Abstract
The purpose of this study was to investigate the permeability of exendin-4-loaded chitosan nanoparticles using the Madin Darby canine kidney (MDCK) cell monolayer as an in vitro model and the rat intestine as an ex vivo model of the human intestinal barrier. A series of formulations of sodium tripolyphosphate (TPP) and chitosan with different molecular weights and degrees of deacetylation was evaluated. The formulation consisting of 0.1% TPP and 0.2% chitosan (400 kDa, 95% degree of deacetylation), which gave optimized monodispersed particle size (303.1±10.36 nm), zeta potential (18.37±1.15 mV) and encapsulation efficiency (38.0±2.6%), was used for further analysis. After determining their biocompatibility, the transport potential of drug-loaded chitosan nanoparticles was evaluated and compared with free exendin-4 using both MDCK cell monolayers and different rat intestinal segments. Mechanisms underlying enhanced transport of exendin-4 in the cell model were also explored. Compared with free exendin-4, the absorption of optimized chitosan nanoparticles was enhanced by 4.7-fold in MDCK cell monolayers and by 2.0–2.78-fold in different rat intestinal segments, with no significant difference between the duodenum, jejunum and ileum. As supported by confocal laser scanning microscopic analysis, the lower enhancement of absorption in the intestine compared to the cell monolayer likely resulted from the chitosan nanoparticle-mediated opening of cellular tight junctions and not through intracellular transport. These findings suggest that the potential application of chitosan nanoparticles as delivery carriers of exendin-4 is limited and may need further modifications.
Exendin-4, an injectable 39-amino-acid peptide found in lizard saliva, is a glucagon-like peptide-1 (GLP-1) receptor agonist, which has been approved for the treatment of type 2 diabetes by the U.S. Food and Drug Administration (FDA) since 2005.1–3) Exendin-4 exerts many beneficial antidiabetic bioactivities, including induction of glucose-dependent insulin secretion, suppression of high glucagon secretion, slowing of gastric emptying to modulate nutrient absorption, reduction of food intake and body weight, improving pancreatic endocrine function and increasing β-cell mass.4,5) More recently, exendin-4 extended-release microsphere, the first once-weekly treatment for type 2 diabetes, was approved by the FDA in 2012. Although improved to some extent by the reduced number and frequency of injections, the therapeutic utility of exendin-4 in this formulation is still limited due to patient non-compliance related to both the expense associated with injection and pain at the site of injection. Therefore, many studies have focused on the development of non-injection routes of administration for this drug, such as the aerosol,6) intranasal7) and sublingual8) routes. Among these, the development of oral exendin-4 preparations remains a great challenge. Gedulin et al. reported that the bioavailability of exendin-4 delivered via the intraduodenal route was 0.0053%, relative to that by subcutaneous (s.c.) administration.8) The minimal bioavailability is assumed to be due to the excessive intestinal enzymatic degradation and poor penetration of the drug across the intestinal membrane.9,10) Our previous study demonstrated that the apparent permeability coefficient (Papp) of exendin-4 across the Madin Darby canine kidney (MDCK) cell monolayer was approximately 1×10−7 cm/s and that this drug was transported mainly via the passive paracellular pathway.11) Moreover, we also found that the addition of an absorption enhancer, such as chitosan, sodium decanoate and ethylenediaminetetraacetic acid, could significantly improve the transport of exendin-4 by 2.2 to 11.9-fold without apparent cytotoxicity.11)
Chitosan is a straight-chain copolymer composed of glucosamine and N-acetyl-glucosamine, which can be obtained by partially deacetylating naturally occurring chitin. The proportions of glucosamine and N-acetyl-glucosamine determine the degree of deacetylation of the polymer, while the length of the chain determines its molecular weight. With a pKa of approximately 6.5 on the amine groups, chitosan is soluble and positively charged at acidic pH but is insoluble at neutral pH. The degree of deacetylation together with chain length fundamentally determines the polymer properties, such as hydrophobicity and the ability to interact electrostatically with negatively charged substances. It has been reported that chitosan can bind to the cell membrane and increase paracellular permeability in a reversible and dose-dependent manner.12) The mechanism includes interactions of chitosan with tight junction proteins occludin and ZO-1, redistribution of F-actin and disturbance of the plasma membrane, which appears to be mediated by the positive charges on amino groups of chitosan.13) Recently, it was found that chitosan treatment of Caco-2 cell monolayers at pH 6.4 could still lead to a redistribution of claudin-4 from the cell membrane to the cytosol followed by lysosomal degradation, ultimately resulting in a decrease in tight junction integrity and an increase in paracellular permeability.14) These new insights suggest that the disruption of tight junctions probably occurs through multiple mechanisms. Besides increasing permeability, chitosan exhibits its mucoadhesive property via interacting with negatively charged sialic acid residues on mucin at physiological pH.15) Incorporating chitosan into micro/nanoparticles also preserves mucoadhesion, which along with the abovementioned features contribute to its widespread evaluation for oral delivery of therapeutic agents. Nguyen et al. developed exendin-4-loaded nanoparticles composed of chitosan and poly(γ-glutamic acid) by using a simple ionic gelation method, and the prepared nanoparticles were then freeze-dried and filled in enteric-coated hard gelatin capsules to release nanoparticles in the proximal segment of the intestine.16) The oral bioavailability of this formulation was found to be approximately 15% relative to its s.c. administered counterpart, but detailed information on the effect of formulation on the intestinal mucosa and absorption mechanism across the intestine were not provided.
The aim of the present study was to investigate the effect of molecular weight and degree of deacetylation on the characteristics of exendin-4-loaded chitosan nanoparticles, as well as evaluate their permeability and mechanism of transport across an MDCK cell monolayer as an intestinal epithelial cell model. In addition, the transport potential of chitosan nanoparticles was studied with different segments of the rat intestine. This paper would provide the new insight into the potential application of chitosan nanoparticles in oral delivery of exendin-4.
MATERIALS AND METHODS
MaterialsExendin-4 was from BCHT Biopharm Co., Ltd. (Changchun, China). Chitosan (molecular weight: 200000–400000 Da; degree of deacetylation: 85% and 95%) was purchased from AK Biotech (Shandong, China). Sodium tripolyphosphate (TPP), 3-(2-benzothiazolyl)-7-(diethylamine) coumarin (6-coumarin) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Hank’s balanced salt solution (HBSS), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), non-essential amino acids (NEAA) solution, penicillin–streptomycin solution and trypsin solution were obtained from Invitrogen (Carlsbad, CA, U.S.A.). Millicell cell culture inserts (24-well, 0.6 cm2 and 0.1 µm) were purchased from Millipore (Billerica, MA, U.S.A.), and 24-well Costar cluster trays were purchased from Corning (Corning, NY, U.S.A.). The fluorescent lipophilic dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was from Molecular Probes (Carlsbad, CA, U.S.A.). All other chemical products were commercially available and of analytical grade.
Methods. Nanoparticle PreparationChitosan nanoparticles were prepared by ionotropic gelation of chitosan with TPP anions.17) Briefly, chitosan of different molecular weights and degrees of deacetylation was dissolved in acetic aqueous solution at different concentrations (1, 2, 3 mg/mL), and TPP was dissolved in water at different concentrations (1, 2, 3 mg/mL). Exendin-4 was dissolved in TPP solution at the concentration of 1 mg/mL. Finally, 1 mL of TPP solution was added dropwise to 3 mL of chitosan solution while vortex mixing to obtain a nanoparticle suspension. Nanoparticles were separated by centrifugation at 12000 rpm at 4–8°C for 30 min. The effects of different chitosan pH values were evaluated on nanoparticle characteristics, such as particle size, zeta potential, polydispersity index and exendin-4 entrapment efficiency.
Nanoparticle CharacterizationParticle sizes were measured by dynamic light scattering (DLS) using the Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, U.K.) and 90° angle collection optics. In addition, zeta potentials were determined based on the electrophoretic mobility of the nanoparticles in aqueous medium with the same instrument.
To estimate the exendin-4 entrapment efficiency, nanoparticles were centrifuged at 12000×g for 30 min, and the protein content of the supernatant was measured by the micro-BCA Assay (Thermo Fisher Scientific, Pierce, Waltham, MA, U.S.A.). The entrapment efficiency of exendin-4 was calculated by comparing the actual and theoretical amount of exendin-4 loaded into nanoparticles, and the results were presented as means±standard deviation (S.D.).
MDCK Cell CultureThe MDCK cell line was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultivated in standard conditions with DMEM medium supplemented with 10% FBS, 1% NEAA and 1% penicillin–streptomycin and cultured in a humidified incubator at 37°C with 5% CO2. Experiments were performed with cells following 70–85 passages.
Cytotoxicity StudiesCytotoxicity of the nanoparticles was evaluated using an MTT assay. MDCK cells were seeded in 96-well plates (Corning) at a density of 5×103 cells/well and cultured for 24 h. Subsequently, exendin-4 and exendin-4-loaded nanoparticles were added to the wells at various concentrations. After 2 h of co-incubation at 37°C, 100 µL of MTT (0.5 mg/mL) was added to each well and incubated for a further 4 h at 37°C. After incubation, the medium was removed, and 100 µL of dimethylsulfoxide (DMSO) was added to the residual precipitates. The absorbance of the resulting DMSO solution was determined at 590 nm using an iMark microplate reader (Bio-Rad, Hercules, CA, U.S.A.). Cell viability was expressed as a percentage of the absorbance relative to that of the control. Control cells were not exposed to any materials. Experiments were performed with five replicate wells for each sample and control.
Confocal Laser Scanning MicroscopyMDCK cells were grown on cover glass (Thermo Fisher Scientific) at a density of 3×105 cells/well. When the cells were confluent, either fluorescein isothiocyanate (FITC)–exendin-4 or FITC–exendin-4-loaded CS nanoparticles were added to the plates for 2 h at 37°C. Cells were then washed five times with ice-cold phosphate buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min at 37°C and then counterstained with 5 µM DiI for 10 min at 37°C. Thereafter, the stained cells were observed using a Carl Zeiss LSM 710 Confocal Laser Scanning Microscope. The excitation wavelengths of FITC–exendin-4 were 488 nm and 543 nm, respectively.
Transmembrane Permeability StudiesMDCK cells were seeded in Millicell 24-well cell culture insert plates at a density of 7.5×104 cells/well. During the transmembrane permeability experiments, the integrity of cell monolayers was assessed by transepithelial electrical resistance (TEER) measurements at fixed times using a Millicell ERS meter (Millipore, Bedford, MA, U.S.A.). MDCK cell monolayers (TEER ≥300 Ω·cm2) were washed with HBSS and then either a solution of exendin-4 or exendin-4-loaded nanoparticles, in which the concentration of exendin-4 was 50 µg/mL, was added to the donor (upper) compartment at 37°C for 2 h. The flux of exendin-4 in the receiver (lower) compartment was measured with LC-MS/MS (Agilent Technologies, Santa Clara, CA, U.S.A.),11) and the Papp (cm/s) was calculated by the following equation: Papp (cm/s)=(dQ/dt)×(1/A×C0), where dQ/dt is the increase in the amount of drug in the receiver chamber per time interval, C0 is the initial concentration in the donor compartment, and A is the permeation area of the cell culture insert.
Rat Everted Gut Sac ExperimentsWistar male rats, weighing 230–260 g, were obtained from the Experimental Animal Center of Norman Bethune College of Medicine (Jilin University, Changchun, China). The rats were cared for in accordance with the guidelines of the Animal Use Committee of Jilin University for the use and care of laboratory animals. Rats were housed in groups of 6–8 under a 12-h light/dark cycle and allowed food and water ad libitum. Everted gut sacs were prepared as previously described.18,19) Briefly, male rats were fasted overnight (approximately 18 h) before the experiment but had free access to water. After each rat was anesthetized with 1% pentobarbital sodium (4.0 mL/kg, intraperitoneal injection), a midline laparotomy was performed to expose the abdomen. The intestinal segments of interest were identified (duodenum, jejunum and ileum) and cut into sacs of 10 cm in length. The sacs were immediately placed in 37°C HBSS and carefully everted using a glass rod, and the mucus was removed by careful stripping. One end of the segment was tied with a silk thread and filled with fresh HBSS (1 mL). The sacs were kept in 37°C HBSS or HBSS solution containing exendin-4 or exendin-4-loaded nanoparticles (50 µg/mL), and then samples were taken after 60 min from inside the sacs for analysis by LC-MS/MS.11)
Lactate Dehydrogenase (LDH) AssayTo evaluate the epithelial damage in the rat everted gut sac model, LDH release into the receival solution was investigated at 15, 30, 45 and 60 min in the ileum sac. LDH activity was determined using a commercial kit (CytoTox96® Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI, U.S.A.). The LDH release level in the samples was expressed as a percentage of that detected in HBSS only.
Statistical AnalysisAll results are presented as the mean±S.D. of more than three replicates. Differences between two means were analyzed using the Student’s t-test. A p value <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Formulation and Characterization of NanoparticlesChitosan nanoparticles are formed through inter/intramolecular cross-linking of the amino groups of chitosan with the negatively charged phosphate groups of the anionic cross-linking agent TPP. It has been found that chitosan and TPP concentrations, chitosan to TPP weight ratio and the pH value of the aqueous chitosan solution all have large influences on the nanoparticle synthesis, particle size, zeta potential and encapsulation efficiency of drugs.
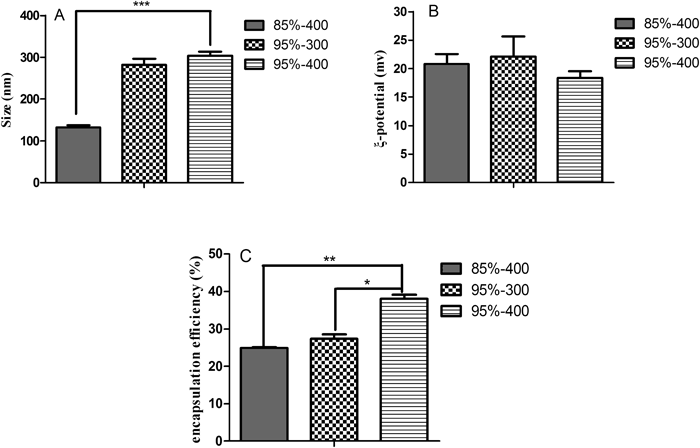
First, a series of formulations was evaluated to identify the range of concentrations of TPP and chitosan where nanoparticles can be formed. It is noteworthy that, in all formulations, the pH of the chitosan solution was maintained at 4 to ensure full ionization of all amino groups in the chitosan chain.20) As shown in Table 1, the molecular weights and degrees of deacetylation of chitosan showed significant effects on nanoparticle synthesis. The chitosan nanoparticles could only be produced in a specific concentration range of chitosan and TPP, beyond or below which large aggregations occurred or no particles were formed. For the experimental group of 400 kDa and 85% degree of deacetylation chitosan, the suitable concentrations of chitosan were 0.2% (with 0.1 and 0.2% TPP) and 0.3% (with 0.2% TPP). In the case of the 300 kDa and 95% degree of deacetylation chitosan group, the suitable concentrations of chitosan were 0.1% (with 0.1% TPP) and 0.2% (with 0.1% and 0.2% TPP). For the 400 kDa and 95% degree of deacetylation chitosan group, the suitable concentrations of chitosan were 0.2% (with 0.1% TPP) and 0.3% (with 0.1% and 0.2% TPP). No particles were formed at the TPP concentration of 0.3% for all three types of chitosan. It has been reported that when solid nanometric structures are formed, the chitosan : TPP weight ratio should normally be within the range of 3 : 1–6 : 1.17,21) However, conditions for the formation of nanoparticles may differ significantly depending on the degree of deacetylation and molecular weight of chitosan employed, as evidenced in this study and other reports.22,23)
Table 1. Effect of Concentrations of Chitosan and TPP on Nanoparticle Synthesis
| Chitosan concentration (w/v) |
|---|
| 0.1% | 0.2% | 0.3% |
|---|
| TPP concentration (w/v) | TPP concentration (w/v) | TPP concentration (w/v) |
|---|
| 0.1% | 0.2% | 0.3% | 0.1% | 0.2% | 0.3% | 0.1% | 0.2% | 0.3% |
|---|
| 85%,a) 400 kDa | − | − | − | + | + | − | − | + | − |
| 95%,a) 300 kDa | + | − | − | + | + | − | − | − | − |
| 95%,a) 400 kDa | − | − | − | + | − | − | + | + | − |
a) Deacetylation degree of chitosan. In all formulations, chitosan solution at pH 4.0 was used. −, No nanoparticles were formed. +, Nanoparticles were formed.
Once the concentration range of particle formation was defined, the effect of pH on the characteristics of nanoparticles was examined. As shown in Fig. 1, the particle size increased from 115 nm to 141 nm, and the zeta potential was decreased from +32.2 to +21.0 mv upon increasing pH from 2 to 4. Between pH 4–5, the sizes and zeta potentials of the nanoparticles did not change significantly. When the pH level increased to 6, a larger particle size of 173 nm and a lower zeta potential of +6.0 mv were observed. The amino group in chitosan is known to have a pKa value of 6.5,24) which leads to protonation in acidic to neutral solutions with a charge density dependent on pH. A higher pH value reflects a lower hydrogen ion concentration, and therefore fewer protonated amino groups, which may explain the observed decreases in charge density and zeta potential of particles with increasing pH in the current study. On the other hand, fewer protonated amino groups in the chitosan chain would lead to less chemical cross-linking with anions of TPP, resulting in less dense and larger particles. The slight change in size and zeta potential at pH range between 4 and 5 was likely closely related to the negative charge numbers of TPP and protonation degree of amino groups. This observation is further supported by the fact that, at pH 4–5, the charge numbers per free TPP molecule has been shown to reach a plateau phase with a value of 2.8–3.0, while more than 95% of amino groups in the chitosan chain are protonated.20)

Based on the concentration range amendable to particle formation, the effects of deacetylation degree and molecular weight of chitosan on the characteristics of nanoparticles were also examined. Nanoparticles were produced with 0.2% chitosan and 0.1% TPP and the pH of 4. As shown in Fig. 2 increasing both the degree of deacetylation and molecular weight of chitosan increased the particle size and simultaneously improved the encapsulation efficiency of exendin-4, whereas only a slight change in zeta potential was observed. The similar zeta potentials in the range of 18–25 were assumed to have resulted from the same protonation degree of amino groups under the same pH. Although the difference in degree of deacetylation was believed also to have an effect, it may not have rendered a significant contribution. In agreement with other studies, the higher molecular weight chitosan, which resulted in larger sized particles, showed a higher capacity for drug entrapment due to the longer chain of chitosan molecules and greater electrostatic interactions between chitosan and the drug.25,26) In other words, the increase in entrapment efficiency from 24.9 to 38.0% was likely related to an increase in electrostatic interactions occurring between chitosan and exendin-4 molecules due to more positively charged points on chitosans with higher degrees of deacetylation.27) Interestingly, the enhancement in size of nanoparticles from 131 to 310 nm as the result of a higher degree of deacetylation degree was inconsistent with other studies, but the related mechanism will require further investigation. In considering the comprehensive effects on mean diameter, zeta potential and encapsulation efficiency, the formulation consisting of 0.1% TPP and 0.2% chitosan (400 kDa, 95% degree of deacetylation and solution pH 4) was chosen for subsequent analyses. And the resultant drug-loaded carrier displayed a sustained release profile with the cumulative release amounts of drugs 13.7±3.9%, 24.1±5.8%, 33.9±6.5% and 39.8±5.8% at 0.5, 1.0, 2.0 and 3.0 h in simulated intestinal fluid (pH 6.8), respectively.
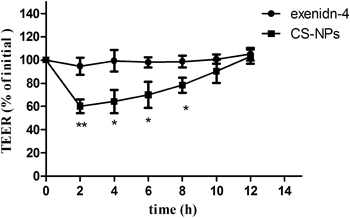
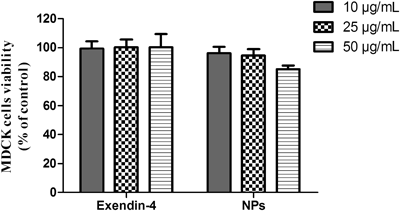
The potential cytotoxicity of chitosan nanoparticles was evaluated by both the MTT assay and TEER measurements. Results of the MTT assay showed that chitosan nanoparticles over a concentration range of 10–50 µg/mL had no adverse effects on cell viability (Fig. 3). The percentage of viable cells was still more than 85% even at a dose of 50 µg/mL of nanoparticles, which is considered to be an acceptable level of biocompatibility. TEER measurements also were used to illustrate the effect of chitosan nanoparticles on the integrity of the MDCK cell monolayer. As shown in Fig. 4, the TEER value decreased to about 60% of the original value after 2 h of incubation of the cell monolayer with exendin-4-loaded chitosan nanoparticles, suggesting an increase in permeability. However, the TEER value gradually increased after removing nanoparticles from the cell monolayer and finally recovered at 10 h after the cells were washed. The reversible effect of nanoparticles on the permeability of the cell monolayer was mainly attributed to the reversible opening of cellular tight junctions. It has been reported that chitosan-induced redistribution of claudin-4 protein from the cell membrane to the cytosol is associated with its degradation in lysosomes and a decrease in tight junction integrity, thus resulting in an increase in paracellular permeability.14) The recovery of the TEER value following the removal of chitosan was due to the reconstruction of tight junctions, which has been shown to be dependent on claudin-4 synthesis in the cytoplasm and rearrangement in the cell membrane.14) In the case of exendin-4, there was no observable effect on either cell viability or cell monolayer permeability. These results clearly showed that both exendin-4 and exendin-4-loaded chitosan nanoparticles were safe and biocompatible.
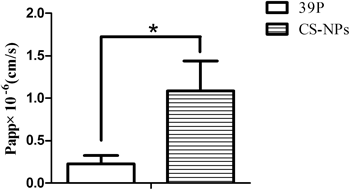
To evaluate the potential permeability enhancement effects of chitosan nanoparticles, the Papp values of exendin-4 both in solution and loaded in chitosan nanoparticles were determined in the MDCK cell monolayer model. As shown in Fig. 5, the Papp of exendin-4 in solution was 0.23±0.10×10−6 cm/s, and that of exendin-4 loaded in chitosan nanoparticles increased up to 1.08±0.36×10−6 cm/s. Although nanoparticle encapsulation improved the permeability of exendin-4 by up to 4.70-fold, the increase was lower in comparison with that of exendin-4 in chitosan solution, which resulted in an 11.9-fold increase in our previous studies. Theoretically, paracellular transport of nanoparticles through the space between cells is not feasible because the interstitial space, ranging from several angstroms to few nanometers, is too small for chitosan nanoparticles to permeate.28) It has also been reported that even in a fully opened state, the width of a tight junction is less then 20 nm.29) Therefore, the limited increase in permeability of exendin-4 resulting from nanoparticle encapsulation was likely attributed to passive diffusion of released exendin-4 from nanoparticles via the paracellular pathway. This reasonable assumption was further supported by TEER measurements, which reflected the chitosan nanoparticle-mediated opening of tight junctions.
In order to further confirm the above conclusion, interactions of free FITC–exendin-4 in solution and FITC–exendin-4-loaded nanoparticles with the MDCK cell monolayer were examined by confocal laser scanning microscopy. Images in Fig. 6 illustrate the cell membrane outlines of the MDCK monolayer by localization of the Dil fluorescent dye (red). The merged images show the green fluorescence of free FITC–exendin-4 or FITC–exendin-4-loaded nanoparticles located between the intercellular spaces, and the yellow color represents the co-localization of FITC–exendin-4-loaded nanoparticles and the cellular membrane. The results suggested that the vast majority of FITC–exendin-4-loaded chitosan nanoparticles were not transported via an intracellular pathway in the MDCK cell model. Combined with the analysis above, it is reasonable to conclude from these results that the absorption enhancement of chitosan nanoparticles was mainly due to the passive diffusion of exendin-4 released from nanoparticles via the paracellular pathway by opening cellular tight junctions. Although adequate evidences to support the passive paracellular diffusion, we still can not exclude the possible contribution of transcellular transport of nanoparticles, such as endocytosis followed by exocytosis, which need further investigation. In addition, preventing exendin-4 from enzymatic degradation via encapsulation may also partly contribute to the absorption enhancement of chitosan nanoparticles through the formation of drug depot in paracellular site. In our pre-formulation study, it was found, in the presence of chymotrypsin, about 67.7% of drug loaded in chitosan nanoparticles remained unaffected within 1 h incubation in pH 6.8 solutions, while the free drug suffered a complete degradation under the same experimental conditions.

Considering that differences exist between the MDCK cell monolayer model and intestinal tissue, the effect of chitosan nanoparticles on permeability of exendin-4 was further evaluated with rat everted gut sacs. The rat small intestine was divided into three segments: duodenum, jejunum and ileum. Before the rat everted gut sac study, the biocompatibilities of both free exendin-4 in solution and exendin-4-loaded nanoparticles were investigated over a time course using the LDH assay in ileum segments. As shown in Fig. 7, the levels of LDH released in the presence of exendin-4 both in solution and in nanoparticles were 106% and 115% at 60 min, respectively, which were comparable to that of the control. The results suggested that the safety and biocompatibility of these reagents were acceptable.

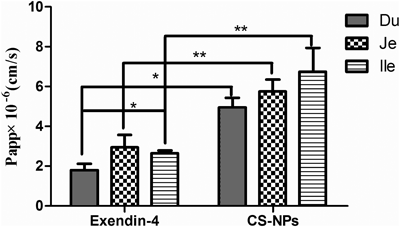
Next, Papp values of exendin-4 both in solution and loaded in nanoparticles were further evaluated. As shown in Fig. 8, the order of Papp values of exendin-4 solution reflecting absorption in different intestinal segments were: duodenum<ileum<jejunum (1.82±0.30×10−6, 2.64±0.49×10−6 and 2.96±0.62×10−6 cm/s, respectively). Except the duodenum and ileum, there were no statistically significant differences between duodenum and jejunum or between ileum and jejunum. However, the order of Papp values of exendin-4 loaded in chitosan nanoparticles in the three segments were duodenum<jejunum<ileum (4.95±0.49×10−6, 5.75±0.59×10−6 and 6.74±1.21×10−6 cm/s, respectively), with no statistically significant differences among them. The fold enhancements of the Papp value of exendin-4 after loading into chitosan nanoparticles in the duodenum, jejunum and ileum were 2.78, 2.00 and 2.55, respectively, again with no statistically significant differences. These results demonstrated that the encapsulation of exendin-4 in nanoparticles could enhance its permeation in the three different segments of the small intestine to some extent. In general, microvilli extending from the apical surface of intestinal cells, circular folds and villi within the intestinal wall will increase the surface area for absorption by approximately a few thousand fold.30) Therefore, a higher transport efficiency of nanoparticles across the rat intestine was expected. However, in comparison with the 4.7-fold enhancement in permeability of exendin-4 obtained in the MDCK cell monolayer model, chitosan nanoparticles showed a lower enhancement efficiency in rat intestinal segments, which was likely due to filtration by the mucus layer secreted by the surface mucous cells.31) In addition, the released exendin-4 may also have suffered enzymatic degradation during the infiltration through the mucus layer.30) Nguyen et al. developed an exendin-4 formulation using enteric-coated hard gelatin capsules containing freeze-dried exendin-4-loaded chitosan/poly(γ-glutamic acid) nanoparticles, which could release the loaded content in the proximal segment of the intestine.16) The oral bioavailability of this formulation, relative to its s.c. administered counterpart, reached up to approximately 15%.16) This high oral bioavailability could be attributed to the high local concentration of exendin-4-loaded nanoparticles at the release site. However, the effect of formulation on the intestinal mucosa and the related mechanism will require further studies.
CONCLUSION
In this study, a series of formulations of exendin-4 was evaluated, and the permeability of the optimized exendin-4-loaded chitosan nanoparticles across MDCK cell monolayers and rat intestine was investigated. After confirming the biocompatibility of the nanoparticles, the enhanced absorption of exendin-4 by use of chitosan nanoparticles was demonstrated in both MDCK cell monolayers and different rat intestinal segments. Compared with MDCK cell monolayers, lower enhancement of absorption was found in the rat intestine with no significant difference between the duodenum, jejunum and ileum. Further investigation into the mechanism showed that the enhanced absorption of exendin-4 mainly resulted from the chitosan nanoparticle-mediated opening of cellular tight junctions and not through intracellular transport. Thus, this study, which shows the limited permeability of exendin-4-loaded chitosan nanoparticles across both the MDCK cell monolayer and rat intestine, raises questions about their potential application as drug delivery vehicles and suggests that further modifications of chitosan nanoparticles are needed.
Acknowledgment
This work was supported by National Natural Science Foundation of China (No. 30901863 and No. 81201764) and the Fundamental Research Funds for the Central Universities (201103184).
REFERENCES
- 1) Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann. Intern. Med., 143, 559–569 (2005).
- 2) Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem., 267, 7402–7405 (1992).
- 3) Norris SL, Lee N, Thakurta S, Chan BK. Exenatide efficacy and safety: a systematic review. Diabet. Med., 26, 837–846 (2009).
- 4) DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care, 28, 1092–1100 (2005).
- 5) Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care, 27, 2628–2635 (2004).
- 6) Lee J, Lee C, Kim TH, Chi SC, Moon HR, Oh KT, Lee ES, Lee KC, Youn YS. Pulmonary administered palmitic-acid modified exendin-4 peptide prolongs hypoglycemia in type 2 diabetic db/db mice. Regul. Pept., 177, 68–72 (2012).
- 7) Kim TH, Park CW, Kim HY, Chi MH, Lee SK, Song YM, Jiang HH, Lim SM, Youn YS, Lee KC. Low molecular weight (1 kDa) polyethylene glycol conjugation markedly enhances the hypoglycemic effects of intranasally administered exendin-4 in type 2 diabetic db/db mice. Biol. Pharm. Bull., 35, 1076–1083 (2012).
- 8) Gedulin BR, Smith PA, Jodka CM, Chen K, Bhavsar S, Nielsen LL, Parkes DG, Young AA. Pharmacokinetics and pharmacodynamics of exenatide following alternate routes of administration. Int. J. Pharm., 356, 231–238 (2008).
- 9) Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit. Rev. Ther. Drug Carrier Syst., 20, 153–214 (2003).
- 10) Hamman JH, Enslin GM, Kotzé AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs, 19, 165–177 (2005).
- 11) Wang M, Sun B, Feng J, Zhang H, Liu B, Li C, Chen Y, Zhang Y, Kong W. Investigation of transport mechanism of exendin-4 across Madin Darby canine kidney cell monolayers. Biol. Pharm. Bull., 35, 745–752 (2012).
- 12) Schipper NG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm. Res., 13, 1686–1692 (1996).
- 13) Schipper NG, Olsson S, Hoogstraate JA, deBoer AG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancement. Pharm. Res., 14, 923–929 (1997).
- 14) Yeh TH, Hsu LW, Tseng MT, Lee PL, Sonjae K, Ho YC, Sung HW. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials, 32, 6164–6173 (2011).
- 15) Deacon MP, McGurk S, Roberts CJ, Williams PM, Tendler SJ, Davies MC, Davis SS, Harding SE. Atomic force microscopy of gastric mucin and chitosan mucoadhesive systems. Biochem. J., 348, 557–563 (2000).
- 16) Nguyen HN, Wey SP, Juang JH, Sonaje K, Ho YC, Chuang EY, Hsu CW, Yen TC, Lin KJ, Sung HW. The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in vivo. Biomaterials, 32, 2673–2682 (2011).
- 17) Zhang H, Oh M, Allen C, Kumacheva E. Monodisperse chitosan nanoparticles for mucosal drug delivery. Biomacromolecules, 5, 2461–2468 (2004).
- 18) Alam MA, Al-Jenoobi FI, Al-Mohizea AM. Everted gut sac model as a tool in pharmaceutical research: limitations and applications. J. Pharm. Pharmacol., 64, 326–336 (2012).
- 19) Li M, Si L, Pan H, Rabba AK, Yan F, Qiu J, Li G. Excipients enhance intestinal absorption of ganciclovir by P-gp inhibition: assessed in vitro by everted gut sac and in situ by improved intestinal perfusion. Int. J. Pharm., 403, 37–45 (2011).
- 20) Shu XZ, Zhu KJ. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm., 54, 235–243 (2002).
- 21) Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv. Drug Deliv. Rev., 47, 83–97 (2001).
- 22) Xu Y, Du Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm., 250, 215–226 (2003).
- 23) Gaspar VM, Sousa F, Queiroz JA, Correia IJ. Formulation of chitosan-TPP-pDNA nanocapsules for gene therapy applications. Nanotechnology, 22, 015101 (2011).
- 24) Filion D, Lavertu M, Buschmann MD. Ionization and solubility of chitosan solutions related to thermosensitive chitosan/glycerol-phosphate systems. Biomacromolecules, 8, 3224–3234 (2007).
- 25) Shahbazi MA, Hamidi M. The impact of preparation parameters on typical attributes of chitosan–heparin nanohydrogels: particle size, loading efficiency, and drug release. Drug Dev. Ind. Pharm., 39, 1774–1782 (2013).
- 26) Vandana M, Sahoo SK. Optimization of physicochemical parameters influencing the fabrication of protein-loaded chitosan nanoparticles. Nanomedicine (Lond.), 4, 773–785 (2009).
- 27) Kafshgari MH, Khorram M, Khodadoost M, Khavari S. Reinforcement of chitosan nanoparticles obtained by an ionic cross-linking process. Iran. Polym. J., 20, 445–456 (2011).
- 28) Nellans HN. (B) Mechanisms of peptide and protein absorption. Adv. Drug Deliv. Rev., 7, 339–364 (1991).
- 29) Adson A, Burton PS, Raub TJ, Barsuhn CL, Audus KL, Ho NF. Passive diffusion of weak organic electrolytes across Caco-2 cell monolayers: uncoupling the contributions of hydrodynamic, transcellular, and paracellular barriers. J. Pharm. Sci., 84, 1197–1204 (1995).
- 30) Daugherty AL, Mrsny RJ. Transcellular uptake mechanisms of the intestinal epithelial barrier Part one. Pharm. Sci. Technol. Today, 2, 144–151 (1999).
- 31) Sonaje K, Lin YH, Juang JH, Wey SP, Chen CT, Sung HW. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials, 30, 2329–2339 (2009).