2014 Volume 37 Issue 4 Pages 597-603
2014 Volume 37 Issue 4 Pages 597-603
The effects of L-ascorbic acid and its stable analogue L-ascorbic acid 2-glucoside on the restoration of liver mass and recovery of liver function after 70% partial hepatectomy (PH), were compared with other natural vitamin C analogues in rats in vivo. L-Ascorbic acid (100 mg/kg/d, intraperitoneally (i.p.))- and L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.)-treated rats showed an approximately 1.3-fold increase in the ratio of liver weight (LW) to body weight (BW), when compared to saline (as control)-, L-dehydroascorbic acid (150 mg/kg/d, i.p.)- and D-isoascorbic acid (150 mg/kg/d, i.p.)-administrated rats on day 3 after PH. Accordingly, 5-bromo-2′-deoxyuridine-labeling index in the regenerating liver was significantly higher in L-ascorbic acid- and L-ascorbic acid 2-glucoside-treated rats compared with saline-, L-dehydroascorbic acid- and D-isoascorbic acid-treated rats on day 1. In control rats, liver-related serum alanine aminotransferase (ALT) activity was rapidly elevated on day 1, and then decreased to near pre-operative levels on day 5 following PH. L-Ascorbic acid and L-ascorbic acid 2-glucoside significantly lowered the serum ALT on day 1 after PH compared with saline-, L-dehydroascorbic acid- and D-isoascorbic acid-administered rats. These results demonstrate that L-ascorbic acid and L-ascorbic acid 2-glucoside significantly promote the regeneration of liver mass and function with full recovery after liver injury.
L-Ascorbic acid, also known as vitamin C, is a nutritional supplement essential for preventing scurvy.1,2) L-Ascorbic acid is classified as a water-soluble vitamin, as are vitamins B6 and B12. L-Ascorbic acid is reversibly oxidized in the body to L-dehydroascorbic acid, which retains full vitamin C activity. Vitamin C is an essential nutrient for the biosynthesis of collagen and L-carnitine, and for the conversion of dopamine to norepinephrine.3,4) The liver is an important target for the antioxidant effects of vitamin C, and plays a role in body vitamin homeostasis. Several reviews have summarized our current understanding of the physiology and pharmacology of vitamin C.5–7)
L-Ascorbic acid and its derivatives can inhibit or stimulate the growth of healthy and tumorous cells in vitro, depending on the cell type.8–12) However, the cellular mechanisms of these actions are poorly understood.
The response of adult rat hepatocytes to L-ascorbic acid has not been investigated with respect to DNA synthesis and proliferation in vitro. More recently, we reported that L-ascorbic acid and its analogous are able to stimulate hepatocyte DNA synthesis and proliferation during short-term (e.g., 4 h) culture in primary cultures of adult rat hepatocytes by interacting with the insulin-like growth factor (IGF)-I receptor site and by activating the receptor tyrosine kinase/extracellular-signal regulated kinase (ERK) pathway.13) However, the effects of L-ascorbic acid and its analogues on liver regeneration in vivo and the mechanisms involved remain to be clarified.
Partial hepatectomy (PH) is the most often used stimulus to study liver regeneration, because in contrast to methods that use hepatic toxins (e.g., carbon tetrachloride), it is not associated with severe inflammation and tissue necrosis, and initiation of the regenerative stimulus is precisely defined (i.e., loss of hepatic tissue).14,15) Furthermore, in vivo investigation of the effects of L-ascorbic acid on hepatic growth response is of particular importance, as L-ascorbic acid and its analogues may improve liver function by stimulating liver regeneration.
The purpose of this study was to evaluate in vivo the effects of L-ascorbic acid and its stable analogue L-ascorbic acid 2-glucoside on the restoration of liver mass and recovery of liver function vs. the effects of natural L-ascorbic acid analogues (L-dehydroascorbic acid and D-isoascorbic acid) in 70% partially hepatectomized rats. To examine whether L-ascorbic acid protects the integrity of the liver after 70% partial hepatectomy, we measured the activity of liver-related cytosolic enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in sera, and examined histological changes in the liver.
Our results demonstrate that administration of L-ascorbic acid or L-ascorbic acid 2-glucoside stimulates liver regeneration following hepatocyte DNA synthesis after 70% partial liver resection, and more rapidly normalizes serum ALT levels compared with saline-, L-dehydroascorbic acid- and D-isoascorbic acid-treated groups.
Seven-week-old male Wistar rats weighing about 200 g were obtained from Tokyo Experimental Animal Co. (Tokyo, Japan). Rats were housed at a controlled temperature of 24°C under a 12-h light-dark cycle, and were fed standard laboratory chow and water ad libitum. Rats were handled humanely in accordance with the Guidelines for the Care and Use of Laboratory Animals of Josai University.
70% Partial Hepatectomy and Drug Administration70% (two-thirds) partial hepatectomy was performed under ether anesthesia according to the method of Higgins and Anderson,16) with minor modifications. Briefly, the left lateral and median hepatic lobes, constituting approximately 70% of the total liver weight, were ligated and resected. In sham-operated controls that were similarly anesthetized, livers were briefly removed from the peritoneal cavity, but not tied or excised.
Rats were randomly divided into 4 groups that received either a solvent (saline), L-ascorbic acid, L-ascorbic acid 2-glucoside, D-isoascorbic acid or L-dehydroascorbic acid. Each group was treated with intraperitoneal administration of either control (saline, 0.5 mL/kg), L-ascorbic acid (100 mg/kg), L-ascorbic acid 2-glucoside (50 mg/kg), L-dehydroascorbic acid (150 mg/kg) or D-isoascorbic acid (150 mg/kg), once per day (at 10 a.m.). Administration was repeated and continued for 1 to 14 d unless otherwise indicated.
Determination of Liver RegenerationAll rats were killed at the indicated time points after 70% partial hepatectomy under ether anesthesia. Regenerating livers were excised, and moist liver weight after regeneration (LW) and body weight (BW) was measured. The ratio of total liver weight after regeneration was normalized against body weight (LW/BW) and was used as an indicator of liver regeneration.17)
5-Bromo-2′-deoxyuridine (BrdU) IncorporationLiver regeneration monitored by mitotic index demonstrates nuclear 5-bromo-2′-deoxyuridine (BrdU) incorporation into hepatocyte DNA after 70% partial hepatectomy, according to a previously described method.18,19) BrdU, a thymidine analogue, is incorporated into hepatocyte nuclei during DNA synthesis (S phase in cell cycle). BrdU incorporation is a useful indicator of the effects of growth factors on cell proliferation in various experimental systems. BrdU (50 mg/kg, intraperitoneally (i.p.)) was injected into rats and 2 h later, the rats were anesthetized with ether and the livers removed. Briefly, livers were fixed in 10% neutralized formalin and embedded in paraffin. Paraffin-embedded tissue sections (5 µm) were deparaffinized and stained with hematoxylin. For assay of the labeling index, deparaffinized liver sections were incubated in 2 N HCl for 30 min, washed several times with phosphate-buffered saline (pH 7.4), and stained with anti-BrdU monoclonal antibody. BrdU was detected using avidin DH-biotinylated horseradish peroxidase complex according to the manufacturer’s instructions (Exalpha, Biologicals, Inc., Shirley, MA, U.S.A.). The number of labeled nuclei out of 1000 nuclei in a randomly selected field was then counted under a light microscope.
In order to detect any histological changes on day 1 after 70% partial hepatectomy, sections of regenerating liver were stained with hematoxylin and eosin.
Determination of Serum Transaminase ActivityIn order to establish whether L-ascorbic acid and its analogues are able to affect the integrity of livers of 70% partially hepatectomized rats, we measured the activities of liver-related cytosolic enzymes in sera. After remnant livers were removed, blood samples were quickly collected from the inferior vena cava. Enzyme assays were performed in normal rats with either control (saline 0.5 mL/kg/d, i.p.), L-ascorbic acid (100 mg/kg/d, i.p.), L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.), L-dehydroascorbic acid (150 mg/kg/d, i.p.) or D-isoascorbic acid (150 mg/kg/d, i.p.) administration. Serum ALT and AST activities were assayed using a commercially available diagnostic kit according to the manufacturer’s instructions (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Enzyme reaction was achieved by adding substrate with diaminobenzidine. One unit of enzyme activity was defined as a change in optical density of 0.001/min/mL of serum.
MaterialsL-Ascorbic acid, L-ascorbic acid 2-glucoside, L-dehydroascorbic acid and dehydroascorbic acid, and insulin-like growth factor (IGF)-I were obtained from Wako Pure Chemical Industries, Ltd. Monoclonal antibodies against IGF-I receptor were obtained from Oncogene Research Products (Cambridge, MA, U.S.A.). The following reagents were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.): 5-bromo-2′-deoxyuridine (BrdU). BrdU was dissolved in 50% dimethylsulfoxide at 120 mg/mL (stock solution). Monoclonal anti-BrdU antibody and BrdU Immunohistochemistry Assay Kit were purchased from Exalpha Biologicals, Inc. (Shirley, MA, U.S.A.). Assay kits for serum ALT and AST were obtained from Wako Pure Chemical Industries, Ltd. All other reagents were of analytical grade.
Statistical AnalysisResults are presented as means±S.E.M. Group comparisons were performed by ANOVA for unpaired data followed by post-hoc analysis using Dunnett’s multiple comparison test. A p<0.05 was considered to be statistically significant.
In order to examine the effects of L-ascorbic acid on regeneration of remnant liver after 70% partial hepatectomy, L-ascorbic acid (100 mg/kg/d, i.p.) and L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.) were administered intraperitoneally to rats, and the effects were compared with those of L-dehydroascorbic acid (150 mg/kg/d, i.p.), D-isoascorbic acid (150 mg/kg/d, i.p.) and insulin-like growth factor (IGF)-I (5 µg/kg/d, i.p.). The ratio of total liver weight after regeneration normalized against body weight was used as an indicator of liver regeneration.17) After 70% partial hepatectomy, LW/BW ratio increased rapidly and reached an initial peak on day 5 in saline-administered control rats, whereas the ratio increased significantly by day 3 in L-ascorbic acid (100 mg/kg/d, i.p.)-treated rats (Fig. 1). The L-ascorbic acid-treated rats showed an approximately 1.3-fold increase over saline-administered controls on day 3 after 70% partial hepatectomy. This response was statistically significant. The ratio of LW/BW peaked for a second time on day 10 after 70% partial hepatectomy and the moist liver weight was almost restored to pre-operative levels. Body weight did not differ significantly between the L-ascorbic acid-administered group and saline-administered controls from days 1 to 14. L-Ascorbic acid 2-glucoside-treated rats (50 mg/kg/d, i.p.) peaked on day 5, and the time course that was associated with the effects of L-ascorbic acid 2-glucoside was similar to that of L-ascorbic acid, as assessed by LW/BW ratio (Fig. 1). LW/BW ratio increased rapidly and reached an initial peak on day 3 in IGF-I (5 µg/kg/d, i.p.)-treated rats, and the time course associated with the effects of IGF-I was very similar to those of L-ascorbic acid and L-ascorbic acid 2-glucoside (Fig. 1). There were no significant differences in ratios calculated from the weight of remnant livers to body weight in rats given L-dehydroascorbic acid (150 mg/kg) or D-isoascorbic acid (150 mg/kg) once per day after 70% partial hepatectomy from days 1 to 14 when compared with saline-administered controls (Fig. 1).

Rats were treated according to the experimental schedule described in Materials and Methods. Saline (control; 0.5 mL/kg), L-ascorbic acid (100 mg/kg), L-ascorbic acid 2-glucoside (50 mg/kg), L-dehydroascorbic acid (150 mg/kg), D-isoascorbic acid (150 mg/kg) or IGF-I (5 µg/kg) was injected intraperitoneally (i.p.) once daily (at 10 a.m.), after which remnant livers were removed rapidly under ether anesthesia at the time points indicated and weighed. Rats (n=5–8) were used in each experimental group. Data are expressed as a percentage of LW/BW on day 0. 100%: Sham-operated rats. Values are expressed as means±S.E.M. * p<0.05 ; ** p<0.01 vs. saline-treated controls.
As the recovery of liver mass following 70% partial hepatectomy peaked 3 d after L-ascorbic acid-treatment, we examined the dose–response effect of L-ascorbic acid on LW/BW ratio at 3 d after hepatectomy, as compared with that of L-ascorbic acid 2-glucoside, L-dehydroascorbic acid, D-isoascorbic acid and IGF-I. As shown in Fig. 2A, the dose–response curve indicates that small doses of L-ascorbic acid (1 and 10 mg/kg/d, i.p.) are less effective, and that higher doses (50 to 150 mg/kg/d, i.p.) stimulate liver regeneration. The maximal effect induced by L-ascorbic acid was achieved with 100 mg/kg. L-Ascorbic acid 2-glucoside showed a normal dose–response curve with a maximal dose of 50 mg/kg. On co-administration of monoclonal antibody against insulin-like growth factor-I receptor (10 µg/kg/d, i.p.) with L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.), the effects of L-ascorbic acid 2-glucoside on LW/BW ratio were completely inhibited. D-isoascorbic acid and L-dehydroascorbic acid (1–150 mg/kg/d, i.p.) did not significantly increase the LW/BW ratio at any of the doses tested. IGF-I as a positive control showed a normal dose–response curve (Fig. 2B). The maximal effect induced by IGF-I was achieved with 5 µg/kg/d, i.p. On co-administration of monoclonal antibody against IGF-I receptor (10 µg/kg/d, i.p.) with IGF-I (1–10 µg/kg/d, i.p.), the effects of IGF-I on LW/BW ratio were completely inhibited. Monoclonal antibody against IGF-I receptor (10 µg/kg/d, i.p.) did not significantly affect LW/BW ratio when co-administrated with saline (data not shown).
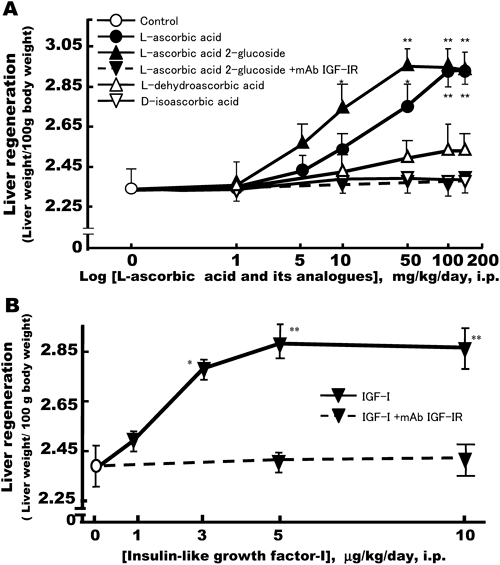
Rats were treated according to the experimental schedule described in Materials and Methods. (A) Control (saline; 0.5 mL/kg/d, i.p.), L-ascorbic acid (1–150 mg/kg/d, i.p.), L-ascorbic acid 2-glucoside (1–150 mg/kg/d, i.p.), with or without monoclonal antibody against IGF-I receptor (10 µg/kg/d, i.p.), L-dehydroascorbic acid (1–150 mg/kg/d, i.p.) or D-isoascorbic acid (1–150 mg/kg/d, i.p.). (B) IGF-I (1–10 µg/kg/d, i.p.) with or without monoclonal antibody against IGF-I receptor (10 µg/kg/d, i.p.). Remnant livers were removed rapidly under ether anesthesia on day 3 after 70% partial hepatectomy, and weighed. The ratio of total liver weight (LW) after regeneration normalized against body weight (BW) was used as an indicator of liver regeneration. Data is expressed as a percentage of LW/BW on day 0. Rats (n=5–8) were used in each experimental group. Values are means±S.E.M. * p<0.05; ** p<0.01 vs. saline-treated controls.
Sections of regenerative liver were stained with hematoxylin and eosin to aid observation of histological changes on day 3 after 70% partial hepatectomy (Figs. 3A, B). Histology showed that there were almost no morphological abnormalities, such as cell swelling, atypical changes in tissue or infiltration of cells, in sections of remnant regenerating livers treated with saline (B), L-ascorbic acid (100 mg/kg/d) (C) and L-ascorbic acid 2-glucoside (50 mg/kg) (D), as compared with sham-operated controls (A) at lower magnification (3A) and higher magnification (3B).
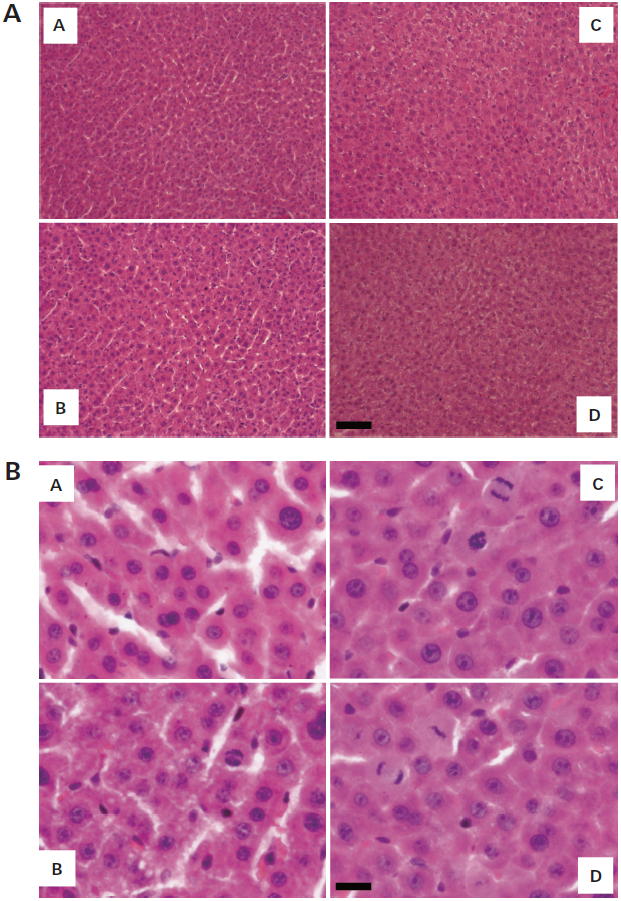
Histological changes in the livers of rats without any treatment (sham operation), or treatment with saline (control), L-ascorbic acid or L-ascorbic acid 2-glucoside, on day 3 after 70% partial hepatectomy were examined as described in Materials and Methods. Liver specimens were fixed in cold 10% neutralized formalin and embedded in paraffin. Paraffin-embedded tissue sections (5 µm) were deparaffinized and stained with hematoxylin and eosin. (A) sham surgery; (B) control (saline; 0.5 mL/kg/d, i.p.); (C) L-ascorbic acid, 100 mg/kg/d, i.p.; (D) L-ascorbic acid 2-glucoside, 50 mg/kg/d, i.p. Bar represents 50 µm (3A) and 10 µm (3B).
In Fig. 4, light micrographs show the typical distribution of hepatocytes undergoing DNA synthesis in the remnant liver of rats after sham operation (A), solvent (B), L-ascorbic acid (C) or L-ascorbic acid 2-glucoside (D) treatment on day 1 after 70% partial hepatectomy. Hepatocytes were pulsed with 5-bromo-2′-deoxyuridine (BrdU) for 2 h in vivo and were visualized by immunochemical staining, whereby dark spots indicate the hepatocytes that are undergoing DNA synthesis. The results show that L-ascorbic acid and L-ascorbic acid 2-glucoside, but not sham-operated mice, significantly stimulate hepatocyte DNA synthesis. The BrdU labeling index of L-ascorbic acid 2-glucoside-treated rats was significantly greater than that of L-ascorbic acid-treated rats on day 1 after 70% partial hepatectomy. Hepatocytes undergoing DNA synthesis in both L-ascorbic acid- and L-ascorbic acid 2-glucoside-treated animals are randomly distributed within hepatic lobes.
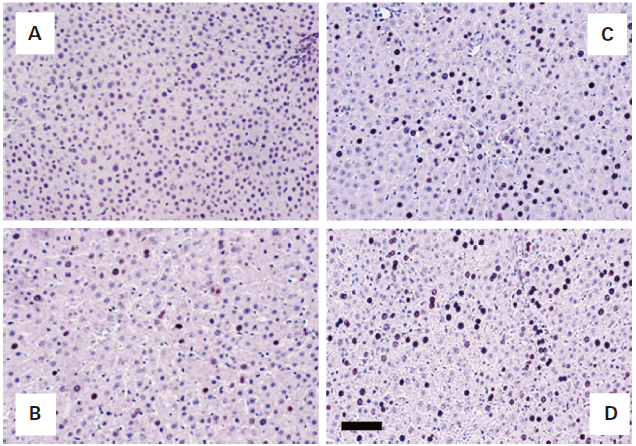
Light micrographs showing typical distributions of hepatocytes undergoing DNA synthesis in remnant livers of rats treated without any treatment (sham operation) or with a saline (control), L-ascorbic acid or L-ascorbic acid 2-glucoside treatment, on day 1 after 70% partial hepatectomy. Cells were pulsed with 5-bromo-2′-deoxyuridine (BrdU) in vivo. Liver sections (5 µm) were stained with hematoxylin following immunohistochemical staining with monoclonal antibody against BrdU, as described in Materials and Methods. Dark spots indicate hepatocytes undergoing DNA synthesis. (A) sham operation; (B) control (saline, 0.5 mL/kg/d, i.p.); (C) L-ascorbic acid, 100 mg/kg/d, i.p.; (D) L-ascorbic acid 2-glucoside, 50 mg/kg/d, i.p. Bar represents 50 µm.
Time course-associated effects of L-ascorbic acid and its analogues on the BrdU labeling index in hepatic parenchymal cells are shown in Fig. 5. Even though BrdU labeling index of sham-operated rat liver sections before 70% partial hepatectomy was less than 0.1%, it increased moderately from the first day after the procedure in solvent-treated controls. Thereafter, it decreased to pre-operative levels at day 4 after 70% partial hepatectomy. In contrast, rats administered 100 mg/kg/d, L-ascorbic acid showed a significant increase in BrdU labeling from 1 to 15% on day 1, which then declined to 5% on day 2. In both groups, the BrdU-labeling indexes returned to pre-operative levels on days 3, 4 and 5 after 70% partial hepatectomy. These results indicate that L-ascorbic acid and L-ascorbic acid 2-glucoside significantly extended the mitogenic activity of hepatocytes in livers after 70% partial hepatectomy when compared with the control group. The effects of L-dehydroascorbic acid (150 mg/kg/d) and D-isoascorbic acid (150 mg/kg/d) were not statistically significant when compared with saline-treated controls (data for D-isoascorbic acid not shown).
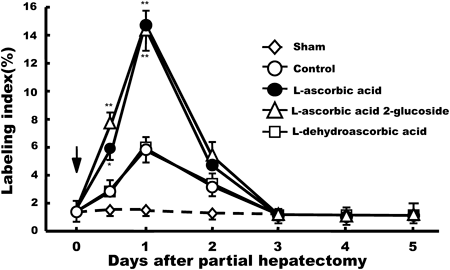
BrdU-mitotic indexes were determined by BrdU incorporation into DNA and from immunohistochemical staining using an anti-monoclonal antibody to BrdU on days 0–5 after partial hepatectomy, as described in the Fig. 4 legend. BrdU labeling index in saline-, L-ascorbic acid- and L-ascorbic acid 2-glucoside-treated rats indicates the percentage of BrdU-positive hepatocyte nuclei out of 1000 nuclei in a randomly selected field under a light microscope. Values are means±S.E.M. in five different rats. * p<0.05; ** p<0.01 vs. saline-treated controls.
Of the known liver-related enzymes, transaminases (ALT and AST) in serum are good markers of liver injury or damage. To examine whether L-ascorbic acid affects liver integrity after partial hepatectomy, we measured ALT and AST activity in sera. As shown in Fig. 6, serum levels of ALT increased rapidly and peaked on day 1 in solvent-administered control rats after 70% partial hepatectomy. ALT activity rapidly returned to pre-hepatectomy levels in saline-treated control animals on day 3, and this continued for a further 11 d. However, on day 1 L-ascorbic acid (100 mg/kg/d, i.p.) and L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.) inhibited the elevation of serum ALT activity induced by 70% partial hepatectomy. The transaminase-lowering effects of L-ascorbic acid and L-ascorbic acid 2-glucoside were significant when compared with saline-treated control groups on days 1 and 3 after 70% partial hepatectomy. On the other hand, L-dehydroascorbic acid (150 mg/kg/d, i.p.) and D-isoascorbic acid (150 mg/kg/d, i.p.) did not reduce serum ALT activity when compared with saline-treated controls (Fig. 6).
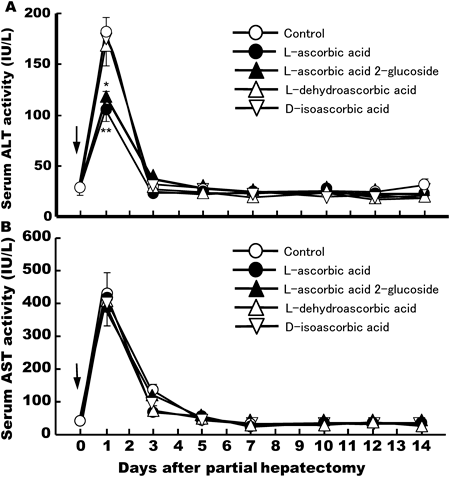
Time course-associated effects of L-ascorbic acid (100 mg/kg/d, i.p.), L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.), L-dehydroascorbic acid (150 mg/kg/d, i.p.) or D-isoascorbic acid (150 mg/kg/d, i.p.), on serum ALT (A) and AST (B) activities assessed on days 0–14 after 70% partial hepatectomy, as described in Materials and Methods. Data from control, L-ascorbic acid-, L-ascorbic acid 2-glucoside-, L-dehydroascorbic acid- and D-isoascorbic acid-treated rats are shown as means±S.E.M. (n=5–6). * p<0.05; ** p<0.01 vs. saline-treated controls.
AST activity also increased significantly on day 1, after 70% partial hepatectomy in control rats and those treated with L-ascorbic acid and its analogue (Fig. 6). On days 1 to 14 L-ascorbic acids and its analogue had no significant effects on elevated AST levels induced by 70% partial hepatectomy, as compared to saline-treated controls.
Dose-Dependent Effects of L-Ascorbic Acid and Its Analogues on Serum Transaminase ActivitiesWe next examined the dose–response effects of L-ascorbic acid and its analogue on liver-specific transaminase ALT and AST activity in serum on day 1 following 70% partial hepatectomy. As shown in Fig. 7, L-ascorbic acid, and L-ascorbic acid 2-glucoside dose-dependently lowered ALT activity with a maximal effect at 100 mg/kg/d, but had no effect on AST activity. L-Dehydroascorbic acid and D-isoascorbic acid did not significantly affect either serum ALT or AST activity in 70% partially hepatectomized rats when compared to saline-treated controls.
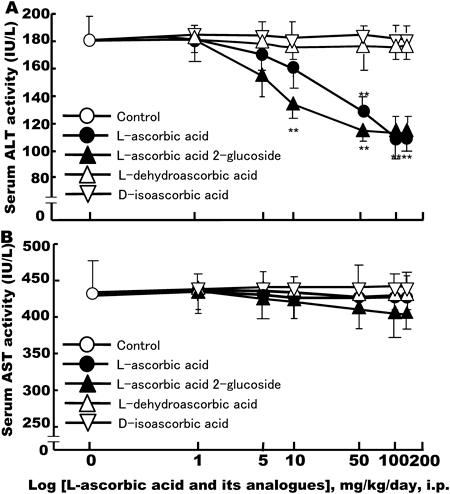
Rats were treated according to the experimental schedule described in Materials and Methods. In L-ascorbic acid-, L-ascorbic acid 2-glucoside-, L-dehydroascorbic acid- or D-isoascorbic acid (each 1–150 mg/kg/d, i.p.)-treated rats, serum ALT (A) and AST (B) activity was assessed on day 1 after 70% partial hepatectomy. Serum ALT and AST activity was determined as described in Fig. 6 legend. Each experimental group contained 5–6 rats. Values are means±S.E.M. ** p<0.01 vs. saline-treated controls.
In this study, we evaluated whether exogenous administration of L-ascorbic acid (100 mg/kg/d, i.p.) and L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.) is able to stimulate liver regeneration in 70% partially hepatectomized rats in vivo.
We showed that restoration was almost complete by the 12th day in saline-administered control rats, based on the ratio of LW to BW (LW/BW) (Fig. 1). These data are consistent with those of Higgins and Anderson.16) In the present study, administration of L-ascorbic acid (100 mg/kg/d, i.p.) or L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.) was found to accelerate remnant liver regeneration after 70% partial hepatectomy when compared with saline-treated control rats. Liver regeneration was also monitored by mitotic index, which demonstrates nuclear 2-bromo-5′-deoxyuridine (BrdU) incorporation into hepatocyte DNA after 70% partial hepatectomy, according to a previously established method.18,19) Administration of L-ascorbic acid (100 mg/kg/d, i.p.) or L-ascorbic acid 2-glucoside (50 mg/kg/d, i.p.) significantly stimulated the BrdU labeling index at days 0.5 and 1 post-hepatectomy compared to saline-treated controls, whereas treatment with L-dehydroascorbic acid (150 mg/kg/d, i.p.) or D-isoascorbic acid (150 mg/kg/d, i.p.) only slightly stimulated the BrdU labeling index (Fig. 5). To our knowledge, this is the first report showing that L-ascorbic acid or L-ascorbic acid 2-glucoside is able to stimulate hepatocyte DNA synthesis during liver regeneration in 70% partially hepatectomized rats in vivo. L-Dehydroascorbic acid, an oxidized form of L-ascorbic acid, did not affect hepatocyte mitogenesis. L-Dehydroascorbic acid was actively imported into the endoplasmic reticulum of cells via glucose transporters. It was trapped therein by reduction back to L-ascorbic acid by glutathione and other thiols.20) In addition, D-isoascorbic acid, which has antioxidant activity but not the biological activity of vitamin C,21) did not affect hepatocyte mitogenesis. These results suggest that the stimulatory effects of L-ascorbic acid and its stable analogue, L-ascorbic acid 2-glucoside on hepatocyte DNA synthesis and cell proliferation are unrelated to their antioxidant or hydrophilic properties.
As shown in Fig. 2A, we found that dose-dependent stimulation of liver regeneration by L-ascorbic acid 2-glucoside and L-ascorbic acid (data not shown) at day 3 after 70% partial hepatectomy, was completely inhibited by co-administration of monoclonal anti-IGF-I receptor antibody. IGF-I as a positive control showed a normal dose–response curve. Co-administration of monoclonal antibody against IGF-I receptor with IGF-I also completely inhibited the LW/BW ratio (Fig. 2B). These results are consistent with our in vitro studies, in which L-ascorbic acid 2-glucoside was found to be more potent than L-ascorbic acid in stimulating hepatocyte DNA synthesis and proliferation through the IGF-I receptor in primary hepatocyte cultures from adult rats.13) Recently, Chen et al. showed that pharmacologically high concentrations of ascorbic acid induced apoptosis in vitro and inhibited tumor growth in vivo, in contrast to the doses we used.22,23)
On the other hand, serum patterns of lactate dehydrogenase (LDH), ALT and AST activities after 70% partial hepatectomy in rats have been reported previously.24) The increase in serum enzyme activity during the onset of liver proliferation could be related to cell necrosis or enhanced cell membrane permeability. Indeed, it has been concluded that increased serum activity of some enzymes after partial hepatectomy may be due to early release either from damaged cells or by cells with altered permeability, probably involving enhanced synthesis and release of enzymes.25)
In order to evaluate the degree of liver injury after 70% partial hepatectomy in vivo, the serum activity of liver-related enzymes, such as ALT and AST, was assayed (Figs. 6, 7). We found that serum ALT and AST activity increase rapidly and significantly after 70% partial hepatectomy on day 1, returning to almost pre-operative levels after 2–3 d in saline-administered control rats. An explanation for this is that in response to damage around the ligated area of the liver, a local inflammatory reaction may occur, leading to temporary increases in serum ALT and AST activity. We found that hepatic resection-induced increases in serum ALT activity, but not AST activity, were significantly inhibited in animals treated with L-ascorbic acid and L-ascorbic acid 2-glucoside, as compared to saline-administered controls, D-isoascorbic acid- or L-dehydroascorbic acid-treated group (Figs. 6, 7). On the other hand, we previously reported that serum ALT and AST activity returned more rapidly to near pre-hepatectomy levels in animals treated with glycyrrhizin, which is known to be a liver-supporting agent, and epidermal growth factor (EGF), after 70% partial hepatectomy on day 1.26,27) In addition, among vitamin A and its natural derivatives, all-trans-retinoic acid (ATRA) stimulated liver regeneration without affecting serum ALT and AST activity after 70% partial hepatectomy on day 1.28) These biochemical data support the notion that moderate inflammatory responses occur during the initial stages of hepatic resection. L-Ascorbic acid and L-ascorbic acid 2-glucoside may act by inhibiting production of specific hepatocyte inflammatory mediators, such as signal transducer and activator of transcription 3 (STAT3) and nuclear factor κ light-chain enhancer of activated B cells.29,30) Glycyrrhizin and EGF may act by inhibiting the production of nonspecific inflammatory mediators, such as prostaglandins and leukotriens.26) However, the reasons why L-ascorbic acid did not affect serum AST activity remain to be elucidated.
From a therapeutic point of view, liver regeneration therapy for fulminant hepatitis is unsatisfactory and so sufficient liver regeneration cannot be expected after resection. In addition, liver function is markedly impaired in hepatectomized patients, from hepatitis, cirrhosis and hepatic cancer with persistent elevation of serum ALT levels.31,32) Hence, it is important to stimulate both the regeneration of mass and the recovery of function in the remnant liver after damage. However, at present, safe and economical therapies that promote proliferation of hepatocytes have yet to be discovered.33–35) Therefore, there is a need for the development of an agent that specifically promotes liver regeneration and recovery of liver function, and that is safe to use during treatment for fulminant liver hepatitis, hepatic failure and hepatic cancer resection.22,32,35,36) Lower doses of L-ascorbic acid and L-ascorbic acid 2-glucoside could be used for this purpose.
In conclusion, we showed for the first time that L-ascorbic acid and its stable analogue L-ascorbic acid 2-glucoside are potent stimulators of normal hepatocyte proliferation and restoration of liver function in vivo after 70% partial hepatectomy via stimulation of IGF-I receptor, but not antioxidant activity, in rats.