Abstract
Telaprevir (TVR) is a protease inhibitor used in combination with pegylated interferon alfa-2b and ribavirin for hepatitis C, and TVR strongly inhibits CYP3A4 and CYP3A5. We reported successful TVR treatment of liver transplant patients with recurrence of hepatitis C during receiving immunosuppressive therapy. Before initiation of triple therapy, all patients switched from tacrolimus to cyclosporine, which has a lower inhibitory effect on CYP3A4 and CYP3A5 than tacrolimus. To avoid graft failure, we measured the cyclosporine blood concentrations at 0, 2, and 6 h after administration to maintain the target level (150–200 ng/mL) within 1 week after initiation of TVR and adjusted the dose of cyclosporine. The dose of cyclosporine was decreased 0.24–0.40 fold in all patients after initiation of TVR treatment. In 3 patients, the dose of TVR was decreased two-thirds of starting dose because of adverse effects, including anorexia and skin rash. However, the HCV RNA level rapidly decreased to undetectable levels within 1 month. Furthermore, all patients completed the TVR therapy in 12 weeks and did not experience liver graft rejection. In addition, we found the rapid elimination of inhibitory effect of TVR on the disposition of cyclospirne in the all four cases and therefore, rapid increase in the dosage of cyclosporine would be required immediately after the end of TVR administration. These results suggest that frequent measurement of cyclosporine levels was important for successful TVR triple therapy and prevention of rejection.
Telaprevir (TVR), a protease inhibitor, is a new drug to treat hepatitis C.1–3) Triple therapy with pegylated interferon alpha 2b (PEG-IFN α-2b), ribavirin, and TVR for 12 weeks and double therapy with PEG-IFN α-2b and ribavirin for 12 weeks strongly affects the hepatitis C virus (HCV), and 73.0% of patients achieve sustained viral responses (SVRs).4) TVR is metabolized by CYP 3A4 and CYP3A5 and strongly inhibits CYP3A4 and CYP3A5.5,6) Therefore, TVR has strong drug interactions with immunosuppressants like tacrolimus and cyclosporine.7)
Some hepatitis C patients develop liver cirrhosis or hepatocellular carcinoma, which require a liver transplant. After liver transplantation, they have to take immunosuppressive agents to prevent graft loss, and the blood concentrations of these drugs have to be carefully monitored. However, patients can show recurrence of hepatitis C even after liver transplantation.8,9) Therefore, it is difficult to control the blood concentration of immunosuppressive agents with TVR triple therapy in patients with recurrence of HCV infection. To prevent graft rejection and treat hepatitis C, we carefully adjusted the dose of immunosuppressive agents to maintain the target blood concentrations.
This case report describes successful treatment of transplant patients with recurrence of hepatitis C with TVR, PEG-IFN α-2b, and ribavirin triple therapy to prevent liver graft rejection. We carefully controlled the blood concentration of immunosuppressive agents to that of the target level by frequent measurement.
MATERIALS AND METHODS
Treatment ProtocolThe day of initial administration of TVR was set as Day 1. The primary immunosuppressive agent was changed from tacrolimus to cyclosporine around one week before the initiation of TVR administration. The target trough and C2 level of cyclosporine was set between 150 ng/mL and 200 ng/mL and between 600 ng/mL and 800 ng/mL, respectively, beginning around 2 weeks of TVR administration. TVR 750 mg was orally administered twice daily. Around day 7, PEG-IFN α-2b and ribavirin were added to start the triple therapy with TVR. The administration of TVR was terminated after 12 weeks, and then combination treatment with PEG-IFN α-2b and ribavirin was continued for 24 weeks. All periods of HCV treatment were 24 weeks.
Blood SamplesBlood samples for measuring the levels of immunosuppressive agents were collected immediately before the morning dosage and at 2 h and 6 h after the administration between days 1 and 7. After day 8, the blood samples were collected for measuring the morning trough level of immunosuppressants.
Measurements of Blood Concentration of Tacrolimus and CyclosporineWhole blood concentrations of tacrolimus and cyclosporine were determined using chemiluminescence immunoassay (CLIA) using an ARCHITECT® i2000SR analyzer (Abbott Laboratories, Chicago, IL, U.S.A.) and affinity column-mediated immunoassay (ACMIA) using Dimension® (Siemens Healthcare Diagnostics Inc., Newark, DE, U.S.A.), respectively, according to the manufacturer’s instructions.
EthicsThis study was conducted in accordance with the Declaration of Helsinki and its amendments, and the study protocol was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee. Written informed consent was obtained from each patient.
CASE REPORTS
Although TVR strongly inhibits the dispositions of tacrolimus and cyclosporine, the interaction between TVR and cyclosporine is relatively mild compared to that between TVR and tacrolimus.7) In addition, the clinical efficiency of cyclosporine was found to be similar with tacrolimus in the 39 living donor liver transplant patients.10) Therefore, we carefully switched the primary immunosuppressant from tacrolimus to cyclosporine about a week before the initiation of TVR administration to avoid toxicity from calcineurin inhibitors. In addition, the dosage of TVR was decreased to 1500 mg/bid, because previous studies in Japan indicated that the standard dose of TVR 2250 mg/tid was toxic to Japanese patients.11,12)
The clinical characteristics of the patients in this study are summarized in Table 1. All patients showed a recurrence of HCV genotype 1b after liver transplantation. The median (range) of duration between liver transplantation and initiation of TVR treatment was 21 (1–75) months.
Table 1. Pretreatment Profile and Clinical Characteristics
| Case number | I | II | III | IV |
|---|
| Male/Female | Male | Male | Female | Female |
| Age (years) | 62 | 50 | 67 | 68 |
| Body weight (kg) | 68.6 | 120.2 | 53.4 | 43.0 |
| Primary disease for transplantation | HCC | LC | LC, HCC | LC, HCC |
| Milan criteria for HCC treatment | Within | | Within | Within |
| ABO blood type (donor/recipient) | O/O | A/A | B/B | O/O |
| Donor | Cadaveric | Offspring | Spouse | Offspring |
| HCV genotype | 1b | 1b | 1b | 1b |
| Months after liver transplantation | 77 | 22 | 74 | 37 |
| Months after transplantation recurrence of HCV | 75 | 1 | 69 | 33 |
| Post-transplant anti-HCV treatment | PEG-IFN, RBV | Not treated | PEG-IFN, RBV | PEG-IFN, RBV |
| Duration of post-transplant anti-HCV treatment (months) | 2–8, 11–44, 68–71 | | 8–22 | 5–15 |
| Outcome | Withdraw | | SVR* | Withdraw |
| Positive conversion of HCV after SVR (month after liver transplantation) | | | 66 | |
HCC, hepatocellular carcinoma; LC, liver cirrhosis; HCV, hepatitis C virus; LT, liver transplantation; PEG-IFN, pegylated interferon alfa-2b; RBV, ribavirin; SVR, sustained viral response.
Case I: A 62-year-old man who underwent cadaveric donor liver transplantation because of hepatocellular carcinoma after HCV-related liver cirrhosis. HCV RNA was detected in his serum after transplantation; therefore, he was administered anti-HCV therapy with PEG-IFN α-2b and ribavirin at the 2nd post-transplant month. Because of fatigue and nausea, the double therapy (consisting of PEG-IFN α-2b and ribavirin) was interrupted twice; once between months 9 and 10 and once between months 45 and 67. Then, the double therapy for recurrence HCV was withdrawn 75 months after liver transplantation. Before initiation of triple therapy (consisting of TVR, PEG-IFN α-2b, and ribavirin), the calcineurin inhibitor was switched from tacrolimus to cyclosporine about a week before the administration of TVR. The dosage and trough concentration of cyclosporine at the day before the administration of TVR were 150 mg/bid and 212 ng/mL, respectively (Figs. 1A, B). To avoid an excessive increase in the blood concentration of cyclosporine, the dosage of cyclosporine was reduced to one-third of the original on the day of TVR administration (1500 mg/bid). Although the blood concentration of cyclosporine varied, dosage adjustment was carefully performed on the basis of the blood concentration of cyclosporine in the morning during the two weeks after initiation of TVR therapy. On day 7 of TVR therapy, PEG-IFN α-2b (100 µg/week) and ribavirin (400 mg/bid) were also added to constitute the triple therapy. The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) decreased to normal levels during the first 7 d with TVR, without PEG-IFN α-2b and ribavirin. The levels of uric acid, serum creatinine, and blood urea nitrogen (BUN) moderately increased; an increase in the dosage of allopurinol from 100 mg/d to 200 mg/d was effective against TVR-related kidney injury. The patient successfully completed the 12-week regimen of the triple therapy; subsequently, the administration of TVR was discontinued. Immediately after the termination of TVR administration, the dosage of cyclosporine was increased to 100 mg/bid to maintain a sufficient trough level to prevent rejection. Over the course of 100 d, the patient did not experience any severe adverse reactions related to TVR and cyclosporine and such as graft rejection.

Case II: A 50-year-old man who underwent living-donor liver transplantation from his offspring because of HCV-related liver cirrhosis. Because the serum HCV RNA level rapidly increased after the transplantation, he received liver-supporting therapy with monoammonium glycyrrhizinate for 1 month. The calcineurin inhibitor was carefully switched from tacrolimus to cyclosporine about 10 d before the administration of TVR. Before TVR administration (1500 mg/bid), the dosage and trough concentration of cyclosporine were 100 mg/bid and 197 ng/mL, respectively (Figs. 2A, B). Because of interactions between cyclosporine and TVR, the dosage of cyclosporine was reduced to half on day 1 of TVR administration. We carefully adjusted the dosage of cyclosporine on the basis of the measurement of morning blood concentration during 3 weeks after administration of TVR, because of the unstable pharmacokinetics of cyclosporine. Finally, the dosage of cyclosporine was 50 mg/qod and the trough level of cyclosporine was 215 ng/mL at day 21. Hepatorenal syndrome and diabetic nephropathy deteriorated his renal function at surgery; therefore, the initiation of PEG-IFN α-2b (150 µg/week) and ribavirin (800 mg/bid) was carefully set back to day 14 to avoid further kidney dysfunction. Fatigue and anorexia worsened from 1 month after initiation of TVR treatment, and the patient developed intense pruritus on his back from day 51 after TVR treatment. Because of these complications, the dose of TVR was decreased to 1000 mg/bid; subsequently, these adverse reactions subsided. Although the blood concentration of cyclosporine decreased to 141 ng/mL at the 20th day after reducing the TVR dosage, the dosage of cyclosporine of 50 mg on alternate days (qod) was unchanged without any rejection episode. On the 19th day, the uric acid level was 7.0 mg/dL; therefore, the patient was administered febuxostat (10 mg/d). Moreover, the serum creatinine level moderately increased, but it decreased after reduction of TVR dose. The patient completed the 12-week TVR therapy. After the end of TVR administration, the dosage of cyclosporine was gradually increased from 50 mg/qod to 50 mg/qd, and finally to 75 mg/qd to maintain the target trough level. At that point, the blood concentration of cyclosporine was stable. The calcineurin inhibitor was switched again from cyclosporine to tacrolimus. Over the course of 100 d, the patient showed no rejection episode.

Case III: A 67-year-old woman who underwent living-donor liver transplantation from her spouse because of hepatocellular carcinoma after HCV-related liver cirrhosis, which was within the Milan criteria. The patient experienced a recurrence of hepatitis C at 6 months after transplantation; therefore, she was treated with PEG-IFN α-2b and ribavirin for 14 months and achieved SVR. At the 66th post-transplant month after achieving SVR, she experienced relapse of HCV infection and was followed-up without PEG-IFN α-2b treatment. Before treatment with TVR, PEG-IFN α-2b, and ribavirin, the calcineurin inhibitor was switched from tacrolimus to cyclosporine about a week before TVR administration. The dosage and trough concentration of cyclosporine at the day before the administration of TVR were 100 mg/bid and 129 ng/mL, respectively (Figs. 3A, B). To achieve the target trough concentration of cyclosporine (150–200 ng/mL), the dosage of cyclosporine was gradually decreased from 100 mg/bid to 35 mg/qd within 4 d after initiation of TVR administration (1500 mg/bid). The dosage of cyclosporine decreased to 25 mg/qd when the trough concentration of cyclosporine was greater than 200 ng/mL. On day 8 of TVR therapy, PEG-IFN α-2b (80 µg/week) and ribavirin (400 mg/bid) were also added to constitute the triple therapy. The levels of AST and ALT decreased during the first 7 d with TVR without PEG-IFN α-2b or ribavirin. Administration of allopurinol (50 mg/d) was effective against TVR-related kidney injury such as temporary increases in the levels of uric acid, serum creatinine, and BUN (Fig. 3D). Because of anemia and anorexia, the doses of TVR and ribavirin were decreased to 1000 mg/bid on day 37 and 200 mg/day on day 30, respectively. Subsequently, the severity of these adverse decreased. The patient completed the 12-week TVR therapy. After the final day of TVR administration, the dose of cyclosporine was gradually increased from 25 mg/qd to 50 mg/bid, and finally to 100 mg/bid to maintain the target trough level. At that point, the blood concentration of cyclosporine was stable at the dosage of 125 mg/bid. Over the course of 100 d, the patient showed no rejection episode.

Case IV: A 68-year-old woman who underwent living-donor liver transplantation from her offspring because of hepatocellular carcinoma and liver cirrhosis. The patient experienced hepatitis C recurrence at 5 months after transplantation, and was immediately treated with PEG-IFN α-2b and ribavirin. However, she was withdrawn from this therapy because of nausea at 15 months after liver transplantation. The calcineurin inhibitor was switched from tacrolimus to cyclosporine about a week before the administration of TVR. The dosage and trough concentration of cyclosporine at the day before TVR administration were 50 mg/bid and 109 ng/mL, respectively (Figs. 4A, B). To achieve the target trough concentration of cyclosporine (150–200 ng/mL), the dosage of cyclosporine was decreased from 50 mg/bid to 25 mg/qd at the first day of TVR administration (1500 mg/bid). At Day 7 of TVR therapy, PEG-IFN α-2b (60 µg/wk) and ribavirin (200 mg/d) were also added to constitute the triple therapy. The levels of AST and ALT returned to normal during the first 7 d of TVR treatment without PEG-IFN and ribavirin. The serum creatinine level increased from 1.02 mg/dL to 1.49 mg/dL within 4 d, and BUN and uric acid levels also increased; therefore, the administration of febuxostat (10 mg/d) was initiated. Because renal dysfunction worsened despite the temporal effect of febuxostat, the dosage of TVR was decreased to 1000 mg/bid on day 15, and the dosage of febuxostat was increased to 20 mg/d on day 16. Because of the reduced TVR dosage, the blood concentration of cyclosporine decreased to 141 ng/mL in 7 d. Because the blood concentration of cyclosporine was around the lower limit of the target window, and the renal dysfunction was not completely ameliorated, the dosage of cyclosporine was maintained at 25 mg/qd. After successful completion of the 12-week TVR treatment, the calcineurin inhibitor was switched back from cyclosporine to tacrolimus. The dosage of tacrolimus was set at 1.4 mg/bid. The dosage of ribavirin was adjusted considering adverse reactions such as a skin rash on her back and a decrease in hemoglobin levels. The patient noticed the skin rash on her back on day 23 of TVR treatment, and her hemoglobin level decreased from 10.3 g/dL to 9.1 g/dL within 9 d. These adverse reactions were presumably caused by ribavirin; therefore, the dosage of ribavirin was decreased to 200 mg/qod on day 29. The dosage of ribavirin was further decreased to 200 mg every 3 d on day 43, 200 mg every 4 d on day 57, and finally, she was withdrawn from ribavirin on day 70. No signs of rejection were observed over the course of 100 d of TVR administration.

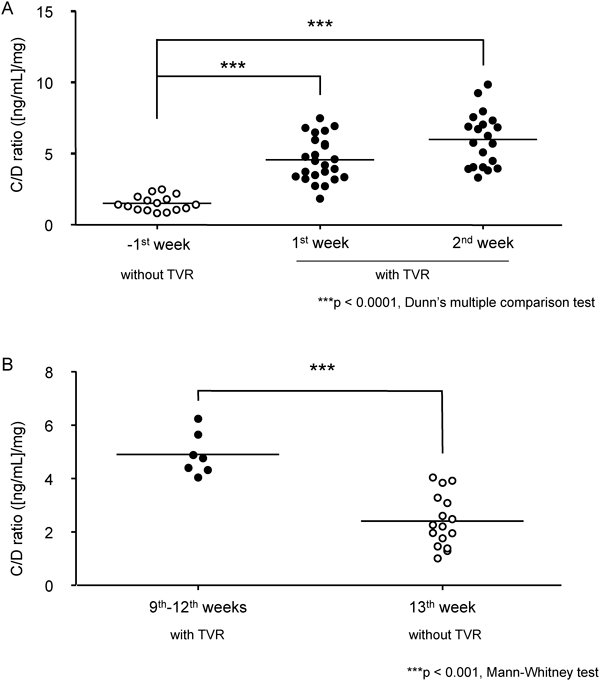
In Case I, the average cyclosporine doses during 7 d before and after beginning administration of TVR were 157.1 mg/bid and 46.4 mg/qd, respectively. In the case of all patients, the dosage of cyclosporine decreased by about 60% after initiation of administration of TVR. The data of concentration/dose ratios (C/D ratio, (ng/mL)/mg) of cyclosporine from all study patients are shown in Fig. 5. When TVR therapy was initiated, the C/D ratio increased 3.04-fold from the −1st week to the 1st week. In addition, the C/D ratio of cyclosporine during the 2nd week significantly increased (p<0.0001). On the other hand, after completion of TVR therapy, the C/D ratio decreased from 4.90 to 2.91.
DISCUSSION
Previously, the standard therapy for liver transplantation patients with chronic hepatitis C has been double combination therapy with PEG-IFN α and ribavirin.13–16) Recently, TVR is a new drug with strong efficacy against hepatitis C when administrated in combination with PEG-IFN and ribavirin.17,18) TVR has strong interactions with drugs metabolized by CYP3A4 and CYP3A5.5,6) Liver transplant patients have to take immunosuppressive agents such as tacrolimus and cyclosporine to avoid graft failure, and these agents are metabolized by CYP3A4 and CYP3A5. The blood concentrations of tacrolimus and cyclosporine increase with the administration of TVR. Therefore, to control the blood concentration of tacrolimus and cyclosporine, treatment with the new TVR therapy is important to prevent recurrence of hepatitis C in patients after liver transplantation. Garg et al. showed TVR increased the area under the curve of tacrolimus and cyclosporine to 70.3-fold and 4.11-fold, respectively.7) Therefore, we switched the immunosuppressive agent from tacrolimus to cyclosporine in this study. On the basis of our predictions, the dosage of cyclosporine was decreased after initiation of TVR administration, and the blood concentration of cyclosporine was unstable. Moreover, the average dosage of cyclosporine decreased to 0.24–0.40-fold before initiation of TVR (data not shown). To maintain the target trough level of cyclosporine, we measured the blood concentration of cyclosporine 3 times a day during the 1st week of TVR administration. This was important to prevent graft rejection. In Case II, the peak blood concentration of cyclosporine was low and the trough level was difficult to decrease. To avoid liver graft rejection, the C2 blood concentration was maintained at the target level.19) On the other hand, the trough level of cyclosporine was kept low to prevent adverse effects on the kidney.20,21) Therefore, we determined that the dosage of cyclosporine would not be 25 mg/d, but rather 50 mg/qod. Thus, we controlled not only the total dosage of cyclosporine but also the timing of administration.20) The dosage adjustment of cyclosporine immediately after the end of TVR treatment is an important issue, because the rapid reduction of blood level of cyclosporine should lead the risk of acute rejection. In the present study, we found the rapid elimination of inhibitory effect of TVR on the disposition of cyclospirne in the all four cases (Fig. 5B). Based on these findings, rapid increase in the dosage of cyclosporine would be required to maintain the immunosuppressive effect throughout the anti HCV therapy with or without TVR.
Recently, we reported the drug interactions with tacrolimus in patients after living-donor liver transplantation.22–24) Uesugi et al. showed that the CYP3A5 genotype in the graft liver and native intestine had an effect on the blood concentration of tacrolimus.25) TVR inhibits not only CYP3A4 but also CYP3A5. We did not examine the CYP3A5 genotype in this study. However, the differences in the CYP3A5 genotype may affect the blood concentration of tacrolimus. Recently, Zheng et al. showed a correlation between CYP3A5 genotype and the risk of cyclosporine-induced nephrotoxicity.26) Therefore, the genotype of CYP3A5 may be important in implementing TVR therapy.
Almost all patients experienced HCV recurrence, and some underwent re-transplantation because of HCV-related liver cirrhosis or hepatocellular carcinoma.27,28) The disease progression of hepatitis C is faster in graft liver than in healthy liver,8,9) and the rate of SVR is lower in transplant patients than in patients who did not receive liver transplants.29) In this study, all patients completed the 12-week TVR triple therapy. In all patients, the HCV RNA levels immediately decreased to the baseline levels (Table 2). This indicated that PEG-IFN α-2b, ribavirin, and TVR therapy were effective against hepatitis C. Moreover, HCV RNA levels decreased and liver function markedly improved with TVR alone. However, SVR was detected 24 weeks after combination therapy with PEG-IFN α-2b, ribavirin, and TVR.4,17,30,31) Therefore, these results did not suggest SVR, but these might be steps to SVR.
Table 2. Transition of HCV RNA before and after the TVR Treatment
| Case | Before (days before TVR treatment)a) | Time after TVR treatment (week) |
|---|
| 1 | 2 | 4 |
|---|
| (log IU/mL) |
| Case I | 7.2 (7) | 4.7 | 3.0 | 1.6 |
| Case II | 6.5 (12) | 2.3 | N.D.b) | N.D. |
| Case III | 6.1 (6) | 2.3 | 1.2> | N.D. |
| Case IV | 4.5 (8) | 1.2> | N.D. | N.D. |
TVR, telaprevir; HCV, hepatitis C virus. a) The latest day before administration of TVR. b) N.D., HCV RNA was not detected by real-time PCR.
The dose of TVR had to be reduced in cases II, III, and IV because of severe adverse effects. TVR is known to elicit a severe rash. In Case IV, the level of hemoglobin decreased, and the dosage of ribavirin was gradually decreased. Cases II and IV showed skin rash, and Cases II and III experienced malaise or anorexia. The latter adverse effects were caused not only by TVR but also by PEG-IFN. Therefore, the adverse reactions might be a synergistic effect of triple therapy. To achieve successful outcome with the PEG-IFN, ribavirin, and TVR triple therapy, maintaining these adverse reactions become less bad may be key points to continuing this therapy. The difference between Case I and the others was the level of HCV RNA after administration of TVR. In Case I, the HCV RNA level rapidly decreased; however, these levels decreased almost immediately in Cases II–IV. In addition, the HCV RNA level in Case IV decreased to limits of detection with only TVR (Table 2). The connection between these adverse effects and therapeutic benefit of this therapy was difficult to determine. Small doses of TVR, for example 1000 mg/d, might be beneficial to treat HCV and to avoid adverse effects in Cases II, III, and IV. Therefore, dose reduction from the standard dose (2250 mg/d) is suitable for Japanese HCV-recurrence patients after liver transplantation.12) In addition, the association between TVR blood level profile and its adverse reactions should be examined in future to individualized dosage adjustment of TVR.
In all patients, the serum creatinine levels were temporarily increased at the 1st week of TVR administration. However, in Case IV, during TVR treatment, the serum creatinine remained at a high level (1.41±0.25 mg/dL). Cyclosporine or TVR might cause renal dysfunction; therefore, cyclosporine was replaced with tacrolimus immediately after TVR therapy. To treat avoid adverse effects of cyclosporine and the risk of re-control of cyclosporine, triple therapy should be administered with tacrolimus as the immunosuppressive agent.
In conclusion, we could treat patients with recurrence of hepatitis C after liver transplantation by TVR therapy to avoid liver graft rejection. Controlling the drug interaction between TVR and cyclosporine was the most important aspect to achieving both treatment of hepatitis C and prevention of liver graft rejection. We selected cyclosporine instead of tacrolimus as the immunosuppressive agent and carefully adjusted the dosage of cyclosporine by frequent measurement of blood concentration. It was risky to change the immunosuppressive agent from tacrolimus to cyclosporine for graft rejection. Therefore, in the future, we will need to control the blood concentration of tacrolimus, which has a strong interaction with TVR, to treat HCV with TVR triple therapy.
Acknowledgment
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and by a Funding Program for Next Generation World-Leading Researchers (NEXT Program: LS073) initiated by the Council for Science and Technology Policy of the Japan Society for the Promotion of Science of Japan.
REFERENCES
- 1) Forestier N, Zeuzem S. Telaprevir for the treatment of hepatitis C. Expert Opin. Pharmacother., 13, 593–606 (2012).
- 2) Fowell AJ, Nash KL. Telaprevir: a new hope in the treatment of chronic hepatitis C? Adv. Ther., 27, 512–522 (2010).
- 3) Kim JJ, Culley CM, Mohammad RA. Telaprevir: an oral protease inhibitor for hepatitis C virus infection. Am. J. Health Syst. Pharm., 69, 19–33 (2012).
- 4) Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J. Hepatol., 56, 78–84 (2012).
- 5) Wilby KJ, Greanya ED, Ford JA, Yoshida EM, Partovi N. A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplant patients. Ann. Hepatol., 11, 179–185 (2012).
- 6) Kiser JJ, Burton JR, Anderson PL, Everson GT. Review and management of drug interactions with boceprevir and telaprevir. Hepatology, 55, 1620–1628 (2012).
- 7) Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology, 54, 20–27 (2011).
- 8) Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl., 9, S28–S34 (2003).
- 9) Sánchez-Fueyo A, Restrepo JC, Quinto L, Bruguera M, Grande L, Sanchez-Tapias JM, Rodes J, Rimola A. Impact of the recurrence of hepatitis C virus infection after liver transplantation on the long-term viability of the graft. Transplantation, 73, 56–63 (2002).
- 10) Tanaka K, Lake J, Villamil F, Levy G, Marotta P, Mies S, de Hemptinne B, Moench C. Comparison of cyclosporine microemulsion and tacrolimus in 39 recipients of living donor liver transplantation. Liver Transpl., 11, 1395–1402 (2005).
- 11) Suzuki F, Suzuki Y, Sezaki H, Akuta N, Seko Y, Kawamura Y, Hosaka T, Kobayashi M, Saito S, Arase Y, Ikeda K, Mineta R, Watahiki S, Kobayashi M, Nakayasu Y, Tsuda H, Aoki K, Yamada I, Kumada H. Exploratory study on telaprevir given every 8 h at 500 mg or 750 mg with peginterferon-alpha-2b and ribavirin in hepatitis C patients. Hepatol. Res., 43, 691–701 (2013).
- 12) Chayama K, Hayes CN, Ohishi W, Kawakami Y. Treatment of chronic hepatitis C virus infection in Japan: update on therapy and guidelines. J. Gastroenterol., 48, 1–12 (2013).
- 13) Mukherjee S, Rogge J, Weaver L, Schafer DF. Pilot study of pegylated interferon alfa-2b and ribavirin for recurrent hepatitis C after liver transplantation. Transplant. Proc., 35, 3042–3044 (2003).
- 14) Neff GW, delaGarza J, Shire N, Nishida S, O’Brien CB, Safdar K, Madariaga J, Schiff E, Ruiz P. The long-term effects of immune suppression on liver transplant recipients with recurrent hepatitis C viral infection. Transplant. Proc., 36, 3065–3067 (2004).
- 15) Ueda Y, Takada Y, Marusawa H, Egawa H, Uemoto S, Chiba T. Individualized extension of pegylated interferon plus ribavirin therapy for recurrent hepatitis C genotype 1b after living-donor liver transplantation. Transplantation, 90, 661–665 (2010).
- 16) Castells L, Vargas V, Allende H, Bilbao I, Luis Lazaro J, Margarit C, Esteban R, Guardia J. Combined treatment with pegylated interferon (alpha-2b) and ribavirin in the acute phase of hepatitis C virus recurrence after liver transplantation. J. Hepatol., 43, 53–59 (2005).
- 17) McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ, PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N. Engl. J. Med., 360, 1827–1838 (2009).
- 18) Kieffer TL, De Meyer S, Bartels DJ, Sullivan JC, Zhang EZ, Tigges A, Dierynck I, Spanks J, Dorrian J, Jiang M, Adiwijaya B, Ghys A, Beumont M, Kauffman RS, Adda N, Jacobson IM, Sherman KE, Zeuzem S, Kwong AD, Picchio G. Hepatitis C viral evolution in genotype 1 treatment-naive and treatment-experienced patients receiving telaprevir-based therapy in clinical trials. PLoS ONE, 7, e34372 (2012).
- 19) Treckmann J, Paul A, Ozcelik A, Saner F, Malago M, Nadalin S, Lang H, Malamutman E, Gerken G, Broelsch CE. Efficacy of C0 and C2 monitoring in adult liver transplant recipients treated with neoral, mycophenolate mofetil, and steroids. Transplant. Proc., 39, 3234–3236 (2007).
- 20) Fukudo M, Yano I, Masuda S, Katsura T, Ogura Y, Oike F, Takada Y, Tanaka K, Inui K. Cyclosporine exposure and calcineurin phosphatase activity in living-donor liver transplant patients: twice daily vs. once daily dosing. Liver Transpl., 12, 292–300 (2006).
- 21) Rodrigo E, Ruiz JC, Angeles de Cos M, Ruiz J, Gago M, Pinera C, Sanchez B, Gonzalez-Cotorruelo J, Gomez-Alamillo C, Arias M. Correlation of C0 and C2 levels with cyclosporine side effects in kidney transplantation. Transplant. Proc., 41, 2328–2331 (2009).
- 22) Hosohata K, Masuda S, Katsura T, Takada Y, Kaido T, Ogura Y, Oike F, Egawa H, Uemoto S, Inui K. Impact of intestinal CYP2C19 genotypes on the interaction between tacrolimus and omeprazole, but not lansoprazole, in adult living-donor liver transplant patients. Drug Metab. Dispos., 37, 821–826 (2009).
- 23) Hosohata K, Masuda S, Ogura Y, Oike F, Takada Y, Katsura T, Uemoto S, Inui K. Interaction between tacrolimus and lansoprazole, but not rabeprazole in living-donor liver transplant patients with defects of CYP2C19 and CYP3A5. Drug Metab. Pharmacokinet., 23, 134–138 (2008).
- 24) Hosohata K, Masuda S, Yonezawa A, Sugimoto M, Takada Y, Kaido T, Ogura Y, Oike F, Uemoto S, Inui K. Absence of influence of concomitant administration of rabeprazole on the pharmacokinetics of tacrolimus in adult living-donor liver transplant patients: a case-control study. Drug Metab. Pharmacokinet., 24, 458–463 (2009).
- 25) Uesugi M, Masuda S, Katsura T, Oike F, Takada Y, Inui K. Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharmacogenet. Genomics, 16, 119–127 (2006).
- 26) Zheng S, Tasnif Y, Hebert MF, Davis CL, Shitara Y, Calamia JC, Lin YS, Shen DD, Thummel KE. CYP3A5 gene variation influences cyclosporine A metabolite formation and renal cyclosporine disposition. Transplantation, 95, 821–827 (2013).
- 27) Brown RS. Hepatitis C and liver transplantation. Nature, 436, 973–978 (2005).
- 28) Berenguer M, Prieto M, Palau A, Rayon JM, Carrasco D, Juan FS, Lopez-Labrador FX, Moreno R, Mir J, Berenguer J. Severe recurrent hepatitis C after liver retransplantation for hepatitis C virus-related graft cirrhosis. Liver Transpl., 9, 228–235 (2003).
- 29) Lavezzo B, Franchello A, Smedile A, David E, Barbui A, Torrani M, Ottobrelli A, Zamboni F, Fadda M, Bobbio A, Salizzoni M, Rizzetto M. Treatment of recurrent hepatitis C in liver transplants: efficacy of a six versus a twelve month course of interferon alfa 2b with ribavirin. J. Hepatol., 37, 247–252 (2002).
- 30) Danta M. Telaprevir triple combination therapy, and dual peginterferon alpha-2a/ribavirin therapy for 24 weeks for those with rapid-early response is not inferior to 48 weeks of therapy for treatment-naive patients with genotype 1 hepatitis C virus infection. Evid. Based Med., 17, 143–144 (2012).
- 31) Ozeki I, Akaike J, Karino Y, Arakawa T, Kuwata Y, Ohmura T, Sato T, Kamiya N, Yamada I, Chayama K, Kumada H, Toyota J. Antiviral effects of peginterferon alpha-2b and ribavirin following 24-week monotherapy of telaprevir in Japanese hepatitis C patients. J. Gastroenterol., 46, 929–937 (2011).






