table 1).
| Group | Bone mineral density (g/cm2) |
|---|---|
| Sham | 1.001±0.011 |
| OVX | 0.969±0.012* |
| OVX-E | 0.994±0.003 |
| OVX-GE | 0.954±0.003**, # |
Note: Data are expressed as means±S.D. Comparisons of data between estriol+puerarin treatment, estriol treatment, ovariectomy and control group were evaluated by one-way ANOVA followed by the Student–Newman–Keuls test. * p<0.05, ** p<0.01, compared with Sham group; # p<0.05, compared with OVX-E group.
Ovariectomy leaded to low BV/TV (p<0.05) and Tb.N. (p<0.05), and high Tb.Sp. (p<0.01). This demonstrated the modeling success. Puerarin improved BV/TV (p<0.05) and Tb.N. (p<0.05) compared with OVX group. In this aspect, puerarin behaved as good as estradiol. No significant difference was found between OVX-E and OVX-GE group. (Fig. 2)

The tibia samples were fixed in 4% paraformaldehyde overnight, and then scanned by microCT. Three-dimensional images were obtained in all spatial directions. The volume of interest (VOI) was drawn using a slice-based method. Data are reported as means±S.D. * p<0.05, ** p<0.01, compared with Sham group; $ p<0.05, $$ p<0.01, compared with OVX group.
Energy to ultimate load decreased significantly after ovariectomy (p<0.05). There was no significant difference between OVX-E and OVX-GE group in the value of energy to ultimate load, which suggested that puerarin enhanced biomechamical property as good as estradiol (Fig. 3).

The tibia samples were same with what scanned by microCT. The secondary spongiosa of the proximal tibia were tested until deformation. The load-deformation curves were recorded during the bending process. Data are reported as means±S.D. * p<0.05, compared with Sham group.
Puerarin sharply enhanced plasma osteocalcin and ALP compared with Sham group (p<0.01, p<0.01, respectively). In addition, there was no difference between all the groups in plasma adiponectin (Fig. 4).

Plasma osteocalcin (A) and adiponectin (C) were performed by ELISA. Plasma ALP (B) was detected by biochemical methods. Data are reported as means±S.D. * p<0.05, ** p<0.01, compared with Sham group; $$ p<0.01, compared with OVX group; ## p<0.01, compared with OVX-E group.
For MTT assay, the osteoblasts in OVX group was less proliferative than that in Sham group (p<0.01). Estradiol enhanced osteoblast viability significantly (p<0.01). The effect of puerarin was less than that of estradiol (p<0.01). But puerarin did increase the proliferative activity of osteoblasts. However, the change didn’t reach a statistical significance. For osteocalcin in culture supernatant, the trend was similar, except that puerarin had more osteocalcin than estradiol group (p<0.05) (Fig. 5).

A: Cell viability was measured by MTT assay. The cells in log phase were plated into 96-well microplates at 1×105/well in αMEM supplemented with 10 mM β-sodium glycerophosphate +0.05 M ascorbic acid +10 nM dexamethasone, and incubated for 24 h prior to treatment at 37°C in 5% CO2. B: Induced osteoblasts were cultured for 72 h. Then culture supernatant was collected for ELISA assay. ** p<0.01, compared with Sham group; $$ p<0.01, compared with OVX group; # p<0.05, ## p<0.01, compared with OVX-E group.
The absence of ovary induced more oil-red-positive cells derived from bone marrow (p<0.01). Both puerarin and estradiol, inhibited this upward tendency (p<0.01, p<0.01, respectively) (Figs. 6A, B). However, puerarin had no effect on lipid accumulation. The lipids in OVX-GE group were higher than that of OVX group (p<0.01) (Fig. 6C). As for adiponectin in cell supernatant was concerned, puerarin suppressed the rising of adiponectin induced by overectomy (p<0.01). However, puerarin’s influence was lower than estradiol (p<0.01) (Fig. 6D).
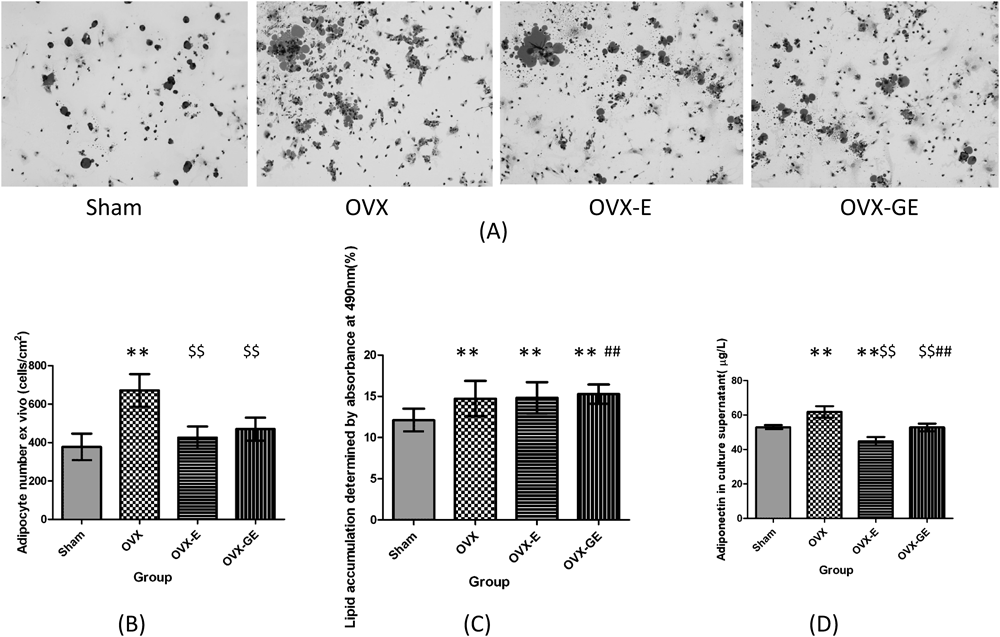
A: The oil-red-positive cells cultured ex vivo. The MSCs were seeded on slices at 1×105/well in αMEM supplemented with 10 µg/mL insulin +10−7 M dexamethasone, and incubated for 72 h at 37°C in 5% CO2 before stained by 0.6% Oil-Red O solution. B: The quantity of the Oil-Red-positive cells. C: The induced adipocytes were plated into 96-well microplates at 1×105/well in the same medium, and stained by Oil-Red O. The spectrophotometry of isopropanol-extracted cells presented the lipid droplets in culture system. D: Induced adipocytes were cultured for 72 h. Then the culture supernatant was collected for ELISA assay. ** p<0.01, compared with Sham group; $$ p<0.01, compared with OVX group; ## p<0.01, compared with OVX-E group.
Our group has ever reported puerarin’s potential effect in the prevention of bone loss. Primary data suggested that 10–50 µg/L puerarin inhibited osteoclastic bone resporption in vitro. Puerarin had some therapeutic effect on the ovariectomy-induced osteoporosis.12,13) Naturally, we wondered puerarin’s osteogenic influence on bone in ovariectomized rats. In this study, we investigated the effect of puerarin on bone mass and marrow adiposity in ovariectomy-induced osteoporosis, and found that puerarin improved bone metablism by modulating osteoblastogenesis and bone marrow adiposity. Therefore, the present findings corroborated the former potential benefit of puerarin in the prevention and treatment of postmenopausal osteoporosis.
Previous study demonstrated that ALP is secreted from the immature osteoblasts, whereas osteocalcin is raised at the later stage of osteoblast differentiation. In this study, ALP activity was considered as an early differentiation marker and osteocalcin as a later differentiation marker, which was relevant to biomineralization.14) Our data suggested that puerarin induced osteoblast differentiation by upregulating both osteocalcin and ALP expression in vivo and in vitro. This result was consistent with the report of Tiyasatkulkovit et al.9) Studies exhibited that puerarin in collagen matrix increased new bone formation locally and could be used for bone graft and bone induction.15) Further, there were data suggested that puerarin stimulated osteoblastic proliferation and Akt activation via phosphatidylinositol 3-kinase (PI3K)-dependent manner,16) or through caspase-3,17) estrogen receptor, p38 mitogen-activated protein kinase (MAPK), and Wnt/β-catenin pathways.18) Further investigation should be conducted on the pathway of how puerarin influences bone formation.
On the other hand, accumulating evidences indicated that the degree of marrow adipogenesis may be an alternative indicator of the severity of osteoporosis in relation to the degree of osteogenesis.19) A reciprocal association between osteoblasts and adipocytes deriving from bone marrow stromal cells prompted us to explore the effect of puerarin on adipogenesis at the same time. Unfortunately, our data indicated that the reciprocal changes in bone and adiposity were not directly coupled with one another. Puerarin decreased the amount of adipocytes and adiponectin in supernatant, but had no significant influence on osteoblasts proliferation and lipid accumulation ex vivo, compared with OVX group. However, puerarin sharply enhanced osteocalcin in vivo and in vitro, and decreased adiponectin dramtically in vitro. We hypothesised that these bone-related hormones were more sensitive than the changes of cells amount. In addition, there are conflicting evidence on the balance of bone and fat in general level, even at the puerarin-specific point. It was reported that growth hormone enhanced adipocytes as well as osteoblast precusor pool size. However, it increased osteoblast differentiation while suppressing bone marrow lipid accumulation.20) For phytoestrogens, icariin prevented ovariectomy-induced bone loss and lowered marrow adipogenesis, comparabley with estrogen.19) Also, puerarin increased adipocyte differentiation, adiponectin expression, and antioxidant response in preadipocytes cell line.21) But it’s showed that only aglycon forms of isoflavones, not glycosylated forms, could induce lipogenesis in the preadipocyte cell line.22) Wang et al. demonstrated that puerarin promoted osteogenesis and inhibited adipogenesis in vitro.23) Our data was different from all the above. We found that the OVX-induced adipocyte-increase and adiponectin-increase in supernatant was suppressed significantly by intake of puerarin. But puerarin had no effect on plasma adiponectin, osteoblastogenesis and lipogenesis in vitro. We analyzed that the cause of these differences was that we obtained bone marrow cells from model rats ex vivo, which most mimicking the real action happened in the body.
In this study, we used the estradiol as a positive control, because its effects on bone mass are well established in ovariectomized rats.24) As expected, estradiol in present study improved bone microstructure, bone strength, BMD, bone biomarker levels in vivo, and osteoblastgenesis in vitro. At the same time, estradiol lowerd the ovarectomy-induced weight increase in vivo, and decreased adipocytes and adiponectin in vitro. Compared with estradiol, our data provided some novel findings in the mechanism for the prevention of osteoporosis by puerarin. Differential effects were shown of puerarin and estradiol on the increase in body weight shown in OVX rats as well as on osteoblasts proliferation and serum osteocalcin levels and adipocytic differentiation, although these compounds were similar properties in the effects on bone density and morphology and intensity of bone in OVX rats. These results strongly suggested that quite differential mechanisms are involved in the effects of puerarin and estradiol on bone of OVX rats.
Phytoestrogen has a double directional adjusting function of estrogenic and antiestrogenic activities, depending on the dosage and the endogenous estrogen status and the type and quantity of estrogen receptor.25) There are two different forms of estrogen receptor (ER), usually named as ERα and ERβ. The dominant subtype in adipocytes is ERα.26) However, isoflavone’s affinity on ERβ is much higher than that of ERα.27,28) It may be the reason that puerarin showed antiestrogenic effect in adipogenesis to some extent. (Estrogen itself has direct effects on adipocytes to inhibit lipogenesis.29)) In addition, there are two ways that ER genes are regulated by. One way is the classic way which is done through estrogen responding expressor combined with DNA-binding domain. Estradiol plays its biological role mainly through this pathway. Another way is a non-classical way, which is by completing a series of signal transductions through the interactions bewteen other transcription factors such as AP-1. Phytoestrogen produced anti-osteoporosis effects through AP-1 passway.30) We speculated that the difference of effect between puerarin and estradiol may be induced by different pathway. Further studies will be needed to fully elucidate the underlying signaling mechanisms by which puerarin prevented ovariectomy-induced osteoporosis.
Overall, the effect of puerarin was comparable with that of estradiol, but a little bit weak in the degree. Considering its low toxicity, less adverse effect, low price, and wide use compared with estrogen, puerarin has very high value in the prevention and treatment of postmenopausal osteoporosis.
This research was funded by National Natural Science Foundations of China, Beijing, China (No. 30901671).